The Pleurotus genus is one of most extensively studied white-rot fungi due to its exceptional ligninolytic properties. It is an edible mushroom that possesses biological effects, as it contains important bioactive molecules. It is a rich source of nutrients, particularly proteins, minerals as well as vitamins B, C and D. In basidiomycete fungi, intensive cultivations of edible mushrooms can often be affected by some bacterial, mold and virus diseases that rather frequently cause dramatic production loss. These infections are facilitated by the particular conditions under which mushroom cultivation is commonly carried out such as warm temperatures, humidity, carbon dioxide (CO2) levels and presence of pests. There is not much bibliographic information related to pests of mushrooms and their substrates. The updated review presents a practical checklist of diseases and pests of the Pleurotus genus, providing useful information that may help different users.
El Pleurotus es uno de los hongos de la podredumbre blanca más extensamente estudiados debido a sus excepcionales propiedades lignocelulolíticas. Es un hongo comestible y también tiene varios efectos biológicos, ya que contiene importantes moléculas bioactivas. Es una fuente rica de nutrientes, particularmente de proteínas y minerales, así como de vitaminas B, C y D. Los cultivos intensivos de hongos comestibles del tipo basidiomicetos a menudo son afectados por enfermedades bacterianas, fúngicas y virales, lo que con frecuencia produce pérdidas significativas en la producción. Estas infecciones son facilitadas por las condiciones particulares bajo las cuales comúnmente se cultivan los hongos, tales como temperaturas cálidas y elevada humedad. Esta revisión presenta una lista práctica y actualizada de enfermedades y plagas frecuentes durante el cultivo del hongo comestible Pleurotus, y proporciona información que puede ser de utilidad para los productores.
Mushrooms are a rich source of nutrients, particularly proteins, mineral vitamins as well as bioactive constituents, such as phenolic compounds, terpenes, steroids and polysaccharides39,61,62. The Pleurotus spp. of basidiomycetes class belongs to a group known as “white rot fungi” as they produce a white mycelium and are generally cultivated on non-composted lignocellulosic substrates15,69. This genus requires little growth time, compared to other mushrooms1.
Mushroom survival and multiplication are associated to a number of factors, which may act individually or have interactive effects among them4,36. Intensive cultivations of edible mushrooms can often be affected by some fungal and bacterial diseases that rather frequently cause dramatic production loss7. These infections are facilitated by the particular conditions under which the mushroom cultivation is commonly carried out, such as warm temperatures, humidity, carbon dioxide (CO2) levels and presence of pests3,4. Due to these reasons, mushroom growers are frequently challenged by mushroom disease of bacterial and fungal origin. While an increasing number of commercial farms cultivate mushrooms, growers have faced serious challenges caused by various viral infections57. Fungal viruses, namely mycoviruses, persistently infect fungal taxonomic groups, including plant pathogenic fungi and mushrooms. The infection has been known to cause few significant phenotypic effects on mushrooms. Although careful farm management and extreme hygiene may prevent major attacks, some diseases are very difficult to control. Moreover, shelf life quality is severely affected by diseases that are still asymptomatic at the time of harvest. The use of disinfectants such as chlorine (household bleach) and the application of selected fungicides is generally practiced in the cultivation of mushrooms, which involve significant costs. Moreover, the use of chemicals in cultivation leaves undesired residues, several of which have been banned from use. Most chemicals that are still allowed have failed to adequately control major mushroom diseases as resistance is easily induced25. Therefore, good alternatives have to be found.
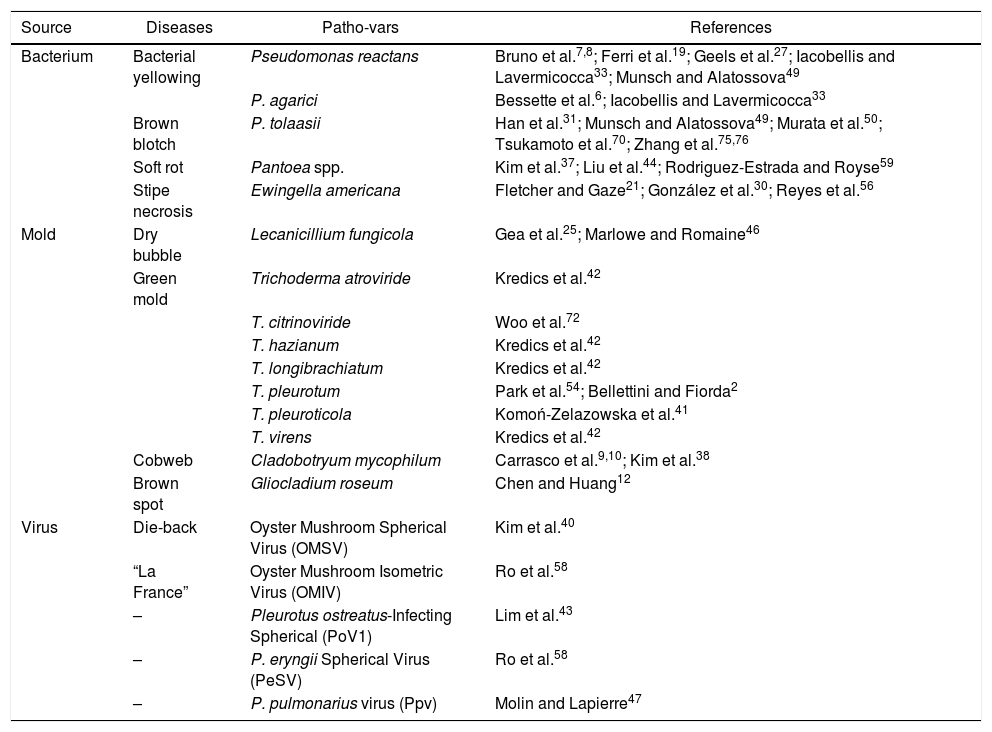
DiseasesBacterial and fungal diseases are a major problem in mushroom cultivation (Table 1); a high percentage of products are lost due to lower productivity, decrease in quality and shortened shelf life.
Diseases and pathogens noxious to Pleurotus spp. mushroom crops
| Source | Diseases | Patho-vars | References |
|---|---|---|---|
| Bacterium | Bacterial yellowing | Pseudomonas reactans | Bruno et al.7,8; Ferri et al.19; Geels et al.27; Iacobellis and Lavermicocca33; Munsch and Alatossova49 |
| P. agarici | Bessette et al.6; Iacobellis and Lavermicocca33 | ||
| Brown blotch | P. tolaasii | Han et al.31; Munsch and Alatossova49; Murata et al.50; Tsukamoto et al.70; Zhang et al.75,76 | |
| Soft rot | Pantoea spp. | Kim et al.37; Liu et al.44; Rodriguez-Estrada and Royse59 | |
| Stipe necrosis | Ewingella americana | Fletcher and Gaze21; González et al.30; Reyes et al.56 | |
| Mold | Dry bubble | Lecanicillium fungicola | Gea et al.25; Marlowe and Romaine46 |
| Green mold | Trichoderma atroviride | Kredics et al.42 | |
| T. citrinoviride | Woo et al.72 | ||
| T. hazianum | Kredics et al.42 | ||
| T. longibrachiatum | Kredics et al.42 | ||
| T. pleurotum | Park et al.54; Bellettini and Fiorda2 | ||
| T. pleuroticola | Komoń-Zelazowska et al.41 | ||
| T. virens | Kredics et al.42 | ||
| Cobweb | Cladobotryum mycophilum | Carrasco et al.9,10; Kim et al.38 | |
| Brown spot | Gliocladium roseum | Chen and Huang12 | |
| Virus | Die-back | Oyster Mushroom Spherical Virus (OMSV) | Kim et al.40 |
| “La France” | Oyster Mushroom Isometric Virus (OMIV) | Ro et al.58 | |
| – | Pleurotus ostreatus-Infecting Spherical (PoV1) | Lim et al.43 | |
| – | P. eryngii Spherical Virus (PeSV) | Ro et al.58 | |
| – | P. pulmonarius virus (Ppv) | Molin and Lapierre47 |
Bacteriosis is an unpredictable disease that can occur during the first or second sporophore flush, causing great yield loss. Destructive disease levels are induced by environmental conditions occurring at high relative humidity levels in growing chambers. Favorable to bacterial blotch outbreaks are the excess of water in the casing layer and a low aeration rate in the growing-house. These conditions can induce the occurrence of morphological variants or aggressive pathovars of Pseudomonas reactans and Pseudomonas tolaasii48.
According to Lo Cantore and Iacobellis45, the etiology of lesions on cultivated Pleurotus ostreatus involves a complex composed by interactions between Pseudomonas spp., P. tolaasii and P. reactans; however individually these bacteria cause different symptoms. P. tolaasii is consistently associated with brown-reddish blotches on P. ostreatus pseudo-tissues. Pseudomonas spp. and P. reactans are mostly associated with superficial yellow lesions on P. ostreatus sporocarps, which in pathogenicity assays have caused yellow discoloration of the sporocarps.
Environmental controls, including low relative humidity, temperature, carbon dioxide level, as well as cleaning cultivation rooms play important roles in diminishing the spread of the disease3. Nair and Bradley51 emphasized the importance of keeping mushroom caps dry by regulating temperature, relative humidity, and ventilation to prevent bacterial propagation.
Bacterial yellowingAmong the diseases observable during the cultivation of Pleurotus eryngii, whatever the growing procedure is, it is yellowing that can cause the most severe damage19. The disease is characterized by a yellow discoloration of the pileus and hydropic, often elongated and coalescing areas on the entire stem. Symptomatic basidiomata then stop growing, turn a reddish-brown color and are affected by rotting. Diseased sporophores exhale an odor, which is almost alcohol-like and pleasant at first, but rapidly becomes offensive and nauseating8.
Pseudomonas agarici and P. reactans are reported as the most likely causal agents of yellowing in both P. eryngii and P. ostreatus (Jacq.) P. Kumm33. P. reactans belong to the V group of fluorescent Pseudomonas and is considered to be saprophytic bacteria inhabiting the mushroom hyphosphere49. According to Bruno et al.8 and Kim et al.37, other bacterial species have been isolated from symptomatic basidiomata (Pseudomonas costantinii, Bacillus cereus, Enterobacter amnigenus, Sphingomonas spp., Staphylococcus epidermidis, Pantoea spp. and Moraxella osloensis). Bessette et al.6 reported that the yellow blotch in P. ostreatus caused by P. agarici formed a clean yellow fluid on the surface of the cluster at first, and then deformed with an increase in severity. The stipes tended to recurve near the base and the sporocarp was upright.
Differences in susceptibility/resistance exhibited by various commercial strains of P. eryngii could play an important role in disease incidence. Yellowing of P. eryngii can occur in all basidioma development phases, from primordia appearance to commercial maturation. Mushroom producers generally try, albeit with disappointing results, to prevent yellowing or halt its development and spread by adding sodium hypochlorite or chemicals containing iodine to irrigation water19. Much research has been done to figure out an adequate method to prevent or control this disease. Controls, such as lowering relative air humidity, and watering with low concentration of chlorine solution (calcium chloride and chlorinated compounds) are currently the most commonly utilized chemicals for blotch disease control. When mushrooms remain wet, however, chlorine has little effect since the bacterial population reproduces at a rate that neutralizes the effect of the oxidizing agent27. Several other disinfectants and antibiotics, such as chloramine T and bronopol, essential oils, and kasugamycin, have also been tried for their ability to control bacterial blotch disease26,71. According to Bruno et al.8, it is safe to say that acetic acid at 87.4 and 69.9mM may be considered an interesting antibacterial tool to prevent and/or halt the yellowing of P. eryngii.
Bacterial brown blotchP. tolaasii is a bacterium, which causes bacterial blotches in the button mushroom Agaricus spp., in Flamulina spp., in oyster mushroom Pleurotus spp., and in Shitake Lentinus edodes31,49. The bacterial brown blotch disease, caused by the bacterium P. tolaasii, has been one of the most serious bacterial diseases for the oyster mushroom. The disease often occurs over a large geographical area. The disease incidence has been different each year. Once the disease occurred on a farm, it became very difficult to control before all of the substrate bags were removed from the farm76.
Tolaasin, an extracellular lipodepsipeptide toxin produced by P. tolaasii, has proven to be the major virulence factor48. When P. ostreatus develops the brown blotch symptom, it continues to rot due to a volatile toxin, tovsin, produced by P. tolaasii only when in contact with P. ostreatus63. Preliminary data also indicates that P. ostreatus fruiting body components activate tolaasin production. It supports the hypothesis that P. tolaasii strains cause disease in P. ostreatus and have mechanisms that enable them to interact specifically with P. ostreatus50. The pathogen causes blotch symptoms on the pileus, forming membrane pores and disrupting the cellular membrane structure.
The disease is characterized by the formation of brown lesions on mushroom caps and by bacterial growth in and discoloration of the stipes. These lesions consist of slightly concave spots, which can be round or spreading31. Typically, spotting occurs at or near the edge of mushroom caps. Blotches occur when mushrooms remain wet for a period of 4–6h after watering, the brown spots and blotches enlarge and coalesce with others. The affected areas are sunken and covered with sticky material. However, the disease affects only the top external layers of the pileus tissues and is restricted to 2–3mm below the pileus surface76.
Bacteria may reach and colonize on the surface of the pileus during the early fruiting body development stage while the young pileus is still in contact with the substrate. Greater bacterial population from spawned substrates may result in more severe infection. Disease incidence of primordia may result directly from the bacterial colonization from the substrate. Disease severity in the pileus is consistent with primordia disease incidence for tested strains with inoculation on spawned substrates. It is important to control the transfer of pathogen from the spawned substrate to the pileus during the early fruiting body development stages in order to manage this disease75.
Huge economic damage is due to a rapid spread of the bacterial pathogen in P. eryngii cultivations and effective biological or chemical control measures are scarce. In fact, some Pseudomonas isolates were screened for their antagonistic ability toward P. tolaasii; however their in vivo suppressive effect was not satisfactorily proven20. Biological control methods with antagonistic microorganisms and/or specific phages have also been investigated. Tsukamoto et al.70 reported that a gram-positive bacterium, strain 9045, detoxifies tolaasins produced by P. tolaasii and significantly suppresses the onset of the disease in P. ostreatus. Another advantage of using strain 9405 is that it is saprophytic to P. ostreatus, in contrast to Pseudomonas fluorescens, which could be pathogenic to cultivated mushrooms by producing various antifungal agents14 and is closely related to P. tolaasii66. According to Zhang et al.75, strain ACCC50618 was resistant to brown blotch disease. However, it has rarely been cultivated by growers, because the fruiting bodies are very fragile and can easily be broken during harvest and transport.
Soft rotThe genus Pantoea includes several species that are generally associated with plants, either as epiphytes or as pathogens73. The gram-negative bacterium Pantoea spp. has been reported as a causal agent of soft rot disease with symptoms of water-soaked lesions on the stipes and pileus of P. eryngii37. The typical symptoms of soft rot disease include a dark brown water drop in the early stages of infection, followed by the development of water-soaked lesions on the stipe and cap of mushrooms within 8 days after the mushrooms are transferred to the cultivation room. The lesions expand gradually and constitute a viscous, mucus-like fluid, finally leading to a mushy soft rot accompanied by an offensive odor during growth59. Liu et al.44 isolated strains belonging to Pantoea beijingensis (growth occurs at 10–37°C) from lesions on the fruiting body of P. eryngii exhibiting symptoms of water-soaked lesions and soft rot in the stipes and pilei.
Compounds containing active chlorine are, at present, the most commonly utilized chemicals for bacterial disease control. Watering with concentrations at 175ppm active chlorine were effective for the reduction of soft rot disease of P. eryngii without affecting mushroom yield44.
Stipe necrosisEwingella americana was identified as an opportunistic pathogen55. Concerning Enterobacteriaceae, little is known about their prevalence and their contribution to the total microbial load of cultivated mushrooms. E. americana was identified as the causal agent of internal stipe necrosis on symptomatic samples collected from mushroom farms. Reyes et al.56 demonstrated the predominance of E. americana in biota of retail fresh P. ostreatus.
The symptoms of internal stipe necrosis appear as a variable browning reaction in the center of the mushroom stipe34. Examined in longitudinal section, the brown tissue extends from the base of the stalk to the cap, but rarely penetrates the cap tissue. Affected mushrooms may be wet in appearance, but frequently, at harvest; the brown tissue is dry and has completely collapsed, leaving a hollow center. In all cases, symptoms are visible only at harvest. The occurrence of internal stipe necrosis disease has occasionally been associated with water-logging of the mushroom stalks at an early development stage, and it is therefore important to maintain good evaporation from the bed surface at all times21. In P. ostreatus, symptoms consisted of soft rot and mild browning of the tissues.
According to González et al.30, E. americana is pathogenic in P. eryngii, although its presence was not dominant in the analyzed samples, being isolated in only 10% of them. However, Reyes et al.56 reported that the presence of this bacterium was high in commercial products. These results indicate that the pathogen is found in crops and increases during storage.
Fungal diseasesDry bubbleLecanicillium fungicola is a devastating pathogen in the mushroom industry, which causes significant losses in the commercial production of Pleurotus spp. This mold causes dry bubble disease in commercially cultivated mushroom. Although its pathogenicity for other species has not been established, it has been isolated from numerous other basidiomycetes. On inoculation of healthy P. ostreatus, these isolates caused disease and could be reisolated25. L. fungicola has also been mentioned as a pathogen of Pleurotus pulmonarius46. However, L. fungicola is not often found on wild mushroom, does not have a wide host range and might more often infect already decaying mushrooms.
Two distinct symptom syndromes are observed on the development stage of the sporophores at the time of infection. Infection of sporophores at the pin or button stage resulted in the development of typical dry bubbles, amorphous masses of sporophore tissue. In contrast, mature sporophores showed cracking and curling of the tissues and depressed, brown, necrotic areas. In advanced stages, a gray weft of mycelium and conidia frequently covered the surface of infected sporophores46.
The control of L. fungicola relies on strict hygiene, regulation of the environment and the routine fungicide spray program. Few chemicals can be used for the control of dry bubble because the host is also sensitive to fungicides. Notably, the development of resistance of L. fungicola has been reported against the fungicides that are used to control dry bubble disease5. The effective and currently legal chemical control for dry bubble disease is Sporgon (active ingredient: prochloraz-manganese). Sensitivity of L. fungicola to Sporgon has decreased, therefore increasing concentrations of Sporgon must be used to combat dry bubble disease46. Another management technique that has been recently researched is the use of volatile 1-octen-3-ol on infected hosts of L. fungicola. While more research is needed to contribute its effects on management, it has been shown that 1-octen-3-ol inhibits the germination of L. fungicola and that enhanced levels can effectively control the pathogen5.
Green moldThe commercial production of oyster mushroom has been seriously affected by green mold epidemics. The causal agents of green mold disease of cultivated oyster mushroom are Trichoderma spp. (T. asperellum, T. atroviride, T. citrinoviride, T. hazianum, T. longibrachiatum, T. pleurotum, T. pleuroticola and T. virens). Trichoderma species are asexual, soil-inhabiting filamentous fungi with teleomorphs belonging to the genus Hypocrea (Ascomycota, Pyrenomycetes, Hypocreales, Hypocreaceae). Trichoderma pleurotum has been found only on cultivated P. ostreatus and its substrate. In contrast, Trichoderma pleuroticola has been found both on wild and cultivated P. ostreatus, as well as on the natural and productive substratum of the oyster mushroom42. Trichoderma green mold infection in edible basidiomycetes has been known for a long time64. The appearance of green fungal sporulation in oyster mushroom substrates are they typical green mold symptoms. In severe outbreaks, no mushrooms are produced from the contaminated substrates54.
Green mold infection of P. ostreatus is supposed to be transmitted by substrate for mushroom cultivation41. Woo et al.72 have observed that Trichoderma species are present at the initial phase of substrate preparation, but later disappear with pasteurization. However, they can be found again in the substrate after inoculation with Pleurotus spp. (spawning), during spawn run (incubation phase) and in the harvesting cycles. The substrate is exposed to green mold infection mostly during spawn run, when the substrate temperature is increased up to 30°C due to the generation of metabolic heat by mushroom mycelia, whereas no growth is observed at 15°C. The optimal pH for Trichoderma spp. growth is acidic-neutral conditions (pH 5–7). This information suggests that adjusting the pH of the substrate to 8–9 might slow down the growth of Trichoderma spp., resulting in a decrease of infection spread. The mycelial growth of Trichoderma spp. is completely inhibited by pasteurization at 60°C for 10h. The mycelial growth of green mold occurred at its maximum in 80% of relative humidity conditions2,72.
Komoń-Zelazowska et al.41 suggested the application of calcium hydroxide on the affected area. The hypothesis of a possible reduction of T. pleurotum infection by substrate alkalization may be further supported by the fact that Pleurotus spp. green mold is not reported to be a severe problem in the United States, where the addition of lime to increase pH to 7.5 is widely practiced. However, this treatment seems to be ineffective against T. pleuroticola. The use of fungicides benomyl, thiabendazole and prochloraz was also reported to be effective24. Prochloraz was shown to be the most effective fungicide for the inhibition of mycelial growth in green molds, because the amount of resistant Trichoderma spp. isolates was the lowest when analyzing this fungicide. Prochloraz, benomyl and propineb were found to inhibit spore germination of benomyl-susceptible isolates in a proper way, while chlorothalonil was effective for benomyl-resistant strains. According to Hatvani32, thymol, ferulic acid, (+)-menthol, and (−)-menthol inhibited green mold growth at concentrations as low as 0.08mgml to 1.25mgml.
CobwebSeveral species of Cladobotryum, including C. dendroides, C. mycophilum, C. varium, C. multiseptatum, and C. verticillatum are known to be the causal agents of “cobweb disease” in the cultivated mushroom Agaricus bisporus and are found in mushroom-growing countries worldwide9,16. However, in 2009–2010, some commercially grown P. eryngii began to show similar symptoms to the fungal disease caused by Cladobotryum mycophilum in A. bisporus23.
The spores are relatively large and multicellular, being easily dislodged from the sporulating colony by external disturbances such as watering, air circulation systems, and harvesting9. Conidia were able to germinate and grow in 4h. This fact suggests that the spores are very readily dispersed in the air and growth of conidia is an important causative factor of cobweb disease in P. eryngii. Air-borne spores from affected crops might contaminate reusable plastic bottles, substrates, and transportation systems, and the pathogen could rapidly spread from farm to farm. This is the main mode of transmission by which the pathogen is distributed to mushroom farms38.
One of the main symptoms is a cobweb-like growth of fungal mycelium over the surface of the mushrooms. The colonies on the surface rapidly overwhelm the mushrooms and develop several spores within 3–4 days. The mycelium can quickly cover the king oyster mushroom debris, pin-heads, stalks, pileus and gills, eventually resulting in decomposition of the entire fruit body. The colonized surface turns pale brown or yellow, accompanied by cracking of the stipe surface. The fruit body eventually turns dark brown and becomes rancid, with an offensive odor33,38.
The fungal pathogen is sensitive to metrafenone (0.025ppm), prochloraz manganese (0.3ppm), chlorothalonil (0.45ppm), benomyl and carbendazim (<1.0ppm) as well as moderately sensitive to thiophanate-methyl (2.0–8.0ppm) and thiabendazole (4.85ppm). To reduce this risk, benomyl and carbendazim should be used with caution only when mushroom growers know for certain that the pathogen they wish to control is sensitive to benzimidazole. In addition, combining the active ingredient with prochloraz manganese and thiophanate-methyl might help to prevent or at least decrease the risk of the pathogen becoming resistant9,10,33,38.
Brown spotGliocladium roseum Bainier were obtained from diseased P. eryngii. Symptoms consist of brow spot curling of the tissues, sometimes even shrinking, and cracking of the infected fruit bodies. The optimum temperature for conidial germination and mycelial growth of G. roseum is 20–32°C. It is also pathogenic to Pleurotus cystidosus and Pleurotus sajor-caju12.
Viral diseasesMycoviruses are widespread in fungi, including plant-pathogenic fungi. In most cases, they have been reported to be cryptic or show few symptoms leading to latent infection in host cells. Interestingly, the symptoms were observed throughout the culture when a certain mushroom spawn was inoculated58. Bacterial diseases such as brown blotch disease by P. tolaasii are generally restricted to a local area of culture. This led to the conclusion that the symptoms originate from virus-infected spawn. Mycoviral infection symptoms include retarded mycelia and fruiting body growth, fruiting body development inhibition, and malformations of the fruiting body40. In 1980, P. pulmonarius virus (Ppv) was isolated from mycelia and basidiocarps of P. pulmonarius47. P. eryngii Spherical Virus (PeSV) was isolated from P. eryngii mushroom, with severe epidemic symptoms. Transmission electron microscope showed that it was spherical with a 31-nm-diameter. Fruiting bodies of P. eryngii showed symptoms such as short and stout stems, and flattened caps with irregular shapes. The mycelia taken from the tissue of basidiocarp exhibited abnormally retarded growth on a solid medium. Curing of the viruses essentially eliminate the symptoms, indicating that they are the causative agents of the disease. Therefore, it is conceivable that many uncharacterized mushroom diseases may be related to mycoviral infection57.
“La France” diseaseRo et al.58 isolated the oyster mushroom isometric virus (OMIV). The characteristics of examined OMIV suggest that it is not related to known P. ostreatus-infecting viruses such as PoV1, which is a spherical virus that contains two dsRNA genomes and has a diameter of 30nm. Typical symptoms of viral disease on oyster mushroom are quite similar to “La France” disease which is a well-known viral disease in A. bisporus, in which fruiting body formation delay, shortening in stipe, abnormal shape and thin mushroom caps are the major symptoms; fruiting bodies are not formed at all on some infected mushroom beds and viral-infected hyphae grow very slowly on agar and their density is very low11.
Die-backPleurotus spp. is generally cultivated under well-controlled environmental conditions, and its cultivation is thus largely free from diseases from external origin. However, mushroom industries often suffer from spawn-related diseases, most notably the die-back disease, which originates from a viral infection40. Yu et al.74 isolated the first single-stranded ssRNA mycovirus, named oyster mushroom spherical virus (OMSV), from a cultivated oyster mushroom, P. ostreatus. The authors detected the virus in all 102 samples collected from 102 different commercial farms with the epidemic. The symptoms are rather complex and the disease spreads fast. An outbreak of such disease in a commercial farm often leads to a complete loss of yield and it is difficult to control. OMSV was not detected in healthy mushrooms, and when OMSV from diseased mushrooms was cured, the epidemic disappeared. This strongly suggests that OMSV is a causative agent of the disease. Another P. ostreatus-infecting spherical dsRNA virus was discovered, being named PoV143. In contrast to OMSV, which is directly related to mushroom disease, infections by PoV1 did not show any distinct morphological or growth phenotypes.
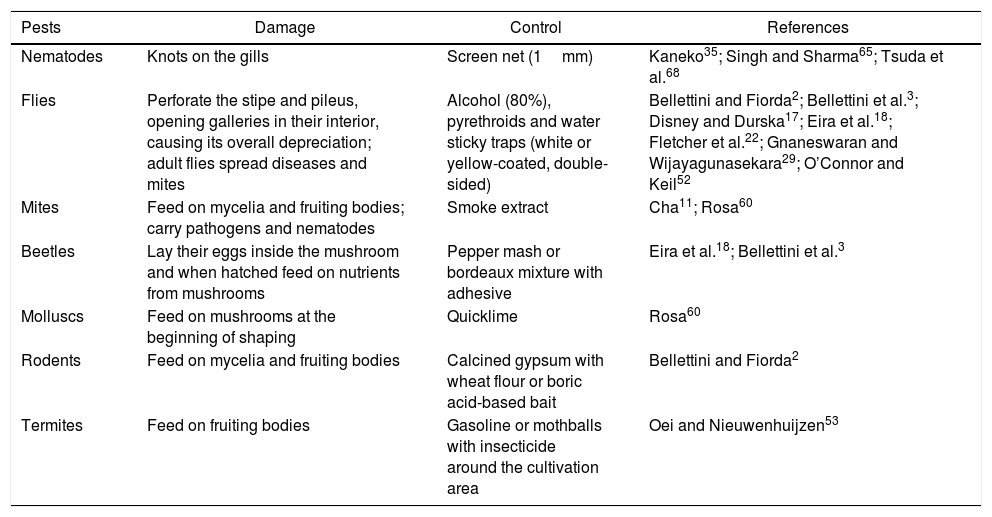
PestsThe presence of insects, mites, crustaceans and other mycetophagous arthropods and synthetic or wood substrate decomposers have been identified by producers as damaging or limiting the proper development of mushrooms (Table 2). Biological cycles of these pests are not well known. The unhygienic conditions of mushroom cultivation provide a congenial atmosphere for many pests and diseases. There is not much bibliographic information related to pests of mushrooms and their substrates.
Pests noxious to Pleurotus spp. mushroom crops
| Pests | Damage | Control | References |
|---|---|---|---|
| Nematodes | Knots on the gills | Screen net (1mm) | Kaneko35; Singh and Sharma65; Tsuda et al.68 |
| Flies | Perforate the stipe and pileus, opening galleries in their interior, causing its overall depreciation; adult flies spread diseases and mites | Alcohol (80%), pyrethroids and water sticky traps (white or yellow-coated, double-sided) | Bellettini and Fiorda2; Bellettini et al.3; Disney and Durska17; Eira et al.18; Fletcher et al.22; Gnaneswaran and Wijayagunasekara29; O’Connor and Keil52 |
| Mites | Feed on mycelia and fruiting bodies; carry pathogens and nematodes | Smoke extract | Cha11; Rosa60 |
| Beetles | Lay their eggs inside the mushroom and when hatched feed on nutrients from mushrooms | Pepper mash or bordeaux mixture with adhesive | Eira et al.18; Bellettini et al.3 |
| Molluscs | Feed on mushrooms at the beginning of shaping | Quicklime | Rosa60 |
| Rodents | Feed on mycelia and fruiting bodies | Calcined gypsum with wheat flour or boric acid-based bait | Bellettini and Fiorda2 |
| Termites | Feed on fruiting bodies | Gasoline or mothballs with insecticide around the cultivation area | Oei and Nieuwenhuijzen53 |
The most dangerous pests are nematodes, which cannot be eradicated without the complete elimination of the crop. Their presence leads to very poor yields or total crop failures. Button mushrooms are generally highly susceptible to nematode infection while oyster mushrooms are relatively resistant65.
A disease that causes knots on the gills of the oyster mushroom P. ostreatus has been reported by Tsuda et al.68. Nematodes inhabit and lay many eggs inside the gill knots. Three nematode species, namely Aphelenchoides composticola, Aphelenchus avenae and Ditylenchus myceliophagus along with some saprophagous (Rhabditis spp.) and predatory (Seinura spp.) nematodes were found in oyster mushroom (Pleurotus spp.). It is surprising that the nematodes causing this disease live in the fruiting body of this fungus, which is known to be nematophagous, that is, with vegetative hyphae that have the ability to immobilize and consume nematodes67. According to the author, the fungus gnat Rhymosia domestica (Mycetophilidae, Diptera) was confirmed to be the vector of the nematodes inside the gill knots.
Kaneko35 controlled this disease by covering the logs used to grow oyster mushrooms with a 1mm mesh screen net; therefore, he suggested that an insect larger than 1mm must take part in the transmission of the disease. The use of pesticides for killing nematodes in mushroom crops is not advisable due to residual hazards as mushroom is a short duration crop. There is a need to exploit the use of some plant products, which have nematicidal properties and at the same time are safe for mushroom mycelium. Nematode management control using Thionazin at the rate of 80ppm is the only recommended nematicide for the control of myceliophagous nematodes without residual toxicity65.
FliesFlies of at least 8 dipterous families (Calliphoridae, Culicidae, Drosophiladae, Cecidomyiidae, Muscidae, Mycetophilidae, Phoridae and Sciaridae) are the main insect disease promoters in mushroom production. They also feed on nutrients from fruiting bodies and are capable of carrying fungal contamination, bacteria diseases and mites52. Lycoriella mali Fitch (Family Sciaridae) is the major pest species of commercial mushrooms throughout the world13. Dipterous larvae can derail the product for consumption, leading to losses of around 20%. Larval stages of Drosophilid funebris and flies Mycodrosophila spp. were also observed in fruiting bodies. D. funebris has naturally been described as scavengers and reported as occasional pests in mushroom Pleurotus spp. houses with poor hygienic conditions22. Bradysia paupera has been reported as a serious insect pest of the mushroom crop in European countries22. However, Gnaneswaran and Wijayagunasekara29 revealed that their abundance and damage to the Pleurotus spp. crop was of minor significance. As a result, young fruiting bodies were wilted and mature ones were feebly attached to the substratum. According to Disney and Durska17, the larvae of Megaselia tamilnaduensis Disney, Megaselia longipennis, Megaselia pleurota Disney and Megaselia scalaris being primarly a mycelium feeder of Pleurotus citrinopileatus Singer, Pleurotus cornucopiae, P. ostreatus and P. sajor-caju, respectively.
Flies, especially in their immature stage (larvae), perforate the stipe and pileus of mushrooms, opening inside galleries, causing its overall depreciation2. For the control of flies in growth rooms, the most common techniques are tapes and traps. Authors recommend chemical methods for pest control, highlighting the use of pyrethroids. Adults of most of these families do not have great flight autonomy, have variable length (a few millimeters to several centimeters), can have thin or stout bodies and are usually attracted by the odor coming from the fermentation exhaled by decomposing material or components used in compounds (sugarcane bagasse, rice straw, etc.). The females lay their eggs directly onto the substrate. Born larvae from their eggs feed on mycelium and subsequently migrate to the mushrooms, destroying the stipe and pileus partially or fully, making them unfit for consumption18.
The use of alcoholic and water sticky traps can be a valuable tool for the grower and a complement to other methods used for the control and prevention of pests in the crop. Sticky traps consist of white or yellow-coated plastic plates with slow drying glue. The color of the plates attracts a large number of species, being the white color particularly attractive to dipterous (flies and mosquitoes). Insects, which land on them become definitely stuck to them. These traps can also be in the form of double-sided tape, used for the same purpose as the plate. The sticky traps should be replaced after saturation by dead insects. The traps must be filled with 80% alcohol and hung along the crop (inside and outside the sheds). The alcoholic or fermentation odor attracts particularly dipterous (flies) with great efficiency. These are the most effective traps, especially during fruiting3.
MitesMites belonging to the class Arachnida are common pests in mushroom crops and can cause total loss of production. Tarsonemus spp. and Histiostoma spp. are major mushroom damaging mites. They develop very fast under high humidity conditions (above 90%) and temperature (25–30°C). The first signals are webs formed between the cultivation shelves, mycelium and fruit body where bacteria and fungi can come in. Mites feed on mycelia and fruiting bodies, causing yield loss and a decrease in mushroom quality. Mites carry pathogens and nematodes, sometimes causing itchy rashes among growers11. For their control, smoke extract can be used over the compound in colonization. An infusion of coriander leaves can also be used60.
Minor pestsBeetles belong to the order Coleoptera and are insects that can affect the edible mushroom cultivation from the beginning of fruiting. These insects lay their eggs inside the mushroom and when hatched can feed on its nutrients. Cyuodes bifacies is a pest of the P. ostreatus28 and it was reported that some mushroom growers had to close down the industry. For their control, a pepper mash or bordeaux mixture can be used with adhesive for beetle immobilization, unabling the deposit of their eggs in the mushroom tissues2.
Molluscs such as slugs, snails and conch can be pests in mushroom cultivation because they are fed with mushrooms at the beginning of shaping. The control is performed with quicklime, inducing dehydration of molluscs60.
Rodents feed directly from mushrooms. For prevention, calcined gypsum can be used along with wheat flour or boric acid-based bait. When consumed by the animal, it blocks their digestive tract causing death. It is important to prevent rodents from climbing onto the shelves containing mushrooms18.
Termites belong to the Isoptera order and represent a constant danger during growing season, as many grow-room structures are made of wood. All termite mounds near the growing room must be eliminated or controlled. For their control, gasoline or mothballs with insecticide are used around the cultivation area53.
Hygiene measuresEmpty facilitiesMaintaining cleanliness inside, outside and in the surroundings of the production unit is recommended8. The technique mostly used in the production of mushroom, basically in high-tech facilities to disinfect the room after the crop cycle is known as “cooking-out”, which consists in applying steam vapor at 70°C for 12h21. This treatment is sufficient to eliminate mushroom pests and diseases in addition to kill mycelium debris. Subsequently, just a wash with water is enough to prepare the room for the next cycle. The interior of the inoculation room should consist of non-biodegradable materials. All the surfaces should be smooth and easy to clean. Shelves should be designed in such a way that the floor beneath can be cleaned easily. Shelves are typically made of galvanized iron or formica53. The handling tools should be heated in boiling water for 1–2min for the complete destruction of nematodes. Workers should frequently disinfect their hands and clothes. Mushroom cultivation can also be disinfected by cleaning with a 10% Clorox solution or 70% ethyl alcohol.
The floor used at the compost preparation room should be cemented, to avoid the direct contact of compost with infested soil. Slightly inclined cemented floors provide a smooth surface that can be easily cleaned and allow excess water to drain. The drainage system of the different rooms should not be connected to prevent a disease in one growing room from easily spreading to other rooms53.
Growing roomsA clean environment is absolutely essential to mushroom production. Basic amenities like clean irrigation water and sewage disposal system and irrigation should be ensured. The area for mushroom cultivation should be such that its air is free from toxic fumes and gases. The surroundings of a farm should be clean and free from possible contamination from insects, molds, etc. Grain and sawdust contain large numbers of contaminants. A single grain kernel may contain thousands of bacteria, fungi and actinomycetes53. Picking should be started from new or cleaner crops toward older crops. The waste from various operations should be collected and disposed of daily from the working areas immediately2. Early termination of infested crops should be done. The growing rooms should be properly ventilated rooms having doors and windows with wire net of 14–16mesh/cm to avoid the entry of insect pests65.
ConclusionsIntensive cultivation of edible mushrooms can often be affected by some bacterial, fungal and viral diseases that, rather frequently, cause dramatic production loss. These infections are facilitated by the particular conditions under which the mushroom cultivation is commonly carried out, such as warm temperatures, high humidity and a low aeration rate. The unhygienic conditions of mushroom cultivation provide a congenial atmosphere for many diseases and pests. Therefore, a clean environment is absolutely essential to mushroom production. The important considerations include previously cleaned implements and maintaining overall hygiene. This review argues that an understanding of the symptoms and treatment controls are needed for a suitable and efficient production of Pleurotus spp.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that no patient data are mentioned in this article.
Right to privacy and informed consentThe authors declare that no patient data are mentioned in this article.
FundingThis research was financially supported by the National Council for the Improvement of Higher Education (CAPES) and Federal University of Paraná (UFPR).
Conflict of interestThe authors declare that they have no conflicts of interest.