The aim of this work was to know the frequency and geographical distribution of genotypes and mating types of Cryptococcus neoformans and Cryptococcus gattii species complexes isolated from human infections in Argentina during the period from April 2009 to April 2011. A multicenter study was conducted, in which 372 isolates were obtained from 61 laboratories throughout the country. Of those, 98.8% of the isolates belonged to the C. neoformans species complex and 1.1% to the C. gattii species complex. Genotype VNI (MATα) was the most frequently isolated (n=326, 87.6%), followed by VNII (MATα) (n=22, 5.9%), the recently described VNII-VNIV (aADα) hybrid (n=14, 3.8%), VNIV (MATα) (n=4, 1.1%), VNIII (αADa) hybrid (n=1, 0.3%), and VNIII (αADα) hybrid (n=1, 0.3%). The Argentine Central region showed the greatest number of cases and genotype diversity. Interestingly, a relative high frequency was observed in genotype VNII (MATα) in the Cuyo, Northeast and Northwest regions and, also in VNII-VNIV (aADα) hybrids in the Northwest region. C. gattii species complex was isolated at a low rate; 3 VGI (MATα) and 1 VGII (MATα) isolates were obtained from the Northwest and Central regions. In conclusion, this study shows that genotype frequencies seem to vary among regions in Argentina and reveals a relatively high frequency of rare hybrids in the Northwest region. Further regional clinical and environmental studies may help to elucidate if those variations in frequencies are associated with the existence of regional ecological niches or any other regional factors.
El objetivo del trabajo fue conocer la frecuencia y la distribución geográfica de genotipos y tipos sexuales de aislados pertenecientes a los complejos de especies Cryptococcus neoformans y Cryptococcus gattii obtenidos de infecciones humanas en Argentina. Entre abril de 2009 y abril de 2011 se realizó un estudio multicéntrico del que se obtuvieron 372 aislados de 61 laboratorios de diferentes zonas del país. El 98,8% de los aislados pertenecieron al complejo C. neoformans y el 1,1% al complejo C. gattii. El genotipo VNI (MATα) fue el más frecuente (n=326; 87,6%), le siguieron VNII (MATα) (n=22; 5,9%), el híbrido VNII-VNIV (aADα) (n=14; 3,8%), VNIV (MATα) (n=4; 1,1%) y los híbridos VNIII (αADa) (n=1; 0,3%) y VNIII (αADα) (n=1; 0,3%). La región Centro mostró el mayor número de casos y la mayor diversidad de genotipos. Cabe destacar que el genotipo VNII (MATα) tuvo una frecuencia relativamente alta en las regiones de Cuyo, Noreste y Noroeste. En esta última región, también fue alta la frecuencia del híbrido VNII-VNIV (aADα). La frecuencia de aislamiento de miembros del complejo C. gattii fue baja: se obtuvieron 3 aislados VGI (MATα) y 1 VGII (MATα) de las regiones Centro y Noroeste. En conclusión, este estudio muestra que las frecuencias de genotipos varían entre las distintas regiones de Argentina y señala la presencia de híbridos poco comunes en una frecuencia relativamente alta dentro de la región Noroeste. Contar con mayor número de estudios clínicos y ambientales regionales podría ayudar a elucidar si tales variaciones están asociadas a la existencia de nichos ecológicos particulares o a algún otro factor regional.
Currently, the taxonomy and nomenclature of the former Cryptococcus neoformans and Cryptococcus gattii species are being revised. On the one hand, Hagen et al. have proposed that these two former species in fact comprise the following seven species: C. neoformans (VNI/VNII/VNB, AFLP1), Cryptococcus deneoformans (VNIV, AFLP2), C. gattii (VGI, AFLP4), Cryptococcus bacillisporus (VGIII, AFLP5), Cryptococcus deuterogattii (VGII, AFLP6), Cryptococcus tetragattii (VGIV, AFLP7) and Cryptococcus decagattii (VGIIIc/VGIV, AFLP10)11. On the other hand, Kwon-Chung et al. have proposed the use of the term “species complex” instead of naming new “species”14. Nowadays, and although Hagen et al. have pointed out that “ignoring the species impedes deciphering the difference among them, which may delay future clinical advances”12, there is still no consensus on nomenclature changes among the scientific community. Here we are using the “species complex” nomenclature that is the most used in clinical routine laboratories because many of them are unable to differentiate the seven species proposed.
Five major molecular types have been recognized within the C. neoformans species complex: VNI, VNII, VNIII, VNIV, and VNB; and four major molecular types have been recognized within the C. gattii species complex: VGI, VGII, VGIII, and VGIV8. Genotyping is used to study the geographical distribution of the etiological agents of cryptococcosis8,20; and genotype differentiation may have clinical relevance3,23. The ability of sexual reproduction is another characteristic commonly studied in the C. neoformans and C. gattii species complexes. These yeasts are heterothallic and their mating type determination (MATα and MATa) is important to study the biological impacts of unisexual versus bisexual mating10. Moreover, different mating type alleles have shown variation in their virulence; suggesting that their determination may have clinical relevance17.
The epidemiology of cryptococcosis in Argentina has been previously described2,9,18, this disease being the second deep yeast infection in the country. Its prevalence in 2010 was estimated at 1.23 per 100000 inhabitants and 4.2 per 1000 HIV-seropositive patients18. Moreover, the ecological niche of C. neoformans and C. gattii species complexes has been previously studied in Buenos Aires city and the Northeast region5,19,21. However, little is known about the frequency and distribution of genotypes and mating types of isolates obtained from human infections in the different regions of Argentina.
The aim of this work was to know the frequency and geographical distribution of genotypes and mating types of isolates belonging to the C. neoformans and C. gattii species complexes obtained from human infections in Argentina during the period from April 2009 to April 2011.
Materials and methodsIsolates and phenotypic identificationA prospective multicenter study, in which 61 laboratories throughout the country participated, was conducted from April 2009 to April 2011. All isolates identified as belonging to the C. neoformans or C. gattii species complex obtained from human samples were included in the study. Only one isolate per patient was considered. Patient data and specimen type were obtained from the derivation form that was sent by the participating laboratories together with the isolate. Each laboratory identified its isolates by conventional methodologies and collected the following patient data: age, gender, occupation and underlying disease. The specimen type was also recorded. Then, each laboratory sent the isolates to the National Reference Laboratory in Clinical Mycology (NRLCM) (Mycology Department, National Institute of Infectious Diseases “Dr. C. G. Malbrán”) for further studies. All isolates were identified again at the NRLCM by standard phenotypic methods13, including micro and macromorphology, urease test, growth at 37°C, phenoloxidase tests on sunflower seed agar, and color reaction test on l-canavanine glycine-bromothymol blue agar (CGB).
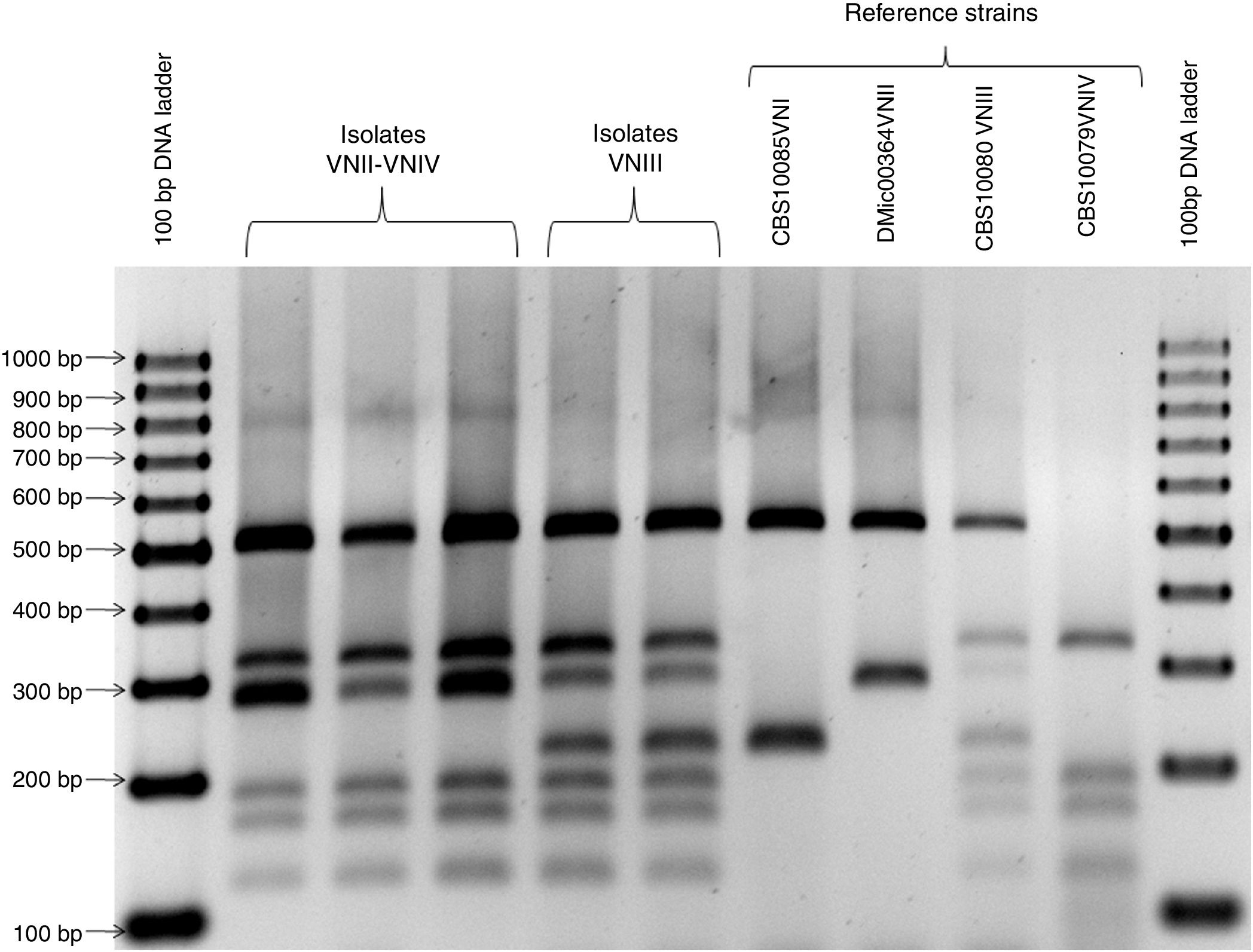
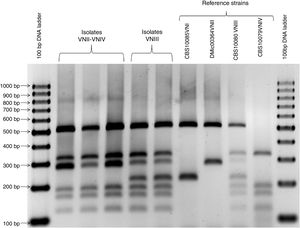
Genotyping and mating typeDNA extraction was performed by using the DNeasy ultraclean microbial kit (MOBIO Laboratories, CA) and following the manufacturer's instructions. DNA was stored at −20°C. All isolates were genotyped by PCR-RFLP of the URA5 gene as described previously20. The mating type was determined by PCR multiplex of the MATα and MATa pheromone gene described by Chaturvedi et al.7 Mating type of hybrids was determined by serotype and mating type-specific STE20 PCRs described by Lengeler et al.16 The following references strains were used as control: CBS10085 (VNI), DMic00364 (VNII), CBS10080 (VNIII), CBS 10079 (VNIV), CBS10078 (VGI), CBS10082 (VGII), DMic031627 (VGIII) and CBS10101 (VGIV).
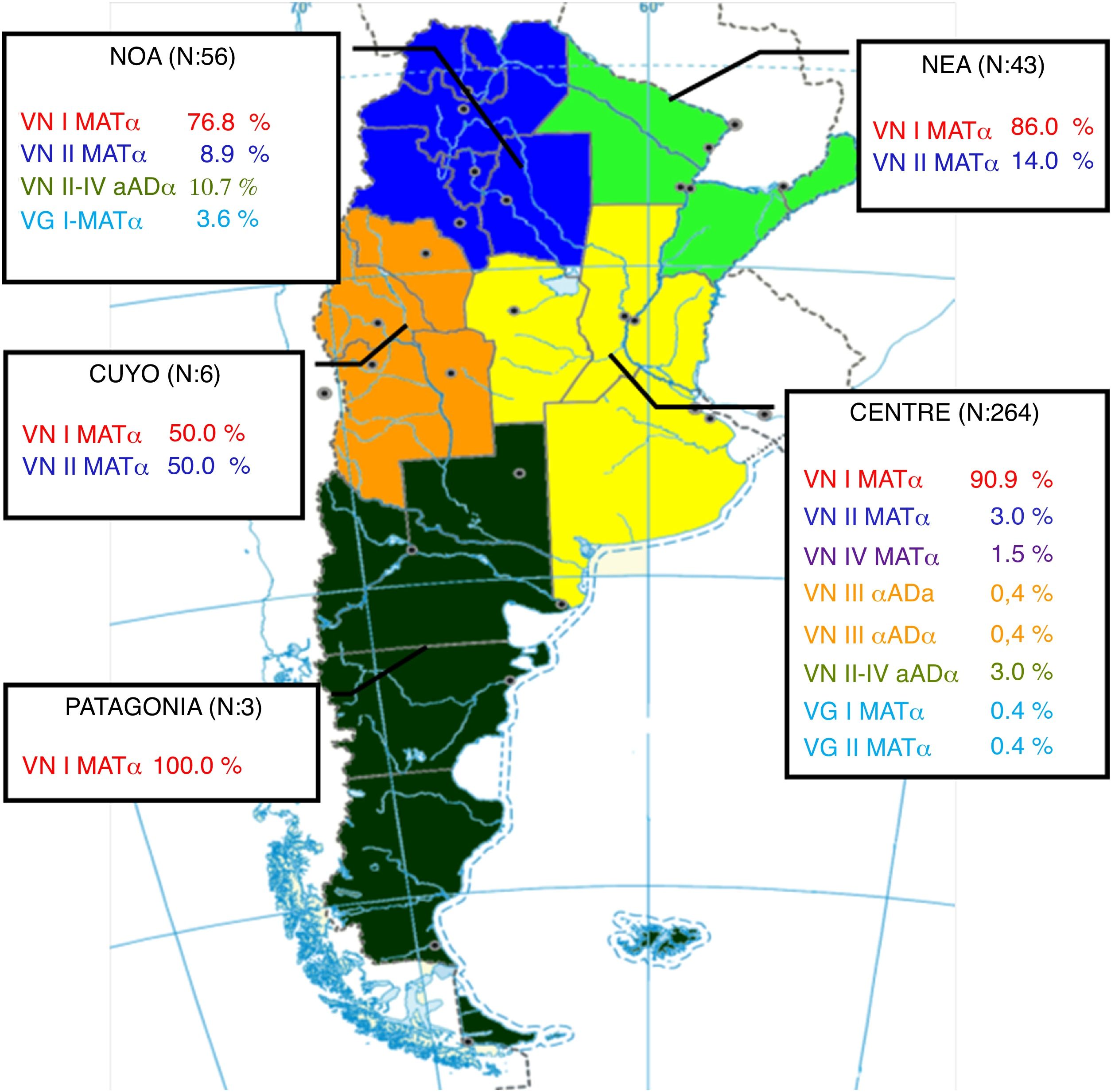
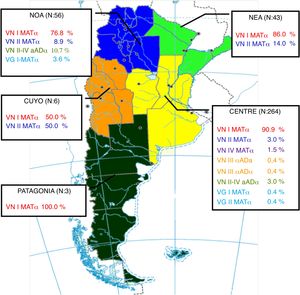
The geographical distribution of genotypes and mating types was analyzed taking into account the following regions: Central: Buenos Aires, City of Buenos Aires, Córdoba, Entre Ríos, Santa Fe; Northwest of Argentina (NWA): Catamarca, Jujuy, Salta, Santiago del Estero, Tucumán; Northeast of Argentina (NEA): Chaco, Corrientes, Formosa, Misiones; Cuyo: La Rioja, Mendoza, San Juan, San Luis; and Patagonia: Chubut, La Pampa, Neuquén, Río Negro, Santa Cruz, Tierra del Fuego.
ResultsA total of 372 isolates from different patients were obtained and analyzed. Patient data, genotyping and mating type results are listed in Supplementary file 2. Epidemiological data of patients were as follows: 78% (n=290) of patients were HIV-seropositive, 13% (n=47) suffered from an autoimmune disease or were immunocompromised, 3% (n=12) had no underlying disease, and in 6% (n=23) the underlying condition was not recorded. Patients’ age ranged from 11 to 86 years and an average age of 38 years; 69% (n=258) were male, 29% (n=107) female, and in 2% (n=7) the gender was not recorded. The patients’occupation was recorded in only 179 (48%) cases; 23% (n=41) were housewives and domestic workers, 21% (n=37) were sellers and commercial assistants, 18% (n=36) were construction workers, 17% (n=29) were office workers, students, teachers and related occupations, 6% (n=11) were motorists, taxi drivers, truck drivers and related occupations, 5% (n=8) were rural employees and 10% (n=17) had other occupations. A total of 277 (74%) isolates were obtained from cerebrospinal fluid (CSF), 58 (16%) from blood, 9 (2%) from bronchoalveolar lavage, 3 (1%) from skin, 11 (3%) from other specimen types and in 14 (4%) cases the specimen type was not recorded.
All isolates showed the following phenotypic characteristics: colonies were smooth, white to cream-colored, mucoid with an entire margin, cells were rounded to globose, urease and phenoloxidase tests were positive, and grew well at 37°C. Of the 372 isolates, 368 were CGB negative whereas 4 isolates were CGB positive.
A total of 368 (98.8%) isolates belonged to the C. neoformans species complex, 326 (87.6%) were genotype VNI, 22 (5.9%) were genotype VNII, 4 (1.1%) were genotype VNIV and 16 (3.8%) were hybrids. Of those hybrids, 14 showed a VNII-VNIV profile and 2 a VNIII profile (see Fig. 1). All VNI, VNII, and VNIV isolates were MATα by using the PCR multiplex of the MATα and MATa pheromone gene. Moreover, those 14 VNII-VNIV hybrids were aADα, one VNIII hybrid was αADa and the other VNIII hybrid was αADα by using the serotype and mating type-specific STE20 PCRs. The remaining 4 (1.1%) isolates belonged to the C. gattii species complex, 3 were genotype VGI and 1 genotype VGII. These 4 isolates were MATα by using the PCR multiplex of the MATα and MATa pheromone gene. Two genotype VGI isolates were obtained from HIV-seropositive patients (1 from sputum and 1 from CSF); the other VGI isolate and the VGII isolate were obtained from patients with no underlying diseases (both from CSF).
The distribution of cases by region was as follows: Central 71%, Northwest of Argentina (NWA) 15%, Northeast of Argentina (NEA) 12%, Cuyo 2%, and Patagonia 1%. The frequency of genotypes and mating type by region is shown in Figure 2.
DiscussionIn this study, the isolates were most frequently obtained from men than from women, the average age of patients was 38 years, and HIV-seropositive was the most frequent underlying disease (78%) in agreement with previous studies2,18. In connection with those results, national data showed that in 2010 the HIV-seropositive diagnosis was most frequent in men than in women, and the average age of people diagnosed with HIV during 2009-2011 was 35 years in men and 31 years in women24. In most cases, the patients’ occupation was not related to high environmental exposure to the fungus. However, we have no data about the patients’ leisure activities.
The most frequently isolated genotype was VNI MATα in agreement with the rest of the world8,9,15,20. Nevertheless, we have found a great diversity of genotypes, including hybrids. Most isolates and the greatest variety of genotypes were found in the Central region. This region is the most populated area in Argentina and includes the largest cities, which are also the most affected by the HIV epidemic24. Interestingly, the other regions seem to have their own epidemiology, probably due to their own population, HIV rate, climate, and regional strains. However, we could not find any correlation between genotypes and the patients’ gender, age, underlying disease or occupation.
C. neoformans VNII MATα was found to have relatively high frequency in Cuyo, NEA and NWA regions. Although the number of isolates obtained from Cuyo was low, the frequency of cryptococcosis in that region is low18 and therefore it is worth noting that half of the cases included in our study were associated with VNII MATα. Hybrid VNII-VNIV aADα was found in higher numbers than hybrid VNIII, and was particularly highly frequent in the NOA region. Two hybrid VNII-VNIV isolates have already been reported from the NEA region in Argentina, both obtained from CSF of HIV-seropositive patients4. However, in that study, the mating type of those isolates was not determined. The hybrid VNII-VNIV aADα has been recently described in a study of potential hybrids from a culture collection1. That study found 8 isolates of this hybrid: 1 obtained from the CSF of an HIV-seropositive patient from Buenos Aires, Argentina, 1 obtained from the CSF of an HIV-seropositive patient from Brazil, 2 obtained from clinical samples of patients from Spain, 2 obtained from the CSF of HIV-seropositive patients from Italy and 2 obtained from pigeon droppings in China. The isolation of this hybrid from different continents suggests that it may have a worldwide distribution.
In this study, all VNIV isolates were MATα; however, one VNIV MATa isolate has been previously reported in Argentina18. Here we first characterized one hybrid VNIII as αADa and other as αADα. We have not found any VNII MATa isolates; however, the isolation of VNII-VNIV aADα hybrids indicates that this allele may be present in the environment. By now, only the environment of the Central and NEA regions has been studied for C. neoformans and C. gattii species complexes5,19,21. There are no environmental studies in the NWA, Cuyo and Patagonia regions.
C. gattii species complex was isolated in a low rate in agreement with other studies in Argentina2,6,18. In this study, we only found genotype VGI and VGII isolates; however, genotype VGIII isolates have been previously reported in Argentina9,19,20. All isolates were MATα in agreement with the rest of the world22. The isolates were only obtained from the NWA and Central regions; however, previous studies have also shown the presence of this species complex in the NEA region5,6,19.
In conclusion, this study shows that genotype frequencies seem to vary between regions in Argentina and reveals the presence of rare hybrids observed in apparently high frequency in the NWA region. These findings highlight the necessity of more regional clinical and environmental studies that may help to elucidate if those variations in frequencies are associated with the existence of regional ecological niches or any other regional factors.
Financial supportThis work has been partially funded by the “Fondos Concursables ANLIS” (FOCANLIS).
Conflict of interestThe authors declare that they have no conflicts of interest.
We would like to thank all participants of the Argentinean group of cryptococcosis.