Anatomopathological studies suggest an association of positive vascular remodeling and vulnerable coronary plaques. The objective of this study was to verify whether there is a correlation between positive vascular remodeling and necrotic core in atherosclerotic coronary lesions.
MethodsWe studied 270 cross sections obtained by Virtual Histology® in 30 patients who had positive remodeling in coronary artery segments with lesions > 50%, identified by coronary angiography. Seven cross sections were assessed per segment of coronary artery, including the cross section with the highest remodeling index, denominated cross section of interest (cross section 4).
ResultsMean age was 60.8±8.8 years, 80% were male and 30% were diabetic. Unstable angina was the most frequent clinical presentation (56.6%) and the left anterior descending artery was the most analyzed vessel (43%). The vessel reference area was 15.5±4.9mm2 and the remodeling index in cross section 4 was 1.2±0.1. Repeated measures analysis of variance showed a higher percentage of necrotic core in the cross section of interest (P < 0.001). We observed a positive correlation of coronary artery remodeling and necrotic core (r = 0.79; P < 0.001).
ConclusionsPositive coronary artery remodeling is associated to the presence of necrotic core, which characterizes vulnerable atherosclerotic plaques. The search for positive arterial remodeling may be a useful strategy for detecting vulnerable plaques before rupture.
Associação entre Remodelamento Vascular e NúcleoNecrótico em Artérias Coronárias: Análise por Ultrassom Intracoronário com Histologia Virtual®
IntroduçãoEstudos anatomopatológicos sugerem a associação de remodelamento vascular positivo e placas coronárias vulneráveis. O objetivo deste estudo foi avaliar se existe correlação entre o grau de remodelamento vascular positivo e o porcentual de núcleo necrótico em lesões ateroscleróticas coronárias.
MétodosForam estudados 270 cortes transversais obtidos pela Histologia Virtual® de 30 pacientes, os quais apresentavam remodelamento positivo em segmento de artéria coronária com lesão > 50%, identificada pela angiografia coronária. Foram avaliados 7 cortes transversais por segmento de artéria coronária, incluindo o corte transversal com o maior índice de remodelamento arterial, denominado corte transversal de interesse (corte transversal 4).
ResultadosA média de idade foi de 60,8±8,8 anos, 80% eram do sexo masculino e 30% diabéticos. Angina instável foi a apresentação clínica mais frequente (56,6%) e a artéria descendente anterior foi o vaso mais analisado (43%). A área de referência do vaso foi de 15,5±4,9mm2 e o índice de remodelamento no corte transversal 4 foi de 1,2±0,1. Análise de variância de medidas repetidas mostrou maior porcentual de núcleo necrótico no corte transversal de interesse (P < 0,001). Observamos correlação positiva do remodelamento arterial coronário com o núcleo necrótico (r = 0,79; P < 0,001).
ConclusõesO remodelamento positivo da artéria coronária está associado à presença de núcleo necrótico, o qual caracteriza placas ateromatosas vulneráveis. A pesquisa de remodelamento arterial positivo pode ser estratégia útil para a detecção de placas vulneráveis antes de sua ruptura.
Acute coronary syndromes present as unstable angina, acute myocardial infarction, or sudden death. Several studies have shown a strong association between these syndromes and atheromatous plaque rupture or erosion, which by exposing the subintimal content, induces the formation of thrombi, and often results in persistent or transient vessel occlusion.1-3
The main histological characteristic of vulnerable coronary plaques is the presence of large lipid droplets coated by a thin fibrous cap, infiltrated by inflammatory cells.4-7 Another characteristic of unstable coronary plaque is the presence of positive remodelling, defined as an increase in vessel diameter at the atheromatous lesion site, which is associated with cardiovascular events in pathological studies.8-12
The currently available data regarding the association between the atheromatous plaque characteristics and vascular remodelling pattern has not been sufficiently studied. The main theory is that positive remodelling occurs early in order to maintain the vessel lumen by accommodating the progressive plaque growth.13,14
Studies using histological sections and intravascular ultrasound images have demonstrated that the physiopathological role of arterial remodelling may be more complex than a mere compensation process, with a strong association between vascular arterial remodelling, local inflammatory response, and plaque vulnerability.15-17
Greyscale intravascular ultrasound is a tool that aids in the diagnosis of coronary artery disease. The images are very useful for the characterization of the extent, distribution, and morphology of atheromatous plaques in vivo, as well as vessel walls.18 However, this method does not allow for an adequate differentiation of the atheromatous plaque content.
The Virtual Histology® (Volcano Therapeutics – Rancho Cordova, United States) intravascular ultrasound with radiofrequency backscatter is an auxiliary tool to the intravascular ultrasound, which transforms the captured ultrasound wave signals into colour images that differentiate the atheromatous plaque components.19
This study aimed to evaluate whether there is a correlation between the degree of positive vascular remodelling and the necrotic core percentage of atheromatous plaque in coronary arteries through evaluation by Virtual Histology®.
METHODSPopulationPatients of both genders aged 18 years and older with significant obstructive lesions in the coronary artery (> 50%) defined by quantitative angiography underwent evaluation with intravascular ultrasound and Virtual Histology® from January 2007 to January 2011 as clinically indicated.
The study was performed in the Haemodynamics Unit of Hospital de Clínicas de Porto Alegre (Porto Alegre, RS, Brazil) and was approved by the Research Ethics Committee of the hospital. The study was performed in accordance with the Declaration of Helsinki for research in human subjects.
Inclusion criteriaPatients who met the following criteria were included:
- –
Significant obstructive lesion in the coronary artery (> 50%) at the coronary angiography;
- –
Presence of positive arterial remodelling in the analysis with greyscale intravascular ultrasound;
- –
Intravascular ultrasound performance with Virtual Histology®.
Patients who had one or more of the following criteria were excluded:
- –
Presence of stent in the evaluated vessel;
- –
Saphenous vein or internal thoracic artery grafts as target vessel;
- –
Ostial target lesion in the left main coronary or right coronary artery;
- –
Presence of lateral branch > 2mm originating from the analyzed vessel segment;
- –
Acute myocardial infarction;
- –
Cardiogenic shock or severe systemic disease;
- –
Images obtained through intravascular ultrasound and/or with Virtual Histology® inadequate for the analysis; and
- –
Impossibility to obtain clinical and demographic data from charts or by telephone contact.
Upon review of the records of intravascular ultrasound of the Hemodynamics Unit of Hospital de Clínicas de Porto Alegre, 113 patients with coronary artery disease submitted to intravascular ultrasound and Virtual Histology® evaluation between January 2007 and January 2011 were selected.
The following variables were collected by reviewing hospital charts or through telephone calls: age, gender, risk factors, and clinical presentation syndrome. Eligible cases were enrolled in the study, and the patients received individual identification codes. Analysis with Virtual Histology® was performed blindly by an interventional cardiologist with extensive experience in the method, in accordance with the American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies.20
The ultrasound images and Virtual Histology® patient data were stored on DVD media and were analyzed after opening the image files in the workstation console of the Volcano S5® intravascular ultrasound equipment (Volcano Therapeutics).
The sequence of analyzed images was chosen by reconstructing the ultrasound images in the longitudinal axis, outlining the region of interest. The cross-sectional analyses were conducted as described:
- •
The diameter and the reference area of the coronary artery were determined by calculating the mean between the diameters and the areas of reference proximal and distal to the analyzed segment, respectively. The proximal and distal reference diameters were obtained from the mean between the highest and lowest axis of the external elastic membrane in mm. The proximal and distal reference areas were determined by the outline of the external elastic membrane in mm2.
- •
The cross-sections used to determine the vessel diameter and reference area were selected considering the symmetry criterion, i.e., the ratio between the smallest and the largest diameter < 0.7, in order to exclude cross-sections with vascular remodelling.
- •
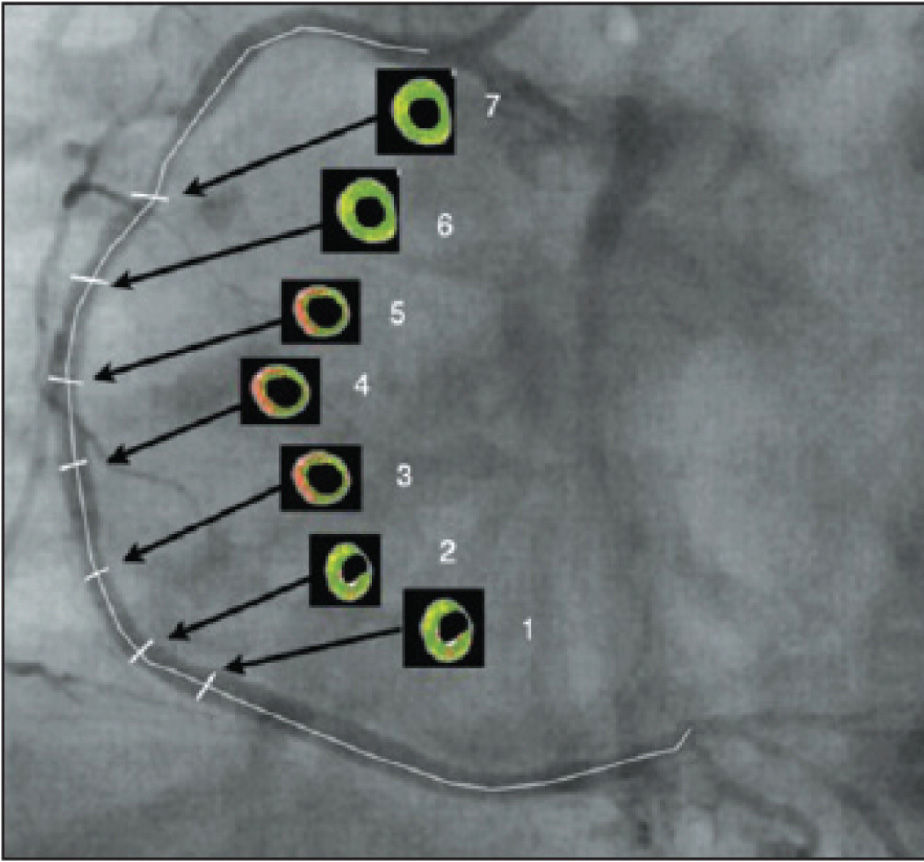
After locating the cross-section with the highest rate of arterial remodelling, termed the cross-section of interest (cross-section 4) and the performance of quantitative measures of intravascular ultrasound and evaluation with Virtual Histology®, the evaluation of six more cross-sections was performed with five frames of difference; three proximal to and three distal to crosssection 4 (Figure 1).
- •
The following measures were determined by ultrasound assessment:
- –
Proximal and distal reference diameters of the coronary artery in mm, for the calculation of reference vessel diameter through simple mean;
- –
Proximal and distal reference areas of the coronary artery in mm2, for the calculation of the reference vessel area through simple mean;
- –
Vessel area of cross-sections in mm2, for the calculation of vessel remodelling index;
- –
Lumen area in cross-sections in mm2;
- –
Atheromatous plaque area of the cross-sections in mm2, calculated by subtracting the area of the vessel from the lumen area; and
- –
Plaque burden of cross-sections in percentages.
- –
The remodelling index was calculated by the ratio between the vessel area in the cross-section analyzed and reference vessel area, defining as positive remodelling a ratio > 1.05.11,12
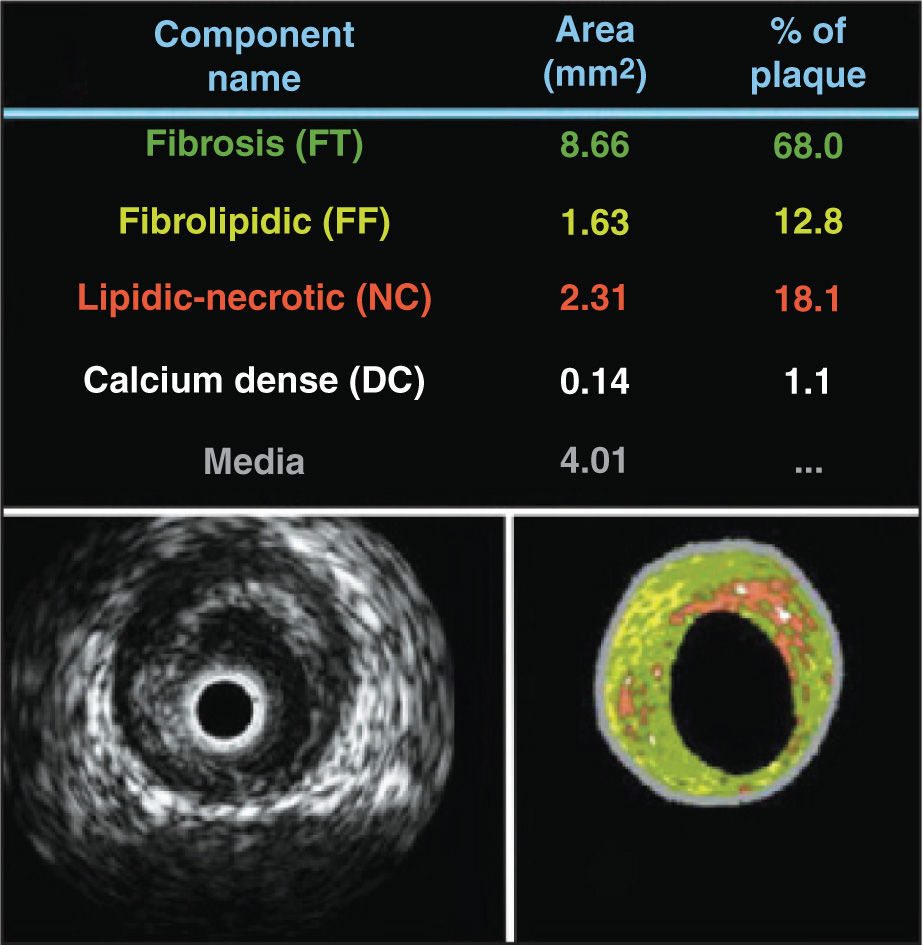
The different compositions of the atheromatous plaque were assessed and characterized by Virtual Histology®, using the program PcVH 2.2 (Volcano Therapeutics). For quantitative analysis of Virtual Histology®, tissue corresponding to the atheromatous plaque was color-coded, which was then divided into four groups according to the main components of the atheromatous plaque: green (fibrotic), yellow-green (fibrolipidic), red (necrotic core), and white (calcium). Data from the analysis by Virtual Histology® were reported as absolute values and as percentages relative to the atheromatous plaque area (Figure 2).
Statistical AnalysisThe ETCETERA module of the WINPEPI program, version 2.32 was used to calculate the sample size. A correlation coefficient of 0.3 was used, with a power of 90% and a significance level of 0.01 to test for the zero difference in the two-tailed test. The sample size was calculated at 159 cross-sections, that is, 22 patients with seven cross-sections each.
The baseline characteristics and the data of the intravascular ultrasound and Virtual Histology® analysis were tabulated and entered into a database created using SPSS software, version 18.0. Quantitative data with Gaussian distribution were described through means and standard deviations. In the presence of asymmetry, median, interquartile range (P25 to P75), and minimum and maximum values were used. Categorical data were described as numbers and percentages.
The variation in the percentage of necrotic core between different cross-sections of the coronary artery was studied by analysis of variance for repeated measures; data were logarithmically transformed before analysis. To evaluate the correlation between the remodelling index and the amount of necrotic core, Spearman’s correlation coefficient was used. SPSS software, version 18.0, was used for the analyses, and the level of significance was set at < = 0.05.
RESULTSIn total, 113 patients were evaluated, of whom only 30 (26.5%) met the inclusion and exclusion criteria. From the 30 patients included in this study, a total of 270 cross-sections were evaluated, derived from previously obtained intravascular ultrasound images with Virtual Histology®; 60 of these cross-sections were used to calculate the reference vessel area.
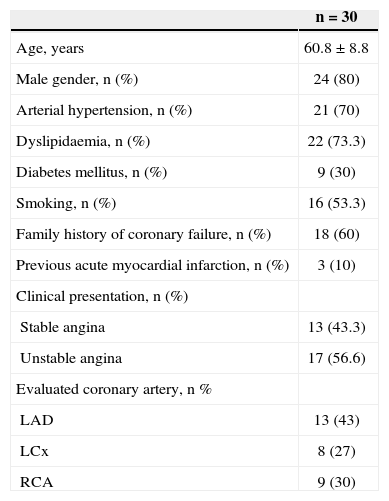
The population’s mean age was 60.8±8.8 years, most were males (80%), and 30% were diabetics. The most common clinical presentation was unstable angina (56.6%) and the left anterior descending artery was the most often studied vessel (43%) (Table 1).
Clinical and angiographic characteristics
| n = 30 | |
|---|---|
| Age, years | 60.8±8.8 |
| Male gender, n (%) | 24 (80) |
| Arterial hypertension, n (%) | 21 (70) |
| Dyslipidaemia, n (%) | 22 (73.3) |
| Diabetes mellitus, n (%) | 9 (30) |
| Smoking, n (%) | 16 (53.3) |
| Family history of coronary failure, n (%) | 18 (60) |
| Previous acute myocardial infarction, n (%) | 3 (10) |
| Clinical presentation, n (%) | |
| Stable angina | 13 (43.3) |
| Unstable angina | 17 (56.6) |
| Evaluated coronary artery, n % | |
| LAD | 13 (43) |
| LCx | 8 (27) |
| RCA | 9 (30) |
LAD, left anterior descending artery; LCx, left circumflex artery; RCA, right coronary artery.
The measurements performed by intravascular ultrasound showed proximal reference area of 16.5±5.2mm2, distal reference area of 14.5±4.9mm2, vessel reference area of 15.5±4.9mm2, and remodelling index in the cross-section of interest (cross-section 4) of 1.2±0.1 (Table 2).
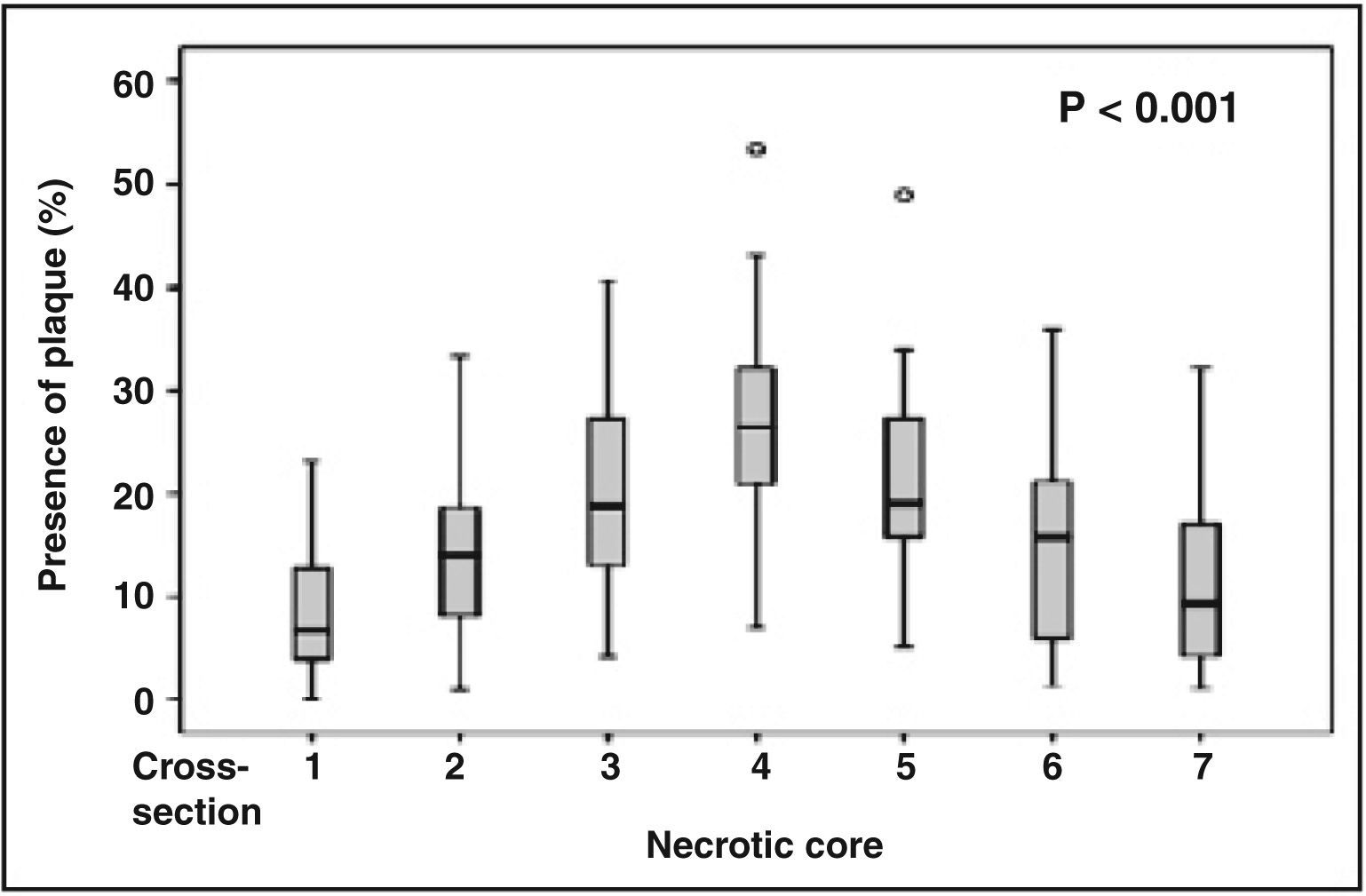
After quantitatively analyzing the percentage of necrotic core in the atheromatous plaque and arranging the results in charts according to the analyzed crosssection, the representation of the amount of necrotic core along the studied segment is visualized. Cross-section 1 represents the most distal cross-section of the vessel, while cross-section 7 is the most proximal. Cross-section 4, or the cross-section of interest, and cross-sections 2, 3, 5, and 6 complement the representation of the chart for the quantitative understanding of the necrotic core in the segment of the coronary artery studied.
The analysis of the variation in the percentage of necrotic core between the seven cross-sections of the coronary artery, using analysis of variance for repeated measures, showed higher amounts of necrotic core in the cross-section of interest, which has the highest rate of vascular remodelling (Figure 3).
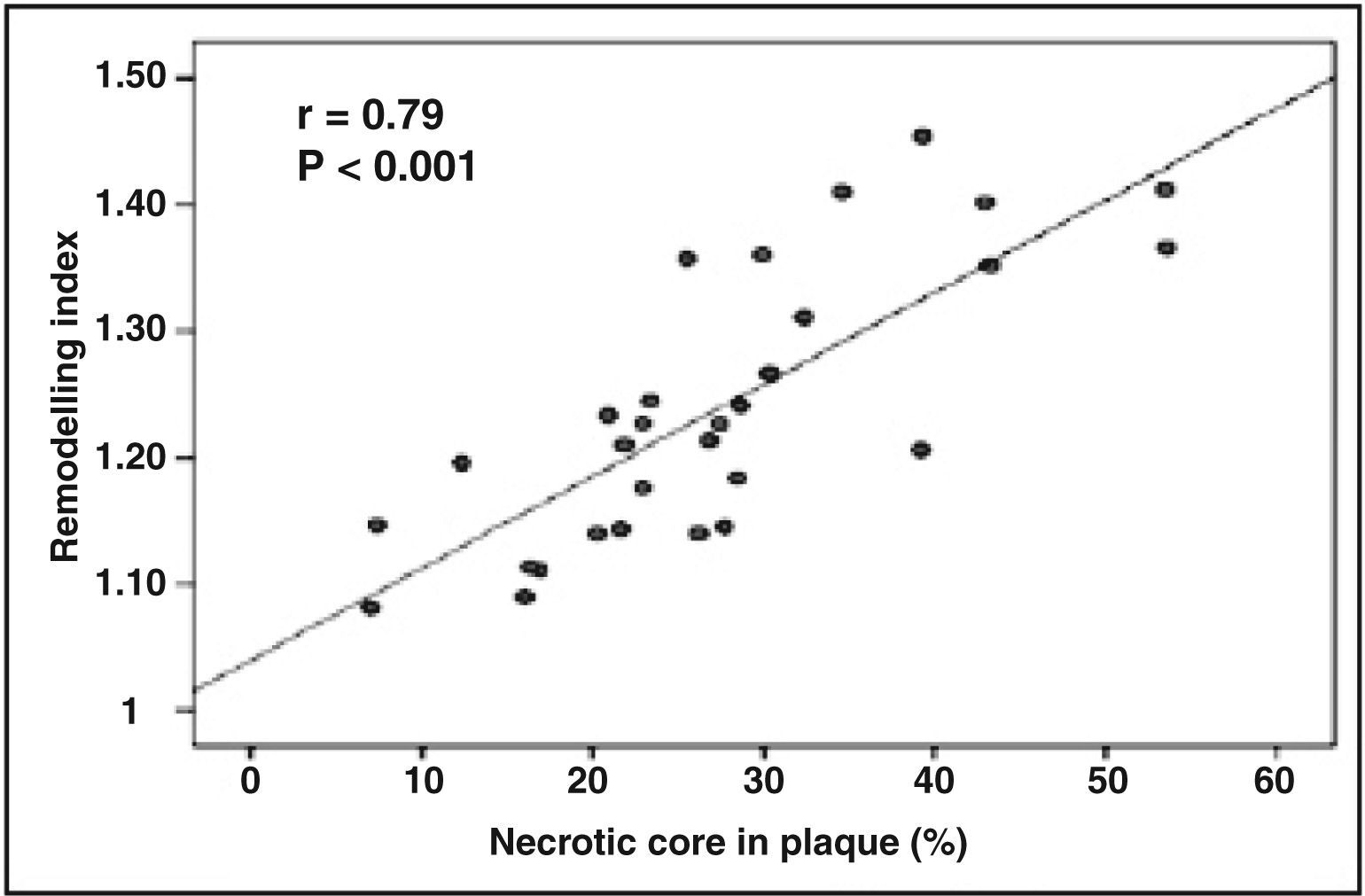
The results obtained when analyzing the linear correlation between the coronary artery remodelling index and the percentage of necrotic core in the atheromatous plaque show a positive correlation r = 0.79 (P < 0.001) (Figure 4).
DISCUSSIONCoronary heart disease as a major cause of morbidity and mortality has been globally investigated in order to better understand the disease. Studies have demonstrated some association between vascular remodelling and atheromatous plaque composition,8−11 and a strong association between plaque accidents (erosion or rupture) and coronary events. Plaques prone to clinical events have a characteristic distribution of their components and presence of positive arterial remodelling.2,9,21−23
The remodelling index is used to quantify arterial remodelling, which is the ratio between the area of the region of interest and the reference vessel area.12 Cross-section areas of the vessel are obtained with intravascular ultrasound and provide a 360-degree planar view of the coronary arteries, differentiating their different structures. Validation studies in vitro have shown good correlation between images generated by intravascular ultrasound and histological tissue analysis.24 However, this imaging modality does not allow for the characterization and differentiation of the atheromatous plaque components.
Virtual® Histology is an adjunct diagnostic method, developed from greyscale intravascular ultrasound. It provides detailed qualitative and quantitative information on the composition of the atheromatous plaque, and has been validated by histological studies.18 This imaging resource has been widely used in clinical research, both in assessing the evolution of atherosclerotic lesions25 and in the demonstration of efficacy of new treatments for atherosclerosis.24−26
The Providing Regional Observations to Study Predictors of Events in the Coronary Tree (PROSPECT) study25 evaluated patients with acute coronary syndromes submitted to three-vessel coronary angiography, supplemented by intravascular ultrasound and by radiofrequency, shortly after percutaneous coronary intervention, and followed-up for 3.4 years for adverse clinical events. Half of the events during patient evolution were related to the non-culprit vessel, and atheromatous plaques with vulnerability characteristics (thin-cap atheroma, plaque burden > 70%, and luminal area < 4mm2) were associated with future adverse cardiovascular events. However, that study did not evaluate the presence of positive remodelling as a predictor of clinical events or plaque vulnerability.
Virtual® Histology allows for the in vivo study of the atheromatous plaque, and it is possible to perform this study in a population at high risk for clinical events. The analysis of atheromatous plaque showed a positive correlation between positive arterial remodelling and percentage of necrotic core in atherosclerotic plaque (r = 0.79; P < 0.001). Similar results were reported in the retrospective study by Rodriguez-Granillo et al.,27 with a population of 41 patients, in which the remodelling index was correlated to the atheromatous plaque components by Virtual Histology® in coronary arteries considered non-culprit and with non-significant stenosis (< 50%). The linear regression analysis showed an association between the atheromatous plaque components and coronary artery remodelling. The size of the necrotic core was significantly higher in coronary lesions with positively remodelled vessel (r = 0.83; P < 0.0001) than in those with negative remodelling.
Moreover, atheromatous plaques in vessels with positive remodelling had morphology more compatible with vulnerable plaque, as 56% had a thin fibrotic cap, whereas in negative remodelling cases, the atheromatous plaques appeared to be more stable, as 64% had pathological intimal thickening and no evidence of fibroatheroma with thin fibrous cap.
In spite of some similar results, the present study and that by Rodriguez-Granillo et al.27 have several differences in methodology and in patient and target lesion selection. In the present study, which addressed the association between coronary artery remodelling and the percentage of necrotic core, the starting point was the search for the point of maximum positive remodelling rather than the point of greatest stenosis, as in the study by Rodriguez-Granillo et al.27 Another relevant difference in methodology was the performance of the full analysis of a segment of coronary artery with a predefined extension in the present study, which allowed for the comparison between the different sections of the variation of atheromatous plaque components in coronary segments studied, while Rodriguez-Granillo et al.27 performed only a few analyses.
As for the selection of the target lesion, the present study assessed lesions with significant obstruction (> 50%) in coronary arteries considered to be culprit vessels in a cardiovascular event, while Rodriguez-Granillo et al.27 studied non-significant obstructive lesions in non-culprit coronary arteries. Therefore, these studies evaluated opposing profiles of target lesion and patients, although the present study assessed patients with predominantly unstable lesions, whereas Rodriguez-Granillo et al.27 studied patients with lesions with stable characteristics.
In the present study, the analysis of the amount of necrotic core in the atheromatous plaque and its intra-section variation in segments of the studied coronary arteries showed a statistically significant difference (P < 0.001), confirming the existence of a variation in the amount of necrotic core along the same atheromatous plaque, where the necrotic core concentrates in segments with the highest degree of positive remodelling.
The association between positive arterial remodelling and necrotic core found in this study emphasizes the interaction between the anatomical and histological components involved in coronary artery disease. The necrotic core has an important role in the release of metalloproteinases, a group of proteolytic enzymes that can degrade and calcify elastin, which is a protein responsible for the elasticity and resilience of blood vessels.20,28 This may therefore be a possible physiopathological explanation to justify the correlation between necrotic core and positive vascular remodelling as a cause-effect association.29
The results of linear regression analysis between the arterial remodelling index and the amount of necrotic core in the present study are in agreement with the series of anatomopathological studies that observed an association between rupture or eroded plaques and the presence of positive arterial remodelling in patients who died due to acute coronary syndrome.30,31
This study demonstrated the importance of positive arterial remodelling and necrotic core in the atheromatous plaque in order to better understand its evolution (which generally translate into clinical events), and to amplify the detection of vulnerable plaques. In this sense, the search for positive arterial remodelling, which was identified in this study as associated with the presence of necrotic core (the main component of a vulnerable plaque), can be a useful tool for the detection of vulnerable plaques prior to plaque instability and its clinical consequences.
Study limitationsThe main limitations of the present study were: retrospective evaluation; difficulty finding a totally healthy segment of coronary artery to be used as reference vessel segment, due to the diffuse nature of coronary artery disease presentation; and the study sample size, which, although small, was sufficient to demonstrate statistically significant correlations.
CONCLUSIONSThe positive remodelling of the coronary artery is associated with the presence of necrotic core, which is characterized by unstable or vulnerable atherosclerotic plaque. The search for positive arterial remodelling can thus be a useful tool for the detection of vulnerable plaque prior to its destabilization.
CONFLICT OF INTERESTThe authors declare no conflicts of interest.