Balloon aortic valvuloplasty (BAV) is used as a palliative strategy in patients who are not eligible for valve replacement surgery, transcatheter aortic valve implantation, or as a bridge to these treatment modalities. The impact of BAV as a salvage procedure for patients in extreme clinical conditions (in extremis) is unknown.
MethodsPatients with severe degenerative aortic stenosis undergoing BAV between July 2008 and January 2013 were evaluated. Patients were divided into the in-extremis group (defined by the presence of two or more of the following organ dysfunctions: mechanical ventilation, hemodynamic instability, dialysis, coagulopathy or severe hepatic dysfunction) and the control group, which included the remaining patients.
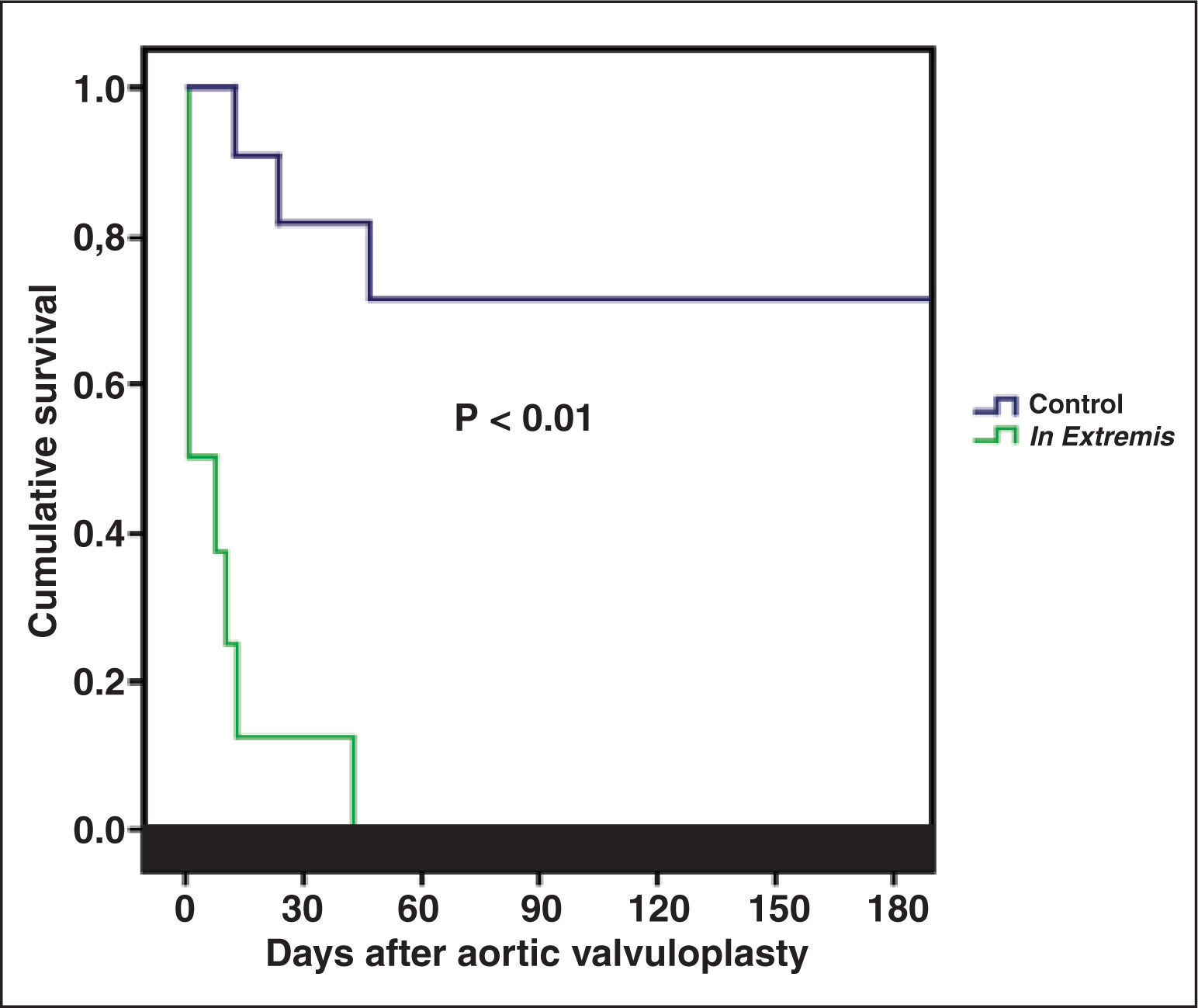
ResultsA total of 19 patients underwent BAV. The clinical condition in-extremis was present in 42.1% of them. Patients from the in-extremis group had a higher EUROSCORE II (41.1 ± 24.7 vs. 15.9 ± 14.0; P = 0.001) and LV ejection fraction lower than the control group (33.9 ± 17.3% vs. 49.0 ± 12.5; P = 0.04). None of the patients in the in-extremis group survived past the hospitalization period, whereas the control group mortality was 27.3% (P < 0.01).
ConclusionsBAV has an unfavorable result in patients with severe degenerative aortic stenosis with two or more organ dysfunctions, that is, patients in extremis.
Valvuloplastia Aórtica por Cateter Balão na Estenose Aórtica Degenerativa:Impacto Terapêutico em Pacientes em Condição Clínica In Extremis
IntroduçãoA valvuloplastia aórtica por cateter balão (VAB) é utilizada como estratégia paliativa em pacientes inelegíveis tanto para troca valvar cirúrgica quanto para implante valvar aórtico transcateter, ou como ponte para essas modalidades de tratamento. Não se sabe o impacto terapêutico da VAB quando realizada como medida de salvamento para pacientes em condições clínicas extremas (in extremis).
MétodosForam analisados pacientes com estenose aórtica grave de etiologia degenerativa submetidos à VAB entre julho de 2008 e janeiro de 2013. Os pacientes foram divididos entre o grupo in extremis (definido pela presença de duas ou mais das seguintes disfunções orgânicas: ventilação mecânica, instabilidade hemodinâmica, terapia renal dialítica, coagulopatia ou disfunção hepática graves) e o grupo controle, que incluiu os demais pacientes.
ResultadosUm total de 19 pacientes realizaram VAB no período. A condição clínica in extremis esteve presente em 42,1%. Os pacientes do grupo in extremis tiveram EUROSCORE II mais elevado (41,1 ± 24,7 vs. 15,9 ± 14,0; P = 0,01) e fração de ejeção do VE mais baixa que o grupo controle (33,9 ± 17,3% vs. 49,0 ± 12,5%; P = 0,04). Nenhum paciente do grupo in extremis sobreviveu ao período intrahospitalar, enquanto que, no grupo controle, a mortalidade foi de 27,3% (P < 0,01).
ConclusõesPara o tratamento de pacientes com estenose aórtica grave de etiologia degenerativa, a VAB tem resultado desfavorável quando indicada para pacientes com duas ou mais disfunções orgânicas, ou seja, em condição clínica in extremis.
Degenerative aortic stenosis is the valvulopathy whose incidence most increases with aging. Its prevalence in individuals over 75 years of age is estimated at 4.6%.1 The prognosis after the onset of symptoms is poor, with survival time between one and three years.2
The treatment of choice for symptomatic patients with severe aortic stenosis secondary to valve degeneration is aortic valve replacement surgery (AoVR).3 However, approximately 30% of these patients do not receive surgical treatment due to the high perioperative risk arising from multiple comorbidities.4 Thus, less invasive modalities have emerged for the treatment of this valvulopathy, among which stand out the transcatheter aortic valve implantation (TAVI) and balloon aortic valvuloplasty (BAV).
TAVI was safe and effective in patients with high surgical risk, providing a reduction in mortality in patients whose surgical procedure was refused by the surgeon because of an excess of clinical comorbidities.5 However, the anatomical prerequisites required, the high cost related to the procedure, and the low number of trained professionals capable of its performance make this option unavailable for most patients.
BAV is a procedure used as a palliative strategy in patients unfit for both surgical valve replacement and TAVI, or as a bridge to these treatment modalities.2,6-8 Its low cost and wide availability in most cardiology centers justify its use for patients with prohibitive surgical risk, despite the high recurrence rate of symptoms and its ineffectiveness in reducing mortality.9,10
The best time to perform the BAV, in the context of symptomatic aortic stenosis, has not yet been defined. In most cases, the procedure is performed with urgency in patients refractory to optimized clinical treatment. In other times, however, BAV is performed as a measure to rescue patients who already present multiple organ dysfunctions, i.e., extreme clinical conditions (in extremis), in an attempt to avoid death, at the expense of an improvement in the cardiac output compromised by the aortic valve stenosis.
The aim of this study was to evaluate the therapeutic impact of BAV in the treatment of degenerative aortic stenosis in patients with and without the clinical condition in extremis.
METHODSThis is a retrospective study, conducted in a single quaternary care service of high complexity cardiology. The research was based on a database analysis and review of the electronic clinical record.
Study populationBetween July 2008 and January 2013, all patients undergoing BAV for treatment of degenerative aortic stenosis at Instituto do Coração, Hospital das Clínicas da Faculdade de Medicina, Universidade de São Paulo (Incor-HCFMUSP), in São Paulo (SP) were reviewed. This study did not include procedures for treatment of congenital aortic stenosis.
ProcedureAll procedures were performed by retrograde route, by puncturing the common femoral artery (right or left), and by the application of a long sheath with diameter 10F. Unfractionated heparin was administered to all patients. The size of the balloon catheter used in each procedure was chosen at the discretion of the surgeon. In some cases, the balloon inflation was preceded by a rapid stimulation (fast pacing), with a temporary pacemaker placed in the right ventricle.
The measurement of the aortic transvalvular gradient was performed before and after the BAV, through intracavitary manometry and transthoracic echocardiography. The sheath was removed immediately after the procedure, with subsequent manual compression for hemostasis.
Procedural success was defined as the successful dilation of the aortic valve with the balloon catheter, in the absence of complications during the procedure.
Data collectionClinical, echocardiographic, and hemodynamic data, and in-hospital outcomes were obtained retrospectively from the electronic case notes of each patient treated at Incor-HCFMUSP. After hospital discharge, the evaluation of cardiovascular events was performed by analyzing the electronic records, with supplementation by telephone contact, when needed.
The patients’ profile of disease severity was estimated by EuroSCORE II and STS Risk Score. Severe pulmonary hypertension was defined as a systolic pressure in pulmonary artery (SPPA) ≥ 60mmHg by echocardiography. Renal failure was characterized as the presence of creatinine clearance ≤ 60mL/min.
The patients were divided in two groups: in extremis (defined by the presence of two or more of the following dysfunctions: mechanical ventilation, hemodynamic instability, renal dialysis therapy, coagulopathy, or severe liver dysfunction) and control, which included the remaining patients.
Statistical analysisThe analysis of clinical, echocardiographic, and hemodynamic data was performed using the software SPSS (IBM Corp., New York, USA). Continuous variables were described as mean and standard deviation and compared by Student’s t-test. Categorical variables were described as frequency, and percentage, and compared by the chi-squared or Fisher’s exact test, when appropriate. The survival analysis was performed by Kaplan-Meier method. A significance level of P < 0.05 was adopted.
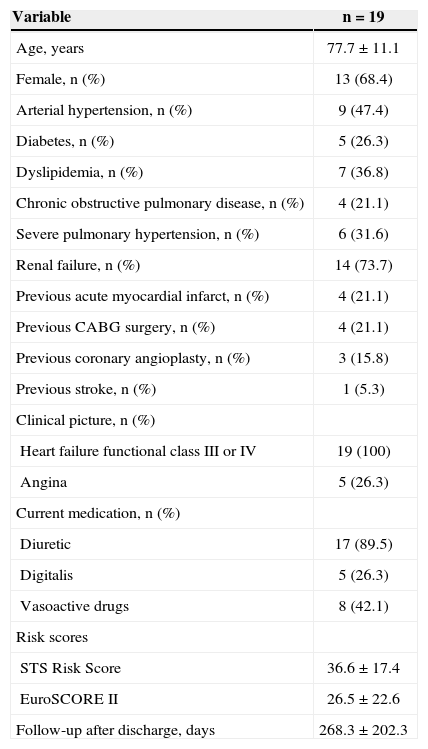
RESULTSA total of 19 patients with severe aortic stenosis with degenerative etiology were treated with BAV in the analyzed period. The study population was composed almost entirely by elderly people, and the mean age was 77.7 ± 11.1 years. The predominant symptom before the procedure was dyspnoea, and all patients were classified as heart failure functional class III or IV. Among the comorbidities, severe pulmonary hypertension was particularly prominent, with 31.6% of patients, and renal failure was present in 73.7%. The mean of STS Risk Score was 36.6 ± 17.4 and that of EuroSCORE II was 26.5 ± 22.6. Other clinical characteristics of the patients are shown in Table 1.
Clinical characteristics
| Variable | n = 19 |
|---|---|
| Age, years | 77.7 ± 11.1 |
| Female, n (%) | 13 (68.4) |
| Arterial hypertension, n (%) | 9 (47.4) |
| Diabetes, n (%) | 5 (26.3) |
| Dyslipidemia, n (%) | 7 (36.8) |
| Chronic obstructive pulmonary disease, n (%) | 4 (21.1) |
| Severe pulmonary hypertension, n (%) | 6 (31.6) |
| Renal failure, n (%) | 14 (73.7) |
| Previous acute myocardial infarct, n (%) | 4 (21.1) |
| Previous CABG surgery, n (%) | 4 (21.1) |
| Previous coronary angioplasty, n (%) | 3 (15.8) |
| Previous stroke, n (%) | 1 (5.3) |
| Clinical picture, n (%) | |
| Heart failure functional class III or IV | 19 (100) |
| Angina | 5 (26.3) |
| Current medication, n (%) | |
| Diuretic | 17 (89.5) |
| Digitalis | 5 (26.3) |
| Vasoactive drugs | 8 (42.1) |
| Risk scores | |
| STS Risk Score | 36.6 ± 17.4 |
| EuroSCORE II | 26.5 ± 22.6 |
| Follow-up after discharge, days | 268.3 ± 202.3 |
CABG = coronary artery bypass graft.
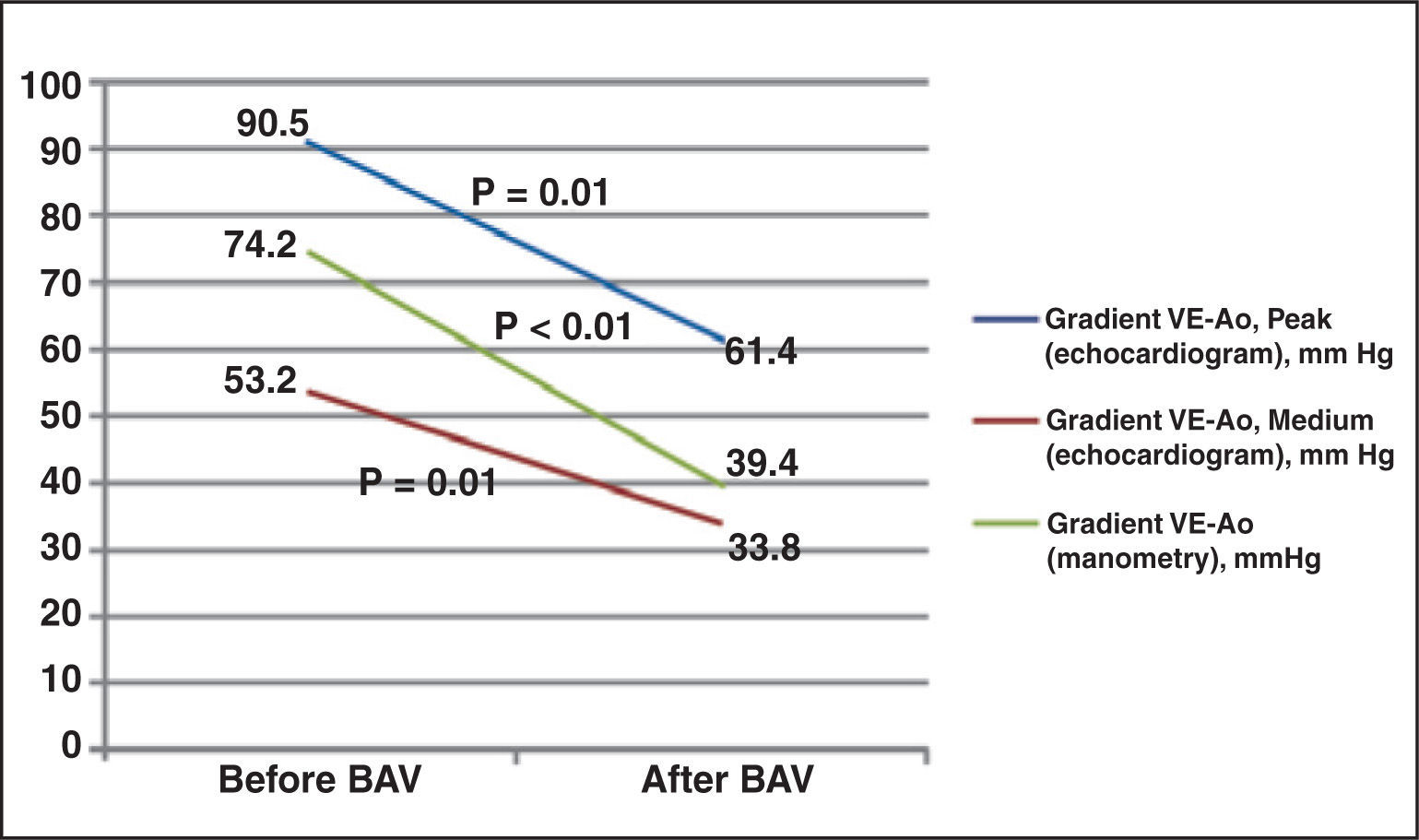
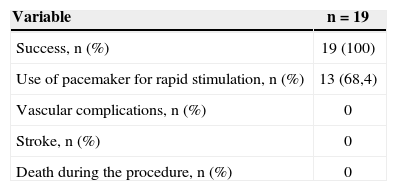
Procedural success was achieved in 100% of the cases. The temporary pacemaker was used to promote rapid stimulation (fast pacing) in 68.4% of patients (Table 2). Significant reduction of the aortic transvalvular gradient was noted, both by echocardiography and by pressure measurements during the procedure (Figure 1).
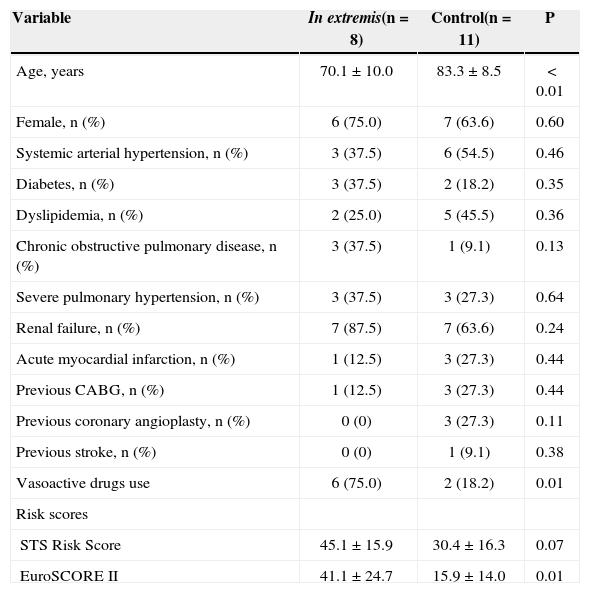
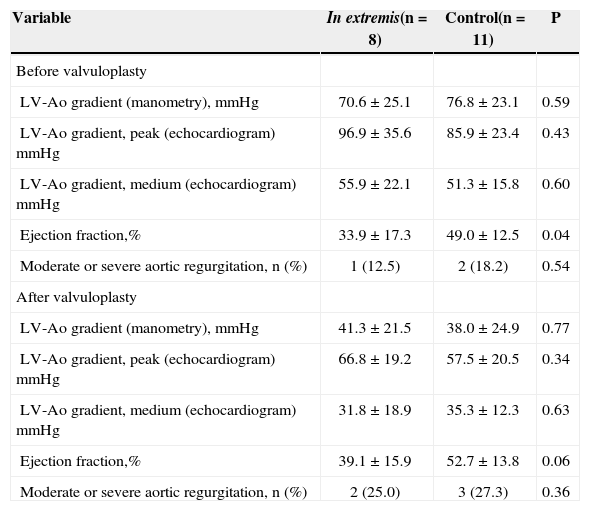
The clinical condition in extremis was present in 42.1% of patients (Table 3). When compared to the control group, patients in the in extremis group were younger (70.1 ± 10.0 vs. 83.3 ± 8.5 years; P < 0.01) and had higher EuroSCORE II (41.1. ± 24.7 vs. 15.9 ± 14.0l; P < 0.01). In addition, the in extremis group had a lower left ventricle ejection fraction before the procedure versus the control group (33.9 ± 17.3% vs. 49.0 ± 12.5%; P = 0.04). Hemodynamic and echocardiographic characteristics are shown in Table 4.
Clinical characteristics by group
| Variable | In extremis(n = 8) | Control(n = 11) | P |
|---|---|---|---|
| Age, years | 70.1 ± 10.0 | 83.3 ± 8.5 | < 0.01 |
| Female, n (%) | 6 (75.0) | 7 (63.6) | 0.60 |
| Systemic arterial hypertension, n (%) | 3 (37.5) | 6 (54.5) | 0.46 |
| Diabetes, n (%) | 3 (37.5) | 2 (18.2) | 0.35 |
| Dyslipidemia, n (%) | 2 (25.0) | 5 (45.5) | 0.36 |
| Chronic obstructive pulmonary disease, n (%) | 3 (37.5) | 1 (9.1) | 0.13 |
| Severe pulmonary hypertension, n (%) | 3 (37.5) | 3 (27.3) | 0.64 |
| Renal failure, n (%) | 7 (87.5) | 7 (63.6) | 0.24 |
| Acute myocardial infarction, n (%) | 1 (12.5) | 3 (27.3) | 0.44 |
| Previous CABG, n (%) | 1 (12.5) | 3 (27.3) | 0.44 |
| Previous coronary angioplasty, n (%) | 0 (0) | 3 (27.3) | 0.11 |
| Previous stroke, n (%) | 0 (0) | 1 (9.1) | 0.38 |
| Vasoactive drugs use | 6 (75.0) | 2 (18.2) | 0.01 |
| Risk scores | |||
| STS Risk Score | 45.1 ± 15.9 | 30.4 ± 16.3 | 0.07 |
| EuroSCORE II | 41.1 ± 24.7 | 15.9 ± 14.0 | 0.01 |
CABG = coronary artery bypass graft.
Hemodynamic and echocardiographic characteristics
| Variable | In extremis(n = 8) | Control(n = 11) | P |
|---|---|---|---|
| Before valvuloplasty | |||
| LV-Ao gradient (manometry), mmHg | 70.6 ± 25.1 | 76.8 ± 23.1 | 0.59 |
| LV-Ao gradient, peak (echocardiogram) mmHg | 96.9 ± 35.6 | 85.9 ± 23.4 | 0.43 |
| LV-Ao gradient, medium (echocardiogram) mmHg | 55.9 ± 22.1 | 51.3 ± 15.8 | 0.60 |
| Ejection fraction,% | 33.9 ± 17.3 | 49.0 ± 12.5 | 0.04 |
| Moderate or severe aortic regurgitation, n (%) | 1 (12.5) | 2 (18.2) | 0.54 |
| After valvuloplasty | |||
| LV-Ao gradient (manometry), mmHg | 41.3 ± 21.5 | 38.0 ± 24.9 | 0.77 |
| LV-Ao gradient, peak (echocardiogram) mmHg | 66.8 ± 19.2 | 57.5 ± 20.5 | 0.34 |
| LV-Ao gradient, medium (echocardiogram) mmHg | 31.8 ± 18.9 | 35.3 ± 12.3 | 0.63 |
| Ejection fraction,% | 39.1 ± 15.9 | 52.7 ± 13.8 | 0.06 |
| Moderate or severe aortic regurgitation, n (%) | 2 (25.0) | 3 (27.3) | 0.36 |
LV = left ventricle; Ao = aorta.
In both groups, there were no deaths during the procedure. None of the eight patients in the in extremis group survived the in-hospital period, while in the control group the in-hospital mortality was 27.3% (P < 0.01). Survival at 180 days is shown in Figure 2.
Three patients (15.8%) of the control group were submitted to BAV as a bridge to other definitive treatment: one patient underwent TAVI by transapical approach and died during the procedure, and two patients underwent AoVR (one of them was discharged and the other died in the immediate postoperative period). All patients who were discharged (42.1%) showed improvement in their heart failure functional class. The mean follow-up was 268.3 ± 202.3 days.
DISCUSSIONThis study was relevant in the analysis of the clinical, echocardiographic, and hemodynamic characteristics, as well as in the evolution of patients with severe degenerative aortic stenosis treated by BAV.
The high in-hospital mortality in the study reflects the clinical profile of extreme severity of the patients, mainly due to the presence of multiple comorbidities. There are no other studies in the literature that include a group of patients as severe as those described in this work. The mean STS Risk Score in the PARTNER study,5 which included only patients at high surgical risk or considered inoperable, was 11.6 ± 6.0, i.e., much lower than that of the present study (36.6 ± 17, 4). Other studies involving only patients undergoing BAV also included patients with less severe conditions compared to the present study, with lower incidence of comorbidities and with lower risk scores.6,7,11-14
Most patients (73.7%) showed renal failure at the time of the procedure, and most had pulmonary artery pressure > 60mmHg. Both conditions have been associated to an increase in mortality in earlier studys.9,15
In a study of 509 patients, Ben-Dor et al. demonstrated that the mortality after 5 months for patients with SPPA > 60mmHg was 49.1%, regardless of the type of treatment received (BAV, TAVI, or AoVR).16
Several studies have correlated the presence of renal insufficiency before the procedure with increased mortality.9,17,18 A study of 262 patients at high surgical risk undergoing BAV showed that creatinine clearance ≤ 60mL/min before the procedure is a strong predictor of mortality.7
The absence of vascular complications in this study may be related to the use of sheaths with small calibre (10F) in all patients, as well as to the immediate withdrawal of the sheath. Despite being related to a shorter hospital stay and a lower rate of blood transfusions in patients undergoing AoBV,19 vascular occlusion devices were not used in this study.
The division of patients according to the presence or absence of the clinical condition in extremis was extremely important for the understanding of the most appropriate time for performing BAV. The guidelines do not address BAV as a salvage therapy in patients in critical clinical condition with multiple organ dysfunction, but do suggest that its performance can be beneficial as a bridge to AoVR.2 In this study, only two patients underwent BAV as a bridge to AoVR, and in these cases, mortality was 50%. Recently, some studies have addressed the use of BAV as a bridge to TAVI, showing excellent results in the short and long term, when compared to BAV alone.6-8
The classification of patients according to the number of organ dysfunctions presented was of great importance to the understanding that there is a subgroup of more severe patients who evolve unfavourably, even after BAV. Patients with two or more organ dysfunctions had significantly higher mortality when compared to patients in the control group. The main reason for the treatment of patients in the group in extremis with BAV was the presence of a clinical condition of extreme gravity, whether or not with cardiac etiology, in the presence of severe aortic stenosis. It was hoped that, in these patients, the reduction of the gradient in the left ventricular (LV) outflow would provide an improvement of their LV function to the point that they could recover from the critical hemodynamic state present during their admission. However, even with the decrease of the aortic transvalvular gradient and the increase in LV ejection fraction after the procedure, these results were not translated into clinical improvement, suggesting that these patients underwent the intervention too late.
Limitations of the studyThe study had some limitations, such as the small number of patients included in the retrospective analysis of data and the fact that it was conducted in a single center.
CONCLUSIONSBalloon aortic valvuloplasty for treatment of severe aortic stenosis with degenerative etiology has unfavorable outcome when indicated for patients with two or more organ dysfunctions, i.e., in a clinical condition in extremis.
CONFLICTS OF INTERESTThe authors declare no conflicts of interest.