Drug-eluting stent trials have predominantly examined male populations of European descent. SPIRIT Women single-arm study evaluates the XIENCE™ V everolimus-eluting stent in complex de novo lesions in a real world female population, including Latin American patients. This analysis provides an insight into how this population responds to stenting when compared to non-Latin American patients.
MethodsOf the 1,572 patients enrolled from 73 non-US sites, 138 (9%) were recruited from Argentina, Brazil and Venezuela.
ResultsTarget lesions had reference vessel diameter ranging between 2.25mm and 4mm and lesion length<28mm. Baseline characteristics were similar between the groups, with exception to a higher prevalence of hypertension, anterior myocardial infarction (MI) and family history of coronary artery disease in the Latin American cohort. Lesions tended to be more complex in Latin American women with a smaller reference vessel diameter, longer lesion length, increased eccentricity and angulation, and more type B2/C lesions. Events were adjudicated according to the guidelines of the Academic Research Consortium. At 1 year, the composite endpoint of death, MI and target vessel revascularization (TVR) was 12.1% in the non-Latin American population and 10.1% in the Latin American population (P=0.58).
ConclusionsAt 1 year, the low rates of adverse cardiac events, including stent thrombosis, target lesion failure, cardiac death, MI and TVR
Avaliação do Stent Coronário Eluidor de Everolimus XIENCE™ Vna População Feminina Latino-Americana do Estudo de Braço Único SPIRIT Women: Acompanhamento Clínico de Um Ano
IntroduçãoOs estudos com stents farmacológicos têm avaliado predominantemente populações masculinas de descendência europeia. O estudo de braço único SPIRIT Women avalia o stent eluidor de everolimus XIENCE™ V em lesões de novo complexas em uma população feminina do mundo real, incluindo pacientes latino-americanas. Esta análise permite compreender como essa população responde ao implante de stent, comparativamente a pacientes não-latino-americanas.
MétodosDas 1.572 pacientes matriculadas em 73 locais fora dos Estados Unidos, 138 (9%) foram recrutadas na Argentina, no Brasil e na Venezuela.
ResultadosAs lesões-alvo tinham diâmetro de referência do vaso entre 2,25mm e 4mm e extensão da lesão<28mm. As características basais foram semelhantes entre os grupos, com exceção de maior prevalência de hipertensão arterial, infarto do miocárdio (IM) de parede anterior e história familiar de doença arterial coronária na coorte latino-americana. As lesões tendiam a ser mais complexas em mulheres latino-americanas, com menor diâmetro de referência do vaso-alvo, maior extensão da lesão, maior excentricidade e angulação e mais lesões tipo B2/C. Os eventos foram adjudicados de acordo com as definições do Academic Research Consortium. Em um ano, o desfecho combinado de morte por todas as causas, IM e revascularização do vaso-alvo (RVA) foi de 12,1% na população não-latino-americana e de 10,1% na população latino-americana (P=0,58).
ConclusõesEm um ano, os baixos índices de eventos cardíacos adversos, incluindo trombose do stent, falha da lesão-alvo, morte cardíaca, IM e RVA nas mulheres latino-americanas foram comparáveis aos das mulheres não-latino-americanas, apesar da maior complexidade das lesões. Esses resultados demonstram a segurança e a eficácia do stent XIENCE™ V nessa pequena coorte de pacientes latino-americanas, à semelhança do que é observado com populações maiores e mais variadas.
Environmental and genetic risk factors, such as abdominal obesity, diabetes, dyslipidemia, smoking, and hypertension have been identified as predisposing factors for the female Latin American population to a significantly increased risk of cardiovascular events. Drug-eluting stents are now widely accepted as safe and effective therapies for patients with coronary artery disease. Drug-eluting stents inhibit neointimal proliferation by locally delivering an anti-proliferative drug, reducing the restenosis rate and the need for repeat revascularization procedures.1
The XIENCE™ V (everolimus-eluting stent system Abbott Vascular, Santa Clara, USA) was extensively evaluated in the SPIRIT clinical trials. SPIRIT FIRST2 and SPIRIT II3, where XIENCE™ V was evaluated, respectively, against the bare metal MULTI-LINK VISION™ RX stent (Guidant Vascular Intervention, Santa Clara, USA) and the paclitaxel-eluting stent TAXUS ™ (Boston Scientific, Natick, USA), demonstrated superiority when compared to the latter in terms of in-stent late loss at 6 months. Subsequently, this improvement in clinical outcomes was demonstrated in up to 5 years of fol lowup in SPIRIT FIRST.4 SPIRIT III demonstrated superiority to XIENCE™ V when compared to TAXUS ™ in terms of in-segment late loss at 8 months and non-inferiority for the secondary endpoint of target vessel failure at 9 months.5 In addition, patients treated with the everolimus eluting stent in this study had a significantly improved event-free survival at 2 years when compared to patients receiving the TAXUS™ stent.6
The aforementioned SPIRIT trials were primarily carried out in male populations of European descent with a relatively low-risk profile and simple lesions. When compared to the rest of the world, the Latin America population has shown a higher prevalence of diabetes and hypertension7 and a high proportion of smokers, especially in big cities.8
The present manuscript reports the clinical follow-up data at 1 year in the Latin American population compared to the non-Latin American (non-Latin American) population of the SPIRIT Women Single Arm Study. This study evaluated the performance of XIENCE™ V in complex de novo lesions in a real world female population (n=1,572). A significant proportion of the study population (138 patients; 9%) was recruited from Latin American sites in Argentina, Brazil and Venezuela, thereby providing insight into how this population responds to the implant of everolimus eluting stents when compared to the non-Latin American patient population.
METHODSStudy design and patient selectionSPIRIT Women is a prospective, single arm, multi-center study aimed at evaluating the performance of XIENCE™ V in the real world, according to its instructions for use, in the treatment of female patients with de novo coronary artery lesions. The study protocol was approved by the medical ethics committee of each participating institution and all patients gave written informed consent.
Between July 2007 and March 2009, 1,572 patients were enrolled at 73 clinical sites outside the United States, of which 9% (n=138) were recruited from sites in Argentina (36), Brazil (82) and Venezuela (20).
Patients (aged>18 years) recruited from the general interventional cardiology population who had been admitted for a PCI procedure, were recruited for the study. Inclusion criteria included patients with evidence of myocardial ischemia, stable or unstable angina, silent ischemia or a positive functional study, or a reversible change in the ECG consistent with ischemia. Patients were required to be suitable candidates for myocardial revascularization and had to agree to undergo clinical follow-up as required per protocol. Patients of child bearing potential should have a negative pregnancy test within 7 days before treatment. In addition, coronary anatomy should allow an optimal treatment with a maximum of 4 stents planned for de novo target lesions with target vessel reference diameter between 2.25mm and 4.0mm, target lesion length≤28mm by visual estimation. Patients were excluded if they had participated in another device or drug study or had completed the follow-up phase of another study within 30 days prior to enrolment or if they had undergone previous stenting, be it a bare metal or drug-eluting stent in the target vessel.
The XIENCE™ V everolimus-eluting stentA detailed description of the device has been published elsewhere.9 In brief, XIENCE™ V everolimuseluting stent system includes MULTI LINK VISION™ metal platform on a delivery system with drug eluting coating. The drug eluting coating is composed of fluorinated acrylic polymers and the anti-proliferative drug everolimus (Certican®, Novartis Pharmaceuticals Corporation, Basel, Switzerland). Stents are available in diameters of 2.25, 2.5, 2.75, 3.0, 3.5 and 4.0mm and lengths of 8, 12, 15, 18, 23 and 28mm.
Study procedureFollowing confirmation of the angiographic inclusion criteria and before implantation of the first stent, patients were entered via an interactive voice response system (ICON Clinical Research, Eastleigh, UK). All registered patients were considered enrolled in the study and were to remain in the study until completion of the required follow-up period. Peri-procedural drug therapy was administered according to standard hospital practice. Unfractionated heparin or bivalirudin was used for procedural anticoagulation. The use of glycoprotein IIb/IIIa inhibitors was left to the discretion of the operator. All patients enrolled in the study were to receive a loading dose≥300mg of clopidogrel. After the procedure the protocol recommended that patients receive a daily dose of 75mg of clopidogrel for a minimum of 6 months and≥75mg of aspirin daily indefinitely.
Follow-upClinical follow-up was scheduled at 30 days, 1 year and 2 years after the procedure and included assessment of angina, adverse event data collection, details of subsequent coronary intervention, protocol required medications and use and changes of concomitant medications.
Study endpointsThe primary endpoint was the composite incidence of all death, myocardial infarction (MI) and target vessel revascularization (TVR) at 1 year. Secondary endpoints included but were not limited to acute success (clinical device and procedure) and stent thrombosis rates.
Source document verificationSource document verification was routinely performed in 20% of random cases and 100% of all reported adverse events during the study, resulting in an overall rate of 30%. For sites with low rates of adverse event reporting, additional monitoring visits and source document verification were performed. All endpoint related events were adjudicated by an independent Clinical Events Committee (CEC) that had access to source documentation.
DefinitionsAll study endpoint events were adjudicated by an independent CEC according to the Academic Research Consortium (ARC) definitions.10 All adverse events were reported bimonthly to an independent Data and Safety Monitoring Board (DSMB) which reviewed data to identify safety issues related to the conduct of the study.
- –
Death: All deaths were considered cardiac unless an unequivocal non-cardiac cause could be established. Specifically, any unexpected death, even in patients with coexisting potentially fatal non-cardiac disease (e.g. cancer, infection), were classified as cardiac.
- –
Cardiac death: Any death due to immediate cardiac cause (e.g. MI, low cardiac output syndrome, fatal arrhythmia). Unwitnessed death and death of unknown cause were classified as cardiac death. This included all procedure-related deaths including those related to concomitant treatment.
- –
Myocardial infarction: MI classification and criteria for diagnosis were defined according to the ARC as follows: for non-procedural/spontaneous MI, troponin or CK-MB levels had to be>2 times the upper normal range; for peri-percutaneous coronary intervention, troponin or CK-MB levels had to be≥3 times the upper normal range; for peri-myocardial revascularization, troponin or CK-MB levels had to be≥5 times the upper normal range. The periprocedural period included the first 48h and 72h after percutaneous coronary intervention and myocardial revascularization, respectively. All late events that were not associated with a revascularization procedure were considered spontaneous. One blood sample was taken from each patient within the post-procedure hospitalization period for the analysis of CKMB or troponin levels.
- –
Target lesion revascularization: TLR is defined as any repeat percutaneous intervention or bypass surgery to treat any segment of the target vessel. The target lesion is defined as a segment 5mm proximal and 5mm distal to the stent.
- –
Target vessel revascularization: TVR is defined as any repeat percutaneous intervention or surgical bypass of any segment of the target vessel. It is defined as the major coronary vessel, which includes the target lesion and its proximal and distal branches.
- –
Target lesion failure: TLF is defined as cardiac death, target vessel MI, or ischemia-driven TLR (percutaneous coronary intervention or myocardial revascularization).
- –
Stent thrombosis: Characterized as acute (< 1 day), subacute (1–30 days) and late (> 30 days) and defined according to the ARC guidelines as definitive (acute coronary syndrome with angiographic or pathologic confirmation of stent thrombosis); probable (unexplained death≤30 days or target vessel-MI without angiographic confirmation); and possible (unexplained death>30 days after stent placement).
- –
Clinical device success: Successful delivery and deployment of the study stent (in case of stent overlapping, a successful delivery and deployment of the first and second stents) at target lesion and successful withdrawal of the stent delivery system with final residual stenosis<50% of the target lesion by quantitative coronary angiography (QCA) (or by visual estimation if QCA unavailable), without using a device outside the assigned treatment strategy. Bailout patients were included as clinical device success only if the above criteria were met.
- –
Clinical procedure success: Successful delivery and deployment of the study stent or stents at the intended target lesion and successful withdrawal of the stent delivery system with final residual stenosis<50% of the target lesion by QCA (or by visual estimation if QCA unavailable) and/or using any adjunctive device without the occurrence of cardiac death, MI not clearly attributed to a non-target vessel and/or TLR during hos pital stay within a maximum of seven days of the index procedure. In multiple lesions setting each lesion must have met clinical procedure success criteria.
All analyses were performed based on the intent to treat population. The study sample size was based on the primary endpoint of the composite rate of all death, MI and TVR at 1 year. A sample size of 1,550 patients would produce a narrow two-sided 95% confidence interval for the clinical endpoints estimates. The ½ width of the 2-sided confidence interval for the primary endpoint would range between 1.5% and 1.7%, assuming the true rate between 10% and 14%. Continuous variables are summarized as mean and standard deviation and compared with a t-test. Binary variables are presented as percentages and compared with the Fisher’s exact test. P-values are not based on a formal hypothesis testing and are displayed for descriptive purpose only.
RESULTSNine percent (138/1572) of the SPIRIT Women population was recruited from sites within Latin America, including 59% (82/138) from sites in Brazil; 26% (36/138) from sites in Argentina and 14% (20/138) from sites in Venezuela. At 1 year, clinical follow-up was obtained in 100% of the Latin American patients and in 98.0% of the patients at non-Latin American sites.
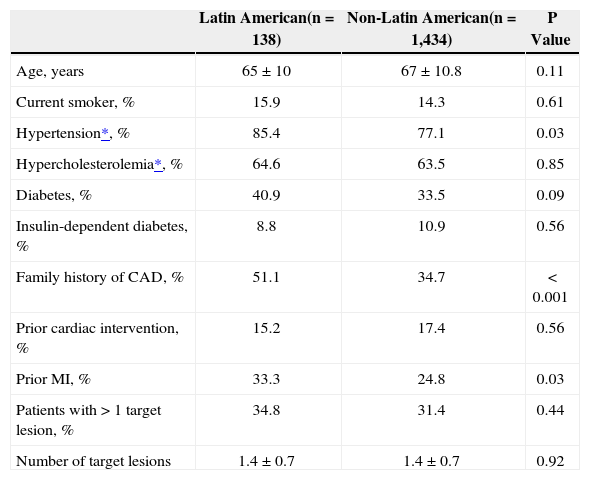
There was no significant difference in the overall mean age between patients recruited from Latin American sites when compared to those from non-Latin American sites (Table 1). However, there was a trend for the Latin American patients to be younger with 46.4% of patients with ages ranging between 18–65 years vs. 38.5% of patients from non-Latin American sites (P=0.08). When compared to non-Latin American patients, Latin American patients had a higher prevalence of hypertension requiring medication (85% vs. 77%; P=0.03), prior myocardial infarction (33% vs. 25%; P=0.03), and family history of coronary artery disease (51% vs. 35%; P<0.001). Latin American patients had an increased body mass index (28.5kg/m2 vs. 27.5kg/m2; P=0.03), which was primarily driven by a lower mean height as there was no difference in mean weight between the two patient populations. Resting diastolic blood pressure was also higher in the Latin American population (77mm Hg vs. 74mm Hg; P=0.004), whereas resting systolic blood pressure was higher in the non-Latin American population (136mm Hg vs. 132mm Hg p=0.03). There was a trend to more frequent diabetes in the Latin American population when compared with the non-Latin American population (40.9% vs. 33.5%; P=0.09), and the non-Latin American population was more likely to be treated with oral hypoglycemic medication (25% vs. 19%; P=0.09) or exercise and diet (6.6% vs. 2.9%; P=0.04). There was a significantly higher number of non-Latin American patients who were diagnosed with renal failure (11% vs. 3%, respectively; P=0.002).
Baseline patient characteristics
| Latin American(n=138) | Non-Latin American(n=1,434) | P Value | |
|---|---|---|---|
| Age, years | 65±10 | 67±10.8 | 0.11 |
| Current smoker, % | 15.9 | 14.3 | 0.61 |
| Hypertension*, % | 85.4 | 77.1 | 0.03 |
| Hypercholesterolemia*, % | 64.6 | 63.5 | 0.85 |
| Diabetes, % | 40.9 | 33.5 | 0.09 |
| Insulin-dependent diabetes, % | 8.8 | 10.9 | 0.56 |
| Family history of CAD, % | 51.1 | 34.7 | < 0.001 |
| Prior cardiac intervention, % | 15.2 | 17.4 | 0.56 |
| Prior MI, % | 33.3 | 24.8 | 0.03 |
| Patients with>1 target lesion, % | 34.8 | 31.4 | 0.44 |
| Number of target lesions | 1.4±0.7 | 1.4±0.7 | 0.92 |
CAD=coronary artery disease; MI=myocardial infarction; n=number of patients.
The administration of GPIIb/IIIa medication prior to the index procedure was much less frequent in the Latin American population (2.9% in Latin American patients vs 14.3% in non-Latin American patients; P<0.0001).
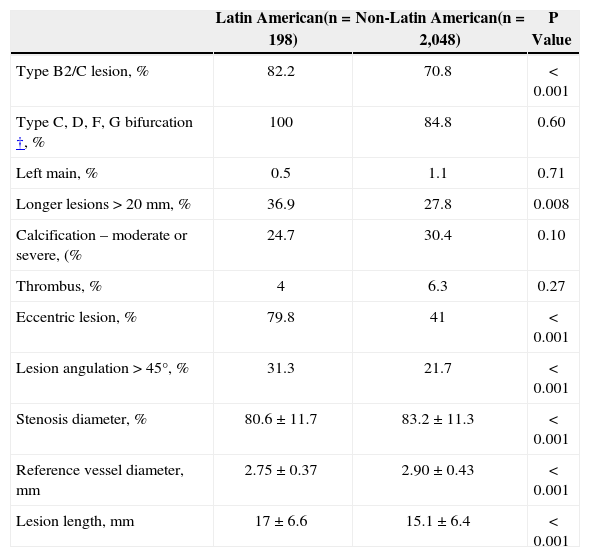
A total of 2,246 lesions were treated (Table 2). There was no difference in the number of target lesions between the two populations. The reference vessel diameter was significantly smaller in the Latin American population (2.75mm vs. 2.90mm; P<0.001), however the stenosis diameter was greater in the non-Latin American population (83.2% vs. 80.6%; P<0.001). Type B2/C lesions (American College of Cardiology/American Heart Association) was higher in Latin American patients when compared to non-Latin American patients (82.2% vs. 70.8%; P=0.001). The prevalence of bifurcation lesions was comparable between the two populations; however eccentricity and angulation were more prevalent in the Latin American population. Target lesions were on average 2mm longer in the Latin American population (17.0mm vs 15.1mm) who had lesions>20mm when compared to 27.8% in the non-Latin American population.
Baseline lesion characteristics*
| Latin American(n=198) | Non-Latin American(n=2,048) | P Value | |
|---|---|---|---|
| Type B2/C lesion, % | 82.2 | 70.8 | < 0.001 |
| Type C, D, F, G bifurcation†, % | 100 | 84.8 | 0.60 |
| Left main, % | 0.5 | 1.1 | 0.71 |
| Longer lesions>20mm, % | 36.9 | 27.8 | 0.008 |
| Calcification – moderate or severe, (% | 24.7 | 30.4 | 0.10 |
| Thrombus, % | 4 | 6.3 | 0.27 |
| Eccentric lesion, % | 79.8 | 41 | < 0.001 |
| Lesion angulation>45°, % | 31.3 | 21.7 | < 0.001 |
| Stenosis diameter, % | 80.6±11.7 | 83.2±11.3 | < 0.001 |
| Reference vessel diameter, mm | 2.75±0.37 | 2.90±0.43 | < 0.001 |
| Lesion length, mm | 17±6.6 | 15.1±6.4 | < 0.001 |
n=number of patients.
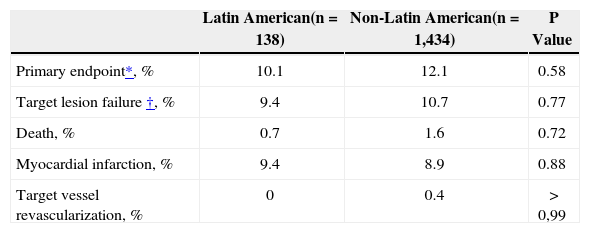
As shown in Table 3 at 1-year the primary composite endpoint of all death, MI and TVR was comparable between the Latin American and non-Latin American populations. In addition, target vessel failure (cardiac death, TV-MI and TLR) was not significantly different between the two populations within 1 year of follow-up. No difference was observed in non-hierarchical event rates within 1 year of follow-up between the two populations. Stent thrombosis rates (definite and probable) were low in the two study populations, with the 1-year cumulative stent thrombosis rate of 0 in Latin American patients and 0.65% in non-Latin American patients.
Hierarchical adverse events at 1 year
| Latin American(n=138) | Non-Latin American(n=1,434) | P Value | |
|---|---|---|---|
| Primary endpoint*, % | 10.1 | 12.1 | 0.58 |
| Target lesion failure†, % | 9.4 | 10.7 | 0.77 |
| Death, % | 0.7 | 1.6 | 0.72 |
| Myocardial infarction, % | 9.4 | 8.9 | 0.88 |
| Target vessel revascularization, % | 0 | 0.4 | > 0,99 |
n=number of patients.
The importance of observational research, particularly post-approval registries with drug eluting stents, has increased in recent years as they provide real world safety data in larger and more representative patient populations than controlled clinical trials. The SPIRIT Women Single Arm study, as a multinational trial, provides not only a large database with a sufficient number of outcomes to be analyzed, but also allows for the comparison of geographical variations among female patients with complex de-novo lesions treated with the XIENCE™ V stent.
Latin America is the fourth largest geographic area in the world, with an estimated population close to 380 million inhabitants distributed in its 12 countries. Latin American citizens represent a conglomerate of different cultures and the socioeconomic status of the population is quite heterogeneous. The use of drug eluting stents has increased significantly in this region, from 2% in 2002 to more than 25% in 2007. 11 Different international clinical trials and observational registries, primarily in patients with heart failure and acute coronary syndromes, have documented that geographic location may affect baseline characteristics, process of care as well as patients´ outcomes. 12−16 However, little information is available in the context of drug-eluting stents. The E-Five Registry, a large prospective, nonrandomized, multicenter, international registry, assessed the safety and effectiveness of the Endeavor™ zotarolimus-eluting stent (Medtronic, Minneapolis, USA) in real-world patients with symptomatic coronary artery disease undergoing percutaneous coronary intervention. Latin American patients, when compared to Europeans had a higher rate of hypertension and prior myocardial infarction, a finding that coincides with the data gathered in this study. 17 We also found a higher prevalence of family history of cardiovascular disease and body mass index, and a trend toward higher diabetes (40.9% vs. 33.5%). Lesion characteristics were also different; being more complex and eccentric, with longer lesion length and smaller diameter vessels among Latin American females when compared to non-Latin American females. However, the rate of use of GPIIb/IIIa was significantly lower in Latin America, a finding that is frequently seen in this region, most likely due to economical factors.
Despite the above-mentioned differences, clinical outcomes were comparable between Latin American and non-Latin American patients, as evidenced also by the E-five registry. Prior reports had shown that the inclusion of Latin American patients was an independent predictor of death. This was observed in the PURSUIT study, where Latin American patients with unstable angina were twice as likely to die within 6 months as their North American counterparts, a difference not explained by the difference in baseline risk. 15
One possible hypothesis explaining these contradictory results may be the greater variability in the realworld standard of care provided to patients with acute coronary syndromes, including the rate of revascularization, when compared to drug-eluting stent registries in which all patients were treated invasively. It should also be considered that most of the patients in SPIRIT Women had a stable clinical condition, representing a population less at risk.
In both the Latin American and non-Latin American populations, we found a lower incidence of major adverse cardiac events when compared to the 15.7% reported by the NHLBI Dynamic Registry, in unselected women treated with drug-eluting stents, even when the lesions characteristics were similar. Also of note, stent thrombosis rates were 50% lower than in the female population of the this registry (1.3%).18
Two complications are of special interest for the female population because of their prevalence and prognostic impact. In-hospital bleeding was low and comparable in both regions. Vascular complications were significantly higher in hospitalized Latin American patients and even higher at 1 year, probably due to the fact that closure devices are not a standard of care.
Limitations of the studyThis study was limited by the single arm design and the inherent lack of a control arm for direct comparison. Lesion characteristics were assessed and reported by the investigator at the time of the procedure without the evaluation of a central laboratory.
All efforts were made to collect all adverse events; 100% of source document was examined for reported adverse events and additional monitoring visits and source document verification were performed for sites with low rates of adverse events reporting.
CONCLUSIONSAt 1 year, low rates of adverse cardiac events, including stent thrombosis, target vessel failure, cardiac death, ARC defined MI and revascularization in the Latin-American cohort were comparable to those of the non-Latin American cohort despite the higher complexity of lesions. These results provide a strong demonstration of the safety and efficacy of XIENCE™ V in this small cohort of Latin American patients, in line with those of larger and more varied populations. This analysis highlights the need for further regional studies and reports.
CONFLICT OF INTERESTJorge Belardi is consultant of Medtronic, Inc. (Minneapolis, USA). Marrianne Stuteville and Cécile Dorange work for Abbott Vascular (Diegem, Belgium). The remaining authors have no conflict of interest to declare regarding this manuscript.
Sponsor: Abbott Cardiovascular Systems, Inc., a subsidiary of Abbott Vascular (Santa Clara, CA, USA)
Principal investigator: Marie-Claude Morice (Paris, France)
Co-principal investigator: Stephan Windecker (Bern, Switzerland)
Steering Committee: Maria Grazia Modena (Italy), Ghada Mikhail (UK), Fina Mauri i Ferré (Spain), Ruth Strasser (Argentina), Liliana Grinfeld (Argentina)
Clinical Events Committee (CEC): K. Koch (Amsterdam, The Netherlands), B. Rensing (Utrecht, The Netherlands), G. Sianos (Thessaloniki, Greece), R. Tölgb (Bad Segeberg, Germany), M. Wiemer (Bad Oeynhausen, Germany), E. McFadden (Cork, Ireland), A. Hoye (Hull, UK)
Data Safety Monitoring Board (DSMB): J. Tijssen (Amsterdam, The Netherlands), T. Lefèvre (Massy, France), P. Urban (Geneva, Switzerland), K. Fox (Edingburgh, UK)
The following Latin American investigators and institutions participated in the SPIRIT Women Trial and number of patients enrolled: Alexandre Abizaid/Instituto Dante Pazzanese de Cardiologia (São Paulo, SP, Brazil) – 46 patients; Pedro Lemos/Instituto do Coração do Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (São Paulo, SP, Brazil) – 24 patients; José M. Torres Viera/Clinica Santa Sofia (Caracas, Venezuela) – 20 patients; Liliana Grinfeld/Hospital Italiano de Buenos Aires (Buenos Aires, Argentina) – 19 patients; Jorge Belardi/Instituto Cardiovascular de Buenos Aires (Buenos Aires, Argentina) – 17 patients; Marcos Marino/Hospital Madre Teresa (Belo Horizonte, MG, Brazil) – 12 patients