An initial experience using the AutoPulse® Non-Invasive Cardiac Support Pump in a catheterisation laboratory is reported. The device was used for a case of cardiopulmonary arrest in the catheterisation laboratory, allowing the percutaneous procedure to continue with simultaneous cardiopulmonary resuscitation. The device provided uninterrupted and effective chest compressions and allowed a team physician to perform other functions during the procedure. There were difficulties related to the setup of the device and to the radiopacity of the electronic components, which prevented some angiographic projections from being obtained. The use of mechanical devices for chest compressions during cardiopulmonary arrest is feasible; however, there is no proof of their benefits when compared to cardiopulmonary resuscitation using manual compressions.
Experiência Inicial com o Uso do Autopulse® em Sala de Hemodinâmica
Os autores apresentam a experiência inicial do uso de dispositivo mecânico de reanimação AutoPulse®. O dispositivo foi utilizado em caso de parada cardiorrespiratória em sala de hemodinâmica, permitindo a continuidade do procedimento percutâneo concomitantemente à ressuscitação cardiopulmonar. O dispositivo proporcionou compressões torácicas ininterruptas e efetivas, bem como liberou um médico da equipe para outras funções durante o procedimento. Houve dificuldades quanto à rapidez na instalação do dispositivo no momento da emergência e em relação à radiopacidade dos componentes eletrônicos, que impediram algumas projeções angiográficas. O uso de dispositivos mecânicos de compressões torácicas durante parada cardiorrespiratória é factível, porém ainda não há comprovação de seus benefícios em relação à ressuscitação cardiopulmonar com compressões manuais.
Cardiorespiratory arrest during procedures in the catheterisation laboratory is a catastrophic event and can greatly affect the outcome of the interventions, as manual chest compressions prevent the continuity of coronary angiography and coronary angioplasty, and they require the assistance of a staff trained in treating cardiorespiratory arrest quickly and effectively. Used in intra-hospital and pre-hospital care, the AutoPulse® (ZOLL Medical Corporation – Chelmsford, MA, USA) mechanical cardiopulmonary resuscitation (CPR) device consists of a pneumatic band coupled to a board placed against the patient’s chest that enables effective and continuous pneumatic compressions, allowing CPR to be performed concurrently with angiography and coronary angioplasty.
CASE REPORTThis is a report of an initial experience with using the AutoPulse® on a 66-year-old non-white male patient who was admitted to the emergency room with anginal pain lasting for an hour and a half. The electrocardiogram showed ST-segment elevation in the inferior wall and an atrioventricular block. The patient was referred to the catheterisation laboratory for emergency intervention; he had a blood pressure of 100/60mmHg, a heart rate of 40bpm, a complete atrioventricular block upon examination, and he was receiving nitroglycerine 0.1 mcg/kg/min by continuous infusion pump. In the emergency room, 300mg aspirin and 300mg clopidogrel were administered.
A temporary pacemaker was implanted in the left femoral vein. Coronary angiography via the right femoral artery route showed that a dominant right coronary artery was occluded in its middle third; the left main coronary artery, left anterior descending artery, and circumflex artery were without significant obstructive lesions, but there was severe left ventricular hypokinesia in the inferior wall, and the mitral valve showed severe regurgitation.
Coronary artery angioplasty was performed immediately after the intravenous administration of 10,000 U heparin. A JR 3.5 6-F guide catheter, 0.014 inch BMW® guide wire (Abbott Vascular – Santa Clara, CA, USA) and 3.0 × 15mm Maverick® balloon catheter (Boston Scientific – Natick, MA, USA), which was inflated to 16atm, were used for pre-dilation, as was a 4 × 20mm Liberté® stent (Boston Scientific – Natick, MA, USA), which was released at 12atm in the middle third of the right coronary artery, successfully resulting in coronary thrombolysis in myocardial infarction (TIMI) 3 flow.
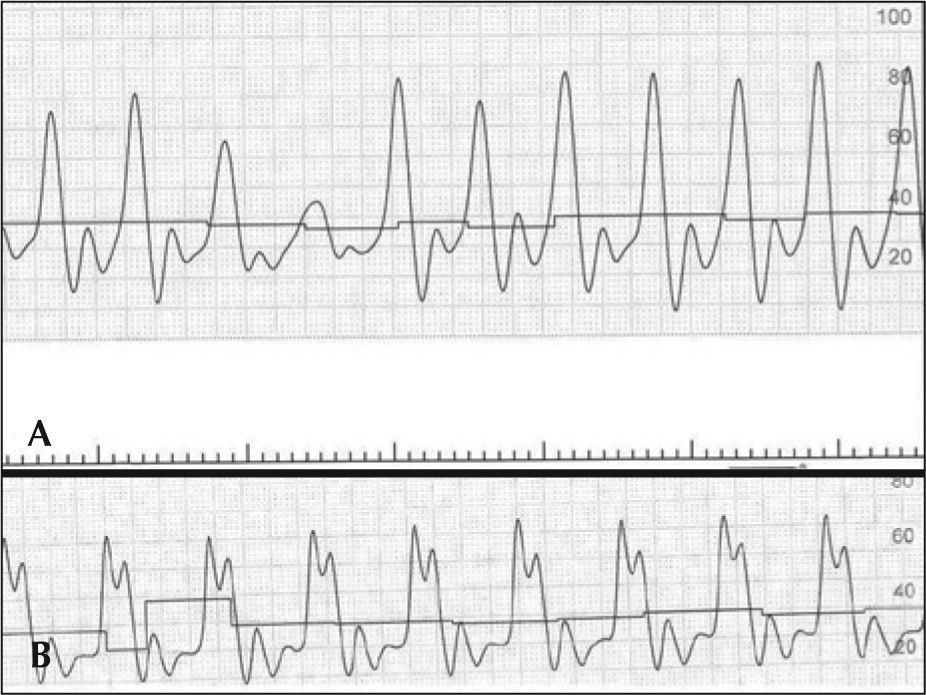
Ten minutes after the end of the procedure, while the patient was still in the catheterisation laboratory, he went into cardiac arrest in ventricular fibrillation, which was reversed with a biphasic shock of 200J. He then suffered multiple cardiorespiratory arrests in ventricular fibrillation and was submitted to successive electric shocks. After tracheal intubation, he went into cardiorespiratory arrest with pulseless electrical activity. Manual compressions were initiated, and after 30 seconds, the AutoPulse® was installed
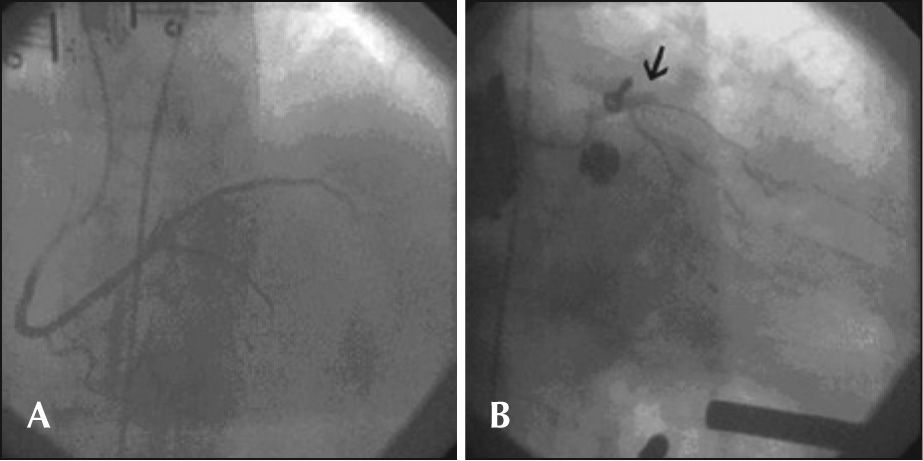
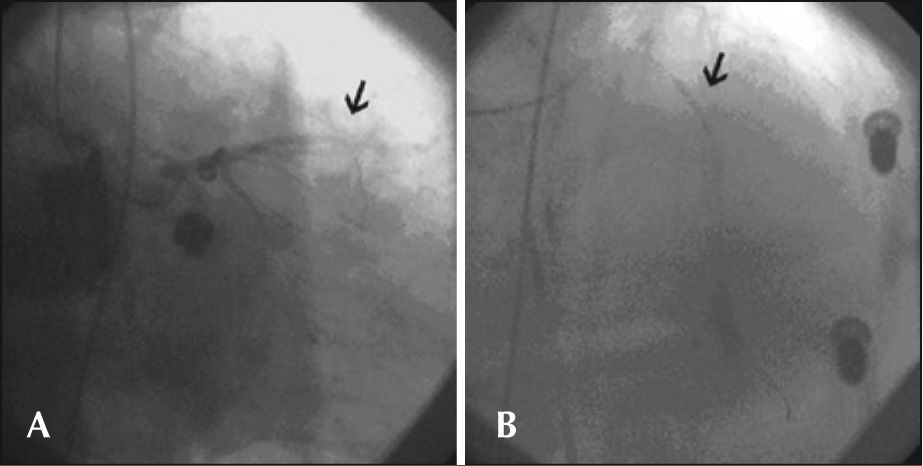
During cardiac compressions with the device, control angiography was started, which showed the right coronary artery with stent patency in the middle third and TIMI 3 flow. Left coronary angiography showed an occluded left anterior descending artery in the proximal third. Using a JL 4 6-F guide-catheter, a 0.014 inch BMW® guide wire was introduced, and aspiration thrombectomy was performed with a PRONTO® (Vascular Solutions, Inc. – Minneapolis, MN, USA) suction catheter to remove thrombi, followed by coronary angioplasty with a 2.5 × 15mm Voyager® balloon catheter (Abbott Vascular – Santa Clara, CA, USA), with no success. After an unsuccessful angiographic procedure, the patient did not respond to CPR and died. These steps are shown in Figures 1 through 3.
An initial experience with the use of the AutoPulse® in the catheterisation laboratory showed that it was possible to continue the procedure with the device attached to the patient. There is insufficient scientific evidence to demonstrate the benefits of employing the AutoPulse® compared to CPR with manual compressions, but the feasibility of percutaneous coronary intervention during CPR was observed.
Series of cases in which the AutoPulse® was employed have shown improvement in haemodynamic pressures1 during CPR and improvement in the restoration of spontaneous circulation.2,3 However, there was no improvement in survival up to hospital discharge or improvement of neurological damage in survivors when compared to manual CPR.4
A prospective, multicentre, randomised trial, comparing the use of the AutoPulse® to manual CPR in the pre-hospital care of cardiac arrest has not demonstrated improved survival at 4 hours, and worse neurological outcomes have been reported when the device was used.5 However, another larger, prospective, multicentre, international, randomised study, the Circulation Improving Resuscitation Care (CIRC) trial, compared survival rates after cardiorespiratory arrest outside of the hospital in patients undergoing CPR procedures with the use of the AutoPulse® or high-quality manual chest compressions. A total of 4,231 patients were included, and it was concluded that mechanical compressions were equivalent to manual compressions regarding survival to hospital discharge.6 Therefore, there is sufficient evidence for the disseminated use of mechanical devices for CPR in cardiorespiratory arrest.7
During CPR, effective and uninterrupted chest compressions are essential. The appropriate return of spontaneous circulation depends on attainment of coronary perfusion during resuscitation, and any interruption in manual chest compressions during CPR for any reason should be avoided.8–10 Manual chest compression pressure by a surgeon decreases 90 seconds after the beginning of CPR.11 Lower levels of pressure attained in manual chest compression were associated with worse prognosis,12 and sufficiently deep compressions prevent neurological damage after successful resuscitation.13
Data on CPR using the LUCAS® device (Medtronic – Redmond, WA, USA), which is analogous to the AutoPulse®, showed better coronary flow14 and cerebral flow15 when compared to manual CPR. An observational study16 reported that the LUCAS® device allowed transluminal coronary angioplasty to be performed during mechanical CPR in 43 patients, of whom 11 were discharged. In this study, there was no comparison with manual CPR.
In the case reported here, the AutoPulse® provided continuous and effective mechanical chest compressions (Figure 3). The equipment released a team physician from manual compressions to perform other functions during the procedure, allowing for concomitant coronary angioplasty resuscitation manoeuvres, which would have been impossible during CPR by manual chest compressions.
The main limitations regarding the use of the device were the difficulty in rapidly setting up the board underneath the patient’s back in the supine position and the radiopacity of the electronic components, which prevented some angiographic projections. The most feasible projections were the anterior-posterior caudal, the posterior-anterior cranial, and right anterior oblique caudal projections, which avoid such components. Caution should be taken to correctly position the pneumatic band, which should be periodically checked during use. There are reports in the literature of patients with findings of ruptured livers and spleens on autopsy after prolonged use of mechanical CPR in cardiac arrest occurring outside the hospital.17
More experience and better training of multidisciplinary teams to use the AutoPulse® in the catheterisation laboratory could result in benefits in cases of cardiorespiratory arrest requiring emergency coronary angioplasty, which, as demonstrated, can be concurrently performed with mechanical chest compressions.
CONFLICTS OF INTERESTThe equipment used in the study was provided by ZOLL Medical Corporation (Chelmsford, USA). The authors declare no conflicts of interest.