Coronary artery disease remains a major public healthcare problem worldwide and percutaneous coronary intervention with drug-eluting stents is the most frequent treatment option for these patients. The objective of this study was to evaluate the rate of clinical events in up to 10 years of clinical follow-up of patients treated with drug-eluting stents.
MethodsWe prospectively enrolled patients with an indication for percutaneous coronary intervention despite their clinical or angiographic presentation. The primary endpoint consisted of the evaluation of the composite rate of major adverse cardiac events (cardiac death, non-fatal acute myocardial infarction or the need of ischemia-guided target-vessel revascularization) in the late clinical follow-up.
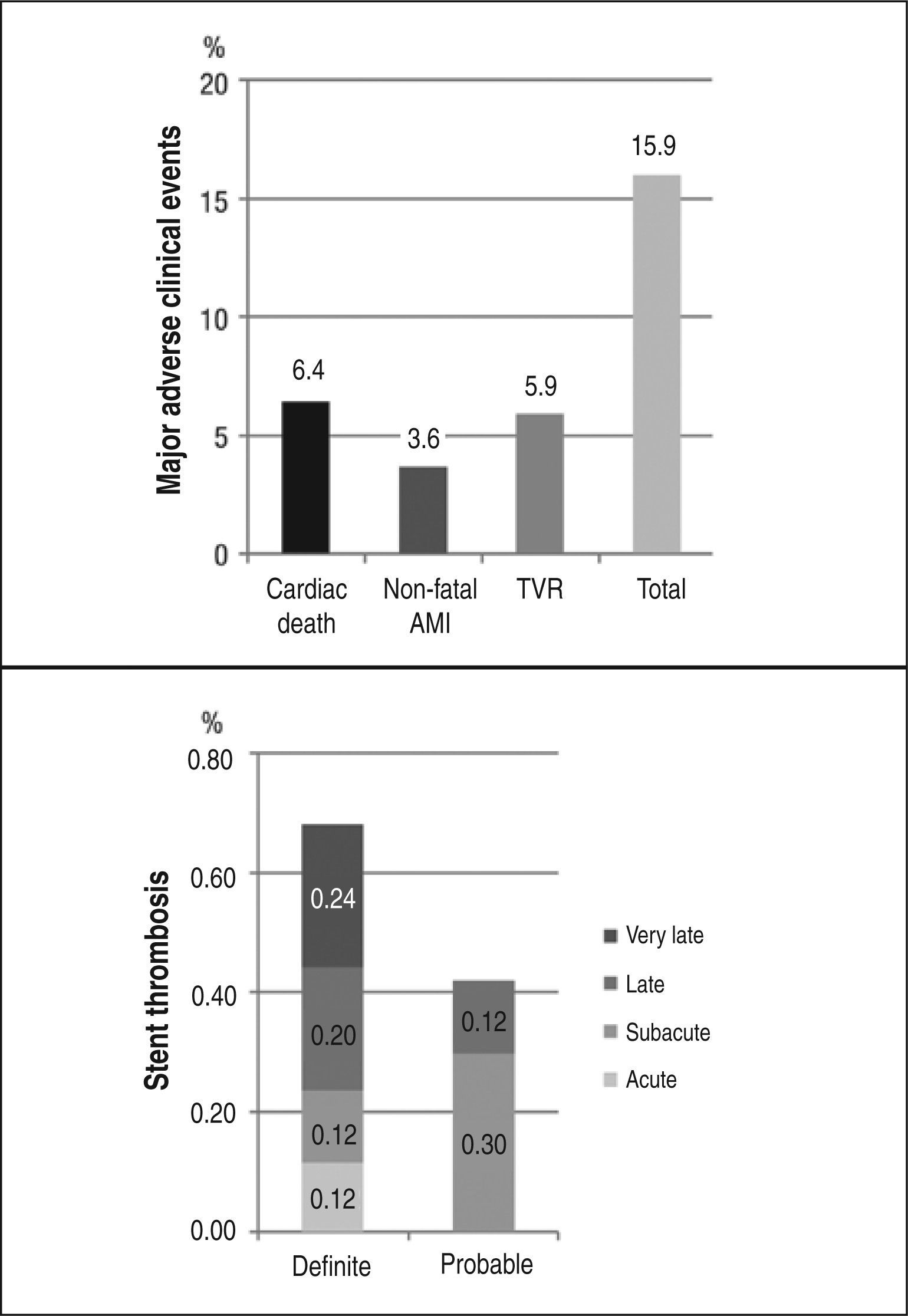
ResultsFrom 2002 to 2012, we included 1,632 patients with mean age of 64.3±10.9years, 71.4% were male and 31.2% had diabetes mellitus. The angiographic and clinical success rates were 99.1% and 96.7%, respectively. Follow-up was obtained in 95.8% of the eligible patients at a mean of 3.4±2.6years. The primary endpoint was observed in 246 patients (15.9%), with rates of cardiac death of 6.4%, myocardial infarction of 3.6% and target-vessel revascularization of 5.5%. Stent thrombosis was observed in 40 patients (2.4%).
ConclusionsIn this clinical practice experience the use of drug-eluting stents demonstrated favorable acute and long-term results in the treatment of a non-selected population of patients with coronary artery disease and variable degrees of clinical and angiographic complexity.
Resultados Tardios de PacientesSubmetidos a Implante de Stents Farmacológicos do Registro SAFIRA
IntroduçãoA doença arterial coronária continua sendo um dos maiores problemas de saúde pública da atualidade e a intervenção coronária percutânea com stents farmacológicos é a forma mais frequente de tratamento desses pacientes. O objetivo deste estudo foi avaliar a taxa de desfechos clínicos no seguimento clínico de até 10 anos dos pacientes tratados com stents farmacológicos.
MétodosPacientes com indicação de ICP foram incluídos de forma prospectiva, independentemente do quadro clínico ou angiográfico de apresentação. O desfecho primário consistiu na avaliação da taxa de eventos cardíacos adversos maiores combinados (óbito cardíaco, infarto agudo do miocárdio não fatal ou necessidade de revascularização do vaso alvo guiada por isquemia) no seguimento clínico tardio.
ResultadosNo período 2002 a 2012, incluímos 1.632 pacientes, com idade de 64,3±10,9 anos, 71,4% eram do sexo masculino e 31,2% portadores de diabetes mellitus. As taxas de sucesso angiográfico e clínico foram de 99,1% e 96,7%, respectivamente. O seguimento clínico foi realizado em 95,8% dos elegíveis em um tempo médio de seguimento de 3,4±2,6 anos. O desfecho primário ocorreu em 246 pacientes (15,9%), com taxas de óbito cardíaco de 6,4%, infarto do miocárdio de 3,6% e revascularização do vaso alvo de 5,5%. A trombose do stent foi verificada em 40 pacientes (2,4%).
ConclusõesNesta experiência da prática clínica diária, a utilização dos stents farmacológicos demonstrou resultados clínicos favoráveis agudos e no longo prazo no tratamento de uma população não selecionada de pacientes com doença arterial coronária e graus variáveis de complexidade clínica e angiográfica.
Among the cardiovascular diseases, coronary artery disease (CAD) remains a major public health problem worldwide. It is estimated that, in the United States, one in three adults, or 81 million people, have some form of cardiovascular disease, including more than 17 million with coronary artery disease and, among them, approximately 10 million with angina pectoris.1,2
Percutaneous coronary intervention (PCI), which has shown significant technological developments over the years, culminating with the advent of drug-eluting stents (DES), is currently the most widely used technique in the treatment of CAD.3-8 First used in lesions of low clinical and angiographic complexity, a gradual expansion of its indications to more complex lesions and patients has been observed, yielding clinical outcomes consistently superior to PCI with bare metal stents.9
However, there are few studies evaluating the long-term follow-up of this technology,10,11 and, in particular, there are few data from the Brazilian population,12 whose own cultural and social characteristics influence the adherence to treatment, and also due to its different genetic composition, compared to populations of large registries from the United States and Europe.13,14
This study aimed to evaluate the long-term results (up to ten years) of an unselected patient cohort undergoing DES implantation in a tertiary hospital in the state of São Paulo.
METHODSThis was a prospective, non-randomized study of consecutive patients with CAD, treated with at least one DES from July 2002 to August 2012 in Complexo Hospitalar, Real e Benemérita Associação Portuguesa de Beneficência, which includes São Joaquim and São José hospitals, Serviço Arie Cardiologia Intervencionista. The protocol for this project was approved by the Research Ethics Committee under No 778-12, and all participants signed the informed consent.
Patients referred for coronary artery bypass graft (CABG), regardless of clinical or angiographic status and who could be treated with DES devices available in the special material sector of the hospital were included. Patients with contraindications to the use of antiplatelet or anticoagulant medication specified by the pharmacological protocol or who refused to sign the consent form were excluded.
Antiplatelet therapyThe protocol included a combination of two antiplatelet agents, acetylsalicylic acid (ASA) and clopidogrel, in loading doses of 200mg and 300mg, respectively, administered 24 hours before the procedure in elective cases. In acute coronary syndrome (ACS) in non-premedicated patients, ASA was chewed at a dose of 300mg and a loading dose of 600mg of clopidogrel was administered. After the appearance of ticagrelor and prasugrel, these medications have been incorporated for use in ACS, or in elective cases of greater angiographic complexity, with PCI performed ad hoc, in loading doses of 180mg and 60mg, respectively. The use of glycoprotein IIb/IIIa inhibitors was at the discretion of the surgeon.
After the procedure, ASA was maintained indefinitely at a dose of 100mg/day. The P2Y12 inhibitors clopidogrel (75mg/day), ticagrelor (90mg twice daily), or prasugrel (10mg/day) were recommended for a minimum of one year.
Data collection and follow-upData were collected through a specific form use by the team and stored in a database especially dedicated to the Safety and efFIcacy of drug-eluting stents in a ReAl-world population (SAFIRA) registry. Patients were followed after discharge by phone interview conducted by a healthcare professional, or by medical appointment; these assessments were performed at 1, 6, and 12 months, and then, annually.
Technical procedureAfter obtaining access, unfractionated heparin was administered at a dose of 100 U/kg. In patients that received glycoprotein IIb/IIIa inhibitors, the initial dose was 70 U/kg, in an attempt to keep the activated clotting time between 200 and 250 seconds.
Pre- and post-procedure serial angiographic analyzes, including qualitative and quantitative assessments of coronary lesions, were performed. The acquisition of angiograms occurred after administration of intra-arterial isosorbide mononitrate 10mg (unless if clinically contraindicated) and included two orthogonal projections, separated by at least 30°, attempting to avoid the overlapping of arteries and to optimize the visualization of the target lesion.
The procedure was monitored by visual analysis and by quantitative coronary angiography and, if necessary, the CAAS 2000 (Pie Medical Imaging BV of Maastricht, The Netherlands) program was used by trained technicians. The scans were recorded at a speed of 15 frames per second on 35mm films, or in DICOM® (Digital Imaging and Communication in Medicine® ) digital format and stored in CDR media.
The location of the target lesion in the coronary tree was defined according to the coronary segment involved: ostial, proximal, middle and distal, with the use of Coronary Artery Surgery Study (CASS), a coronary mapping system, as modified for the Bypass Angioplasty Revascularization Investigation (BARI) study.15
During implantation of DES, the authors aimed to obtain full coverage of the lesion treated, preventing post-dilation damage from the stent edges and, in the case of implantation of two sequential stents, without leaving any space between devices, with 2-3mm overlapping of its stems.
DefinitionsAngiographic success was defined as the implantation of a stent in the target lesion with residual stenosis < 20%, with Thrombolysis in Myocardial Infarction (TIMI) III final flow in the absence of thrombi and arterial dissection. Clinical success was characterized as angiographic success associated with the absence of in-hospital major adverse clinical events.
Stent thrombosis was defined as any ischemic event related to thrombotic occlusion of the stent, classified as definite, probable, and possible, and, as to the temporal evolution, as acute, subacute, late, and very late, according to the Academic Research Consortium (ARC) definition.16
All deaths that occurred were considered as cardiac, unless a noncardiac cause could be clearly established. Acute myocardial infarction (AMI) was defined as an increase in CK-MB greater than three times the reference value, with or without appearance of new Q waves lasting > 0.04 second in two or more contiguous leads on the electrocardiogram. Urgent CABG was classified as an in-hospital unplanned surgery.
Target-lesion revascularization was defined as a reintervention caused by a recurrence of the lesion within the stent or in the segment, including the five mm proximal or distal to the prosthesis; target-vessel revascularization was defined as a percutaneous or surgical reintervention in the target vessel.
Bleeding complications were classified as major and minor according to the TIMI classification.17 Vascular complications included pseudoaneurysm, hematoma, arteriovenous fistulae, external bleeding, retroperitoneal hematoma, arterial thrombosis, abscess at the site of puncture, and mycotic aneurysm.
Study outcomesThe primary endpoint was the assessment of the combined rate of major adverse cardiac events (MACE) (cardiac death, non-fatal AMI, or need for target-vessel revascularization indicated by ischemia) during clinical follow-up. Secondary endpoints included an evaluation of individual components of the primary endpoint, success of the procedure, vascular and bleeding complications, stent thrombosis, probability of MACE-free survival at ten years, and identification of predictors of MACE, of its individual components, and of stent thrombosis.
Statistical analysisContinuous variables were presented as mean and standard deviation, and categorical variables were presented as frequencies (number and percentage). The curves of cumulative event-free survival were estimated by the Kaplan-Meier method.
To determine independent predictors of MACE, of its components, and of stent thrombosis, the multivariate logistic regression model of Cox was used, including variables whose were p-values≤15% in the univariate model. The results were presented with odds ratio and a confidence interval of 95%. The proportional hazards premise was confirmed by Schoenfeld residuals testing; no violations were found.
The analyses were performed by the statistical program STATA® (StataCorp Company – College Station, Texas, USA), version 12.
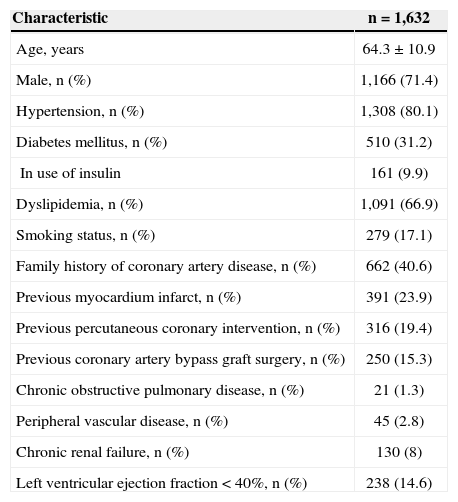
RESULTSFrom July 2002 to August 2012, 1,632 patients were included, with mean age of 64.3±10.9years; 71.4% were males, and 31.2% suffered from diabetes mellitus (9.9% of these were insulin users; Table 1).
Clinical characteristics
| Characteristic | n =1,632 |
|---|---|
| Age, years | 64.3±10.9 |
| Male, n (%) | 1,166 (71.4) |
| Hypertension, n (%) | 1,308 (80.1) |
| Diabetes mellitus, n (%) | 510 (31.2) |
| In use of insulin | 161 (9.9) |
| Dyslipidemia, n (%) | 1,091 (66.9) |
| Smoking status, n (%) | 279 (17.1) |
| Family history of coronary artery disease, n (%) | 662 (40.6) |
| Previous myocardium infarct, n (%) | 391 (23.9) |
| Previous percutaneous coronary intervention, n (%) | 316 (19.4) |
| Previous coronary artery bypass graft surgery, n (%) | 250 (15.3) |
| Chronic obstructive pulmonary disease, n (%) | 21 (1.3) |
| Peripheral vascular disease, n (%) | 45 (2.8) |
| Chronic renal failure, n (%) | 130 (8) |
| Left ventricular ejection fraction<40%, n (%) | 238 (14.6) |
With respect to clinical picture, 872 (53.4%) patients had stable angina; 373 (22.9%) were asymptomatic – 87.1% had positive ischemic evidence, 366 (22.4%) presented with ACS without ST elevation, and 21 (1.3%) were in ACS with ST elevation. From the patients with ACS with no ST elevation, 197 (54%) were classified as high risk, 118 (32%) as moderate risk, and 51 (14%) as low risk.
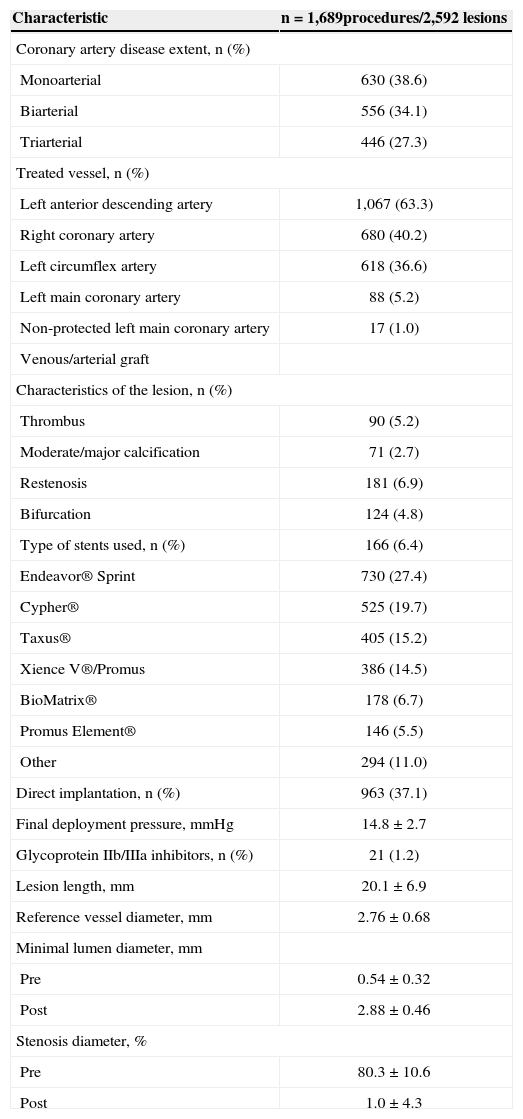
Angiographic and procedural characteristicsThe majority of the population consisted of patients with multiarterial involvement (61.4%), and the most frequently treated vessel was the left anterior descending artery (63.3%). Intervention in left main coronary artery was performed in 83 cases (3.4%), and of these, 17 (1%) were not protected by previous CABG. More complex B2- and C-type lesions were present in 1,639 (63.2%) of all cases (Table 2).
Angiographic and procedural characteristics
| Characteristic | n = 1,689procedures/2,592 lesions |
|---|---|
| Coronary artery disease extent, n (%) | |
| Monoarterial | 630 (38.6) |
| Biarterial | 556 (34.1) |
| Triarterial | 446 (27.3) |
| Treated vessel, n (%) | |
| Left anterior descending artery | 1,067 (63.3) |
| Right coronary artery | 680 (40.2) |
| Left circumflex artery | 618 (36.6) |
| Left main coronary artery | 88 (5.2) |
| Non-protected left main coronary artery | 17 (1.0) |
| Venous/arterial graft | |
| Characteristics of the lesion, n (%) | |
| Thrombus | 90 (5.2) |
| Moderate/major calcification | 71 (2.7) |
| Restenosis | 181 (6.9) |
| Bifurcation | 124 (4.8) |
| Type of stents used, n (%) | 166 (6.4) |
| Endeavor® Sprint | 730 (27.4) |
| Cypher® | 525 (19.7) |
| Taxus® | 405 (15.2) |
| Xience V®/Promus | 386 (14.5) |
| BioMatrix® | 178 (6.7) |
| Promus Element® | 146 (5.5) |
| Other | 294 (11.0) |
| Direct implantation, n (%) | 963 (37.1) |
| Final deployment pressure, mmHg | 14.8±2.7 |
| Glycoprotein IIb/IIIa inhibitors, n (%) | 21 (1.2) |
| Lesion length, mm | 20.1±6.9 |
| Reference vessel diameter, mm | 2.76±0.68 |
| Minimal lumen diameter, mm | |
| Pre | 0.54±0.32 |
| Post | 2.88±0.46 |
| Stenosis diameter, % | |
| Pre | 80.3±10.6 |
| Post | 1.0±4.3 |
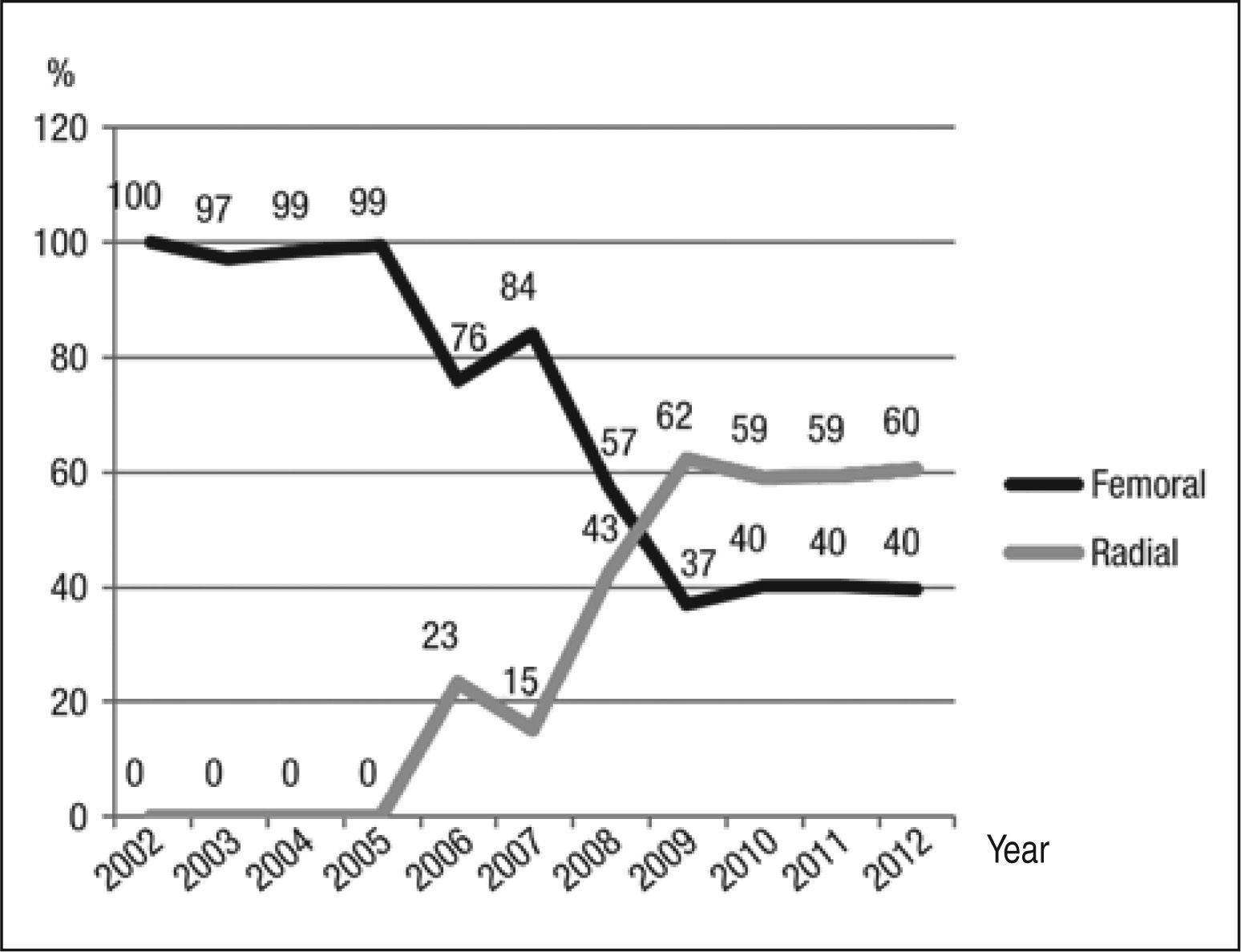
The most used approach was the femoral route, in 973 cases (58%). The use of the transradial approach began in 2006, and was performed in 699 procedures (41%) and, from 2009, this technique has become the preferred access route in this service. The brachial approach was used in 17 cases (1%). Figure 1 shows the rate of utilization of femoral and radial approaches over time. 1,689 procedures were performed, lasting from 8 to 300 minutes (mean 47.8±2.3minutes), and the mean volume of contrast used was 138±63.8mL, ranging from 30 to 350mL. 2,592 lesions were treated, with the use of 2,664 DES, and a mean of 1.03 stents/ lesion. In 963 lesions (37.1%), direct stenting was performed without need for pre-dilatation.
The mean diameter of the stents used was 2.98±1.25mm, with a mean length of 18.8±8.0mm, and the mean final pressure of deployment was 14.8±2.7atm.
In-hospital resultsThe success of clinical and angiographic procedures was 99.1% and 96.7%, respectively. The combined endpoint of death, non-fatal AMI, and urgent CABG occurred in 40 (2.4%) cases, death in eight (0.5%), myocardial infarction in 29 (1.8%), and the need for urgent CABG in three (0.2%) patients. Major bleeding was observed in 21 procedures (1.2%), with 12 (0.7%) hematomata≥5cm and nine (0.5%) cases of drop in hemoglobin≥5g/dL.
Minor bleeding occurred in 26 (1.5%) cases, due to 21 (1.2%) hematomata < 5cm, and in five (0.3%) patients a drop in hemoglobin < 5g/dL was observed. Vascular complications occurred in 60 (3.5%) procedures, with addition of more 13 cases of arteriovenous fistula (0.8%).
The in-hospital stay ranged from one to 32 days, with a mean of 1.5±1.4days.
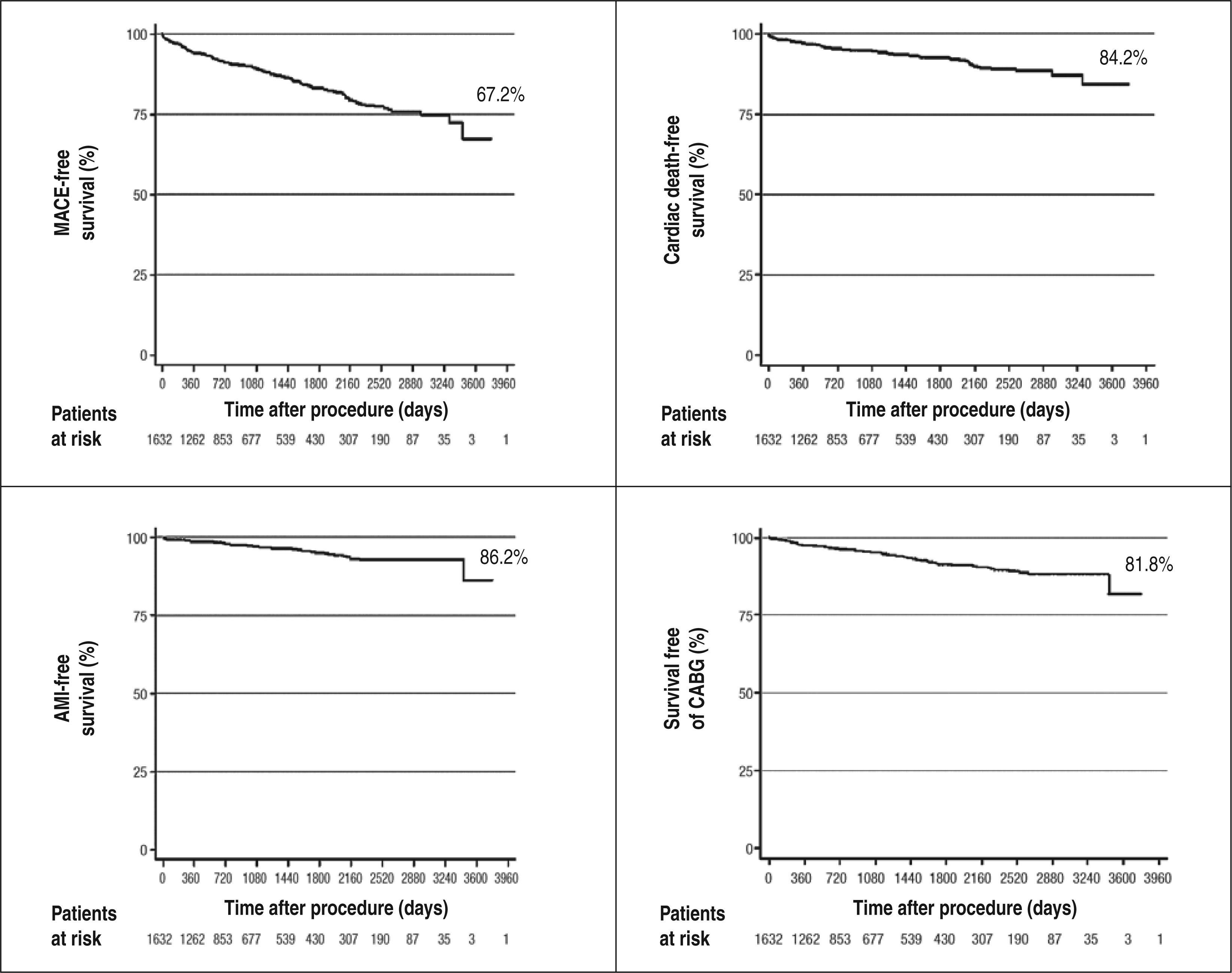
Late resultsThe clinical follow-up was performed in 1,542 patients (95.8% of those eligible). The mean follow-up was 3.4±2.6years (range, one month to ten years) (Figure 2).
The combined endpoint of cardiac death, non-fatal AMI, and ischemia-guided target-vessel revascularization (primary endpoint) occurred in 245 patients (15.9%); the combined endpoint of cardiac death, non-fatal AMI, and target-lesion revascularization occurred in 234 patients (15.1%). The need for target-lesion revascularization occurred in 71 patients (4.6%) and total mortality occurred in 142 (9.2%) cases. Thirty-seven patients (2.4%) underwent CABG, and 27 (1.7%) underwent PCI in a different vessel.
Stent thrombosis was observed in 40 patients (2.4%) throughout the period of evolution. Definite or probable stent thrombosis occurred in 18 patients (1.1%); its temporal distribution is summarized in Figure 2.
Figure 3 shows Kaplan-Meier curves for survival free of MACE, cardiac death, AMI, and target-vessel revascularization, respectively, over ten years of follow-up.
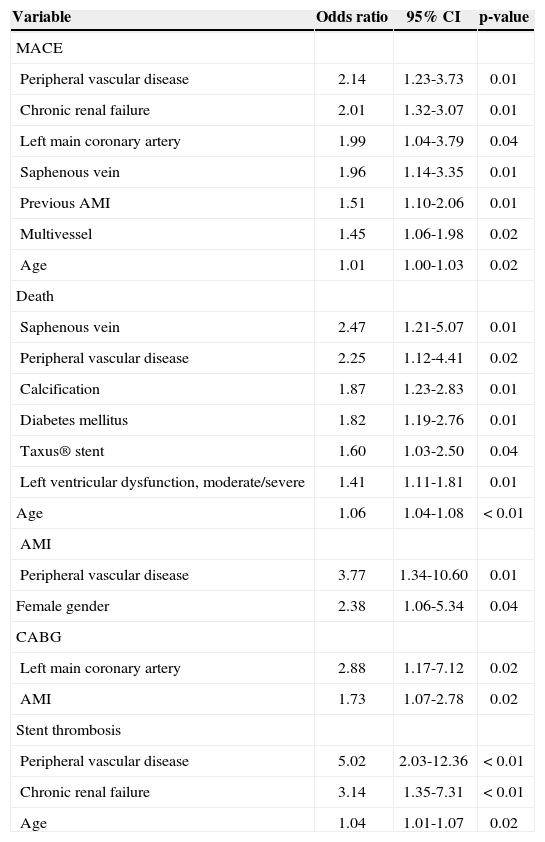
Table 3 presents independent predictors of the combined endpoint of cardiac death, non-fatal AMI, and ischemia-guided target-vessel revascularization, of their individual components, and of stent thrombosis, identified by multivariate analysis.
Independent predictors of major adverse cardiac events (MACE), death, acute myocardial infarction (AMI), target-vessel revascularization, and stent thrombosis
| Variable | Odds ratio | 95% CI | p-value |
|---|---|---|---|
| MACE | |||
| Peripheral vascular disease | 2.14 | 1.23-3.73 | 0.01 |
| Chronic renal failure | 2.01 | 1.32-3.07 | 0.01 |
| Left main coronary artery | 1.99 | 1.04-3.79 | 0.04 |
| Saphenous vein | 1.96 | 1.14-3.35 | 0.01 |
| Previous AMI | 1.51 | 1.10-2.06 | 0.01 |
| Multivessel | 1.45 | 1.06-1.98 | 0.02 |
| Age | 1.01 | 1.00-1.03 | 0.02 |
| Death | |||
| Saphenous vein | 2.47 | 1.21-5.07 | 0.01 |
| Peripheral vascular disease | 2.25 | 1.12-4.41 | 0.02 |
| Calcification | 1.87 | 1.23-2.83 | 0.01 |
| Diabetes mellitus | 1.82 | 1.19-2.76 | 0.01 |
| Taxus® stent | 1.60 | 1.03-2.50 | 0.04 |
| Left ventricular dysfunction, moderate/severe | 1.41 | 1.11-1.81 | 0.01 |
| Age | 1.06 | 1.04-1.08 | < 0.01 |
| AMI | |||
| Peripheral vascular disease | 3.77 | 1.34-10.60 | 0.01 |
| Female gender | 2.38 | 1.06-5.34 | 0.04 |
| CABG | |||
| Left main coronary artery | 2.88 | 1.17-7.12 | 0.02 |
| AMI | 1.73 | 1.07-2.78 | 0.02 |
| Stent thrombosis | |||
| Peripheral vascular disease | 5.02 | 2.03-12.36 | < 0.01 |
| Chronic renal failure | 3.14 | 1.35-7.31 | < 0.01 |
| Age | 1.04 | 1.01-1.07 | 0.02 |
The results of this study demonstrate that in the clinical practice, the use of DES for the treatment of CAD in patients with varying degrees of clinical and angiographic complexity is an effective and safe procedure, providing favorable outcomes during in-hospital and late phases, similarly to the findings obtained by those initial randomized trials of DES.3-8
To the authors’ knowledge, this is one of two national18 published trials presenting a clinical follow up of ten years’ duration of unselected patients treated with DES.
In the analysis of the clinical characteristics of this study, it was observed that the mean age (64.3±10.9years) and the percentage of male patients (71.4%) were in agreement with previously published studies on DES.3-8,19-22 It is worth mentioning the fact that 31.2% of this population consisted of patients with diabetes mellitus, which is a higher index than those found in randomized trials3-8 and in some registries, as the Swedish Coronary Angiography and Angioplasty Registry (SCAAR)22 (23.8%), and RESEARCH23 (22%); this value, however, is in agreement with the REAL10 study from Emilia Romagna, Italy (30.7%).
PCI in the unprotected left main coronary artery was performed only in 17 patients (1.0%) in the present study, and this scenario was also seen in DESIRE registry,18 which analyzed 4,229 patients undergoing DES implantation, showing that a lesion in that vessel was present in 1.5% of treated cases. The findings of these two centers are in agreement with the Central Nacional de Intervenções Cardiovasculares (CENIC) database,24 in which a total of 67,887 PCIs conducted from 2009 to 2011 were registered, and only 1,081 (1.6%) of them were performed for the treatment of lesions located in the left main coronary artery, demonstrating that, in Brazil, the percutaneous treatment of this condition is still reserved for a limited number of patients.
The in-hospital results obtained in this series demonstrate that in current clinical practice, the implementation of PCI is a safe procedure with a high rate of clinical and angiographic success. In fact, the use of coronary stents has provided better immediate results; indeed, their use corrected some important limitations of the coronary angioplasty with balloon catheter, such as acute vessel occlusion and dissections of the arterial wall. In this study, an emergency surgery was necessary only in 0.2% of cases.
Major bleeding occurred in 1.2% of patients – less than the rate found in patients treated with the femoral technique (2.3%) in the meta-analysis by Jolly et al.,25 which analyzed 23 randomized trials (six diagnostic trials) that compared radial and femoral routes.
This fact reinforces the findings on the use of radial access as an important factor in reducing this kind of complication.
The clinical follow-up of ten years (mean 3.4±2.6years) of this study’s population showed that the primary outcome of this study (cardiac death, non-fatal AMI, and ischemia-guided target-vessel revascularization) occurred in 15.9%. Tu et al.,26 in an analysis of 3,751 patients undergoing DES implantation performed in the province of Ontario, Canada, observed, at the end of two years, an incidence of 17.4% for these events. In another important registry, the REAL10 study, which included 3,064 patients who underwent DES implantation with two years of follow-up, this rate was 20.2%.
Total stent thrombosis occurred in 2.4% of the present patients, and thromboses classified as definite or probable reached 1.1% of cases. In the study by Marzocchi et al.,10 angiographically proven thrombosis occurred in 1% of patients. Conversely, Costa Jr. et al.12 reported an overall incidence of 1.6% in a total of 2,365 patients analyzed, and in 1% of their cases, the thrombosis was classified as definite.
In the present series, the curves of event-free survival over a period of up to ten years showed favorable results with the use of DES in clinical practice (67.2% of MACE, 84.2% of cardiac death, 86.2% of non-fatal AMI, and 81.8% of target-vessel revascularization). In the findings through five years of follow-up in the DESIRE registry, these rates were 91.5% for MACE-free survival and 96.7% for target-lesion revascularizationfree survival.11
Using multivariate analysis, seven independent predictors of MACE were identified. Of these, three (peripheral vascular disease, chronic renal failure, and off-label lesions/saphenous vein bypass graft) were also mentioned in the study by Sousa et al.,19 over a followup period of up to ten years. Among the significant predictors of cardiac death, it is worth mentioning the use of the Taxus® stent, with a hazard ratio=1.6 (p=0.04). Stettler et al.20 conducted a meta-analysis involving 38 randomized trials with 18,023 patients and found no difference in the total mortality between bare metal stents and sirolimus- or paclitaxel-eluting stents.
However, the sirolimus-eluting stent showed a 17% relative reduction in the rate of AMI, compared to the paclitaxel-eluting stent (p=0.05). Peripheral vascular disease was also a predictor of AMI (hazard ratio=3.77), a finding that was also observed in the analysis by Sousa et al.19 Regarding target-vessel revascularization, the present study identified, as predictors, lesions located in the left main coronary artery and patients with history of previous AMI.
With respect to stent thrombosis, the predictors were: peripheral vascular disease, chronic renal failure, and age. The study by Iakovou et al.,21 which analyzed 2,229 patients undergoing DES implantation in three European centers, demonstrated that, in addition to the chronic renal failure observed in the present study, diabetes mellitus, bifurcation lesions, premature discontinuation of antiplatelet therapy, and low left ventricular ejection fraction were independent predictors of stent thrombosis.
In the present study, a trend towards a higher rate of thrombosis with the paclitaxel-eluting stent, compared with sirolimus-eluting stent (0.8% vs. 1.7%; p=0.09) was observed. This finding is in agreement with findings in the meta-analysis by Stettler et al.,20 which observed a relative increase of 86% in late definite thrombosis with the paclitaxel-eluting stent, compared with the sirolimus-eluting stent (p=0.0041).
CONCLUSIONSIn this experience of patients in clinical practice, the use of drug-eluting stents demonstrated both favorable acute and long-term clinical outcomes for an unselected population of subjects with varying degrees of clinical and angiographic complexity. These findings, together with previous studies, have proven the efficacy and safety of this technology for the treatment of coronary artery disease.
CONFLICTS OF INTERESTThe authors declare no conflicts of interest.
SOURCE OF FINANCINGNone.