A 82-year-old patient with multiple comorbidities and angina, in spite of optimal drug therapy, was submitted to coronary angiography, which showed three-vessel disease with left main coronary artery involvement and severe coronary artery calcification. Coronary artery bypass grafting surgery was contraindicated and the patient was referred for percutaneous coronary intervention, which was carried out in two stages, with a 15-month interval between them. Firstly, the left coronary artery was treated and rotational atherectomy was performed in the left main coronary artery, left anterior descending and left circumflex arteries, with successful implantation of five drug-eluting stents. Subsequently, the right coronary artery was treated with rotational atherectomy, and four drug-eluting stents were also successfully implanted.
Paciente com 82 anos, portadora de múltiplas comorbidades, com angina do peito apesar de terapia medicamentosa otimizada. Submetida à coronariografia, que evidenciou doença triarterial com envolvimento do tronco da coronária esquerda e intensa calcificação das artérias coronárias. A cirurgia de revascularização miocárdica foi contraindicada, sendo encaminhada para intervenção coronária percutânea, realizada em dois tempos, com intervalo de 15 meses. Primeiramente, a coronária esquerda foi abordada, realizando-se aterectomia rotacional no tronco da coronária esquerda, artérias descendente anterior e circunflexa, com implante de cinco stents farmacológicos com sucesso. Posteriormente, a coronária direita foi tratada com aterectomia rotacional e implante de quatro stents farmacológicos, também com sucesso.
Rotational atherectomy (RA) was developed aiming to address calcified atherosclerotic plaques in the era of conventional balloon angioplasty, in the late 1980s. Subsequently, other atheroablation techniques were developed aimed to reduce balloon angioplasty complications, such as extraction atherectomy and laser; however, the results were disappointing, with high rates of restenosis when used alone or associated with balloon angioplasty.1
With the emergence of the stent, in the 1990s, most of these atheroablation techniques have fallen into disuse or have been abandoned.1 In the drug-eluting stents era, RA resurfaced, aiming to minimize inappropriate device expansion and/or malapposition, which are factors related to adverse clinical outcomes, such as stent thrombosis and restenosis. However, its use is uncommon, accounting for less than 5% of percutaneous coronary interventions (PCI).2
Case reportA 82-year-old female patient with a history of angina for 10 years and functional class worsening in the last five months was treated. Despite optimal medical treatment, she was admitted with acute myocardial infarction without ST-segment elevation (NSTEMI). She had systemic arterial hypertension, dyslipidemia, rheumatoid arthritis, chronic renal failure not requiring dialysis (estimated creatinine clearance of 43.6mL/min), chronic anemia of unknown cause, and episodes of paroxysmal atrial fibrillation. She reported bronchospasm with beta-blocker use and was receiving verapamil 240mg/day, oral nitrate 80mg/day, trimetazidine 60mg/day, ivabradine 15mg/day, furosemide 40mg, clopidogrel 75mg/day, rosuvastatin 20mg, and sublingual nitrate, when necessary.
Laboratory tests disclosed hemoglobin, 11.2g/dL; hematocrit, 35%; urea, 45mg/dL; creatinine, 0.99mg/dL; total cholesterol, 213mg/dL; high-density lipoprotein (HDL), 42mg/dL; low-density lipoprotein (LDL), 129.4mg/dL; triglycerides, 208mg/dL; and fasting glucose, 94mg/dL. The test for occult blood in the stool was negative.
An electrocardiogram showed sinus bradycardia, left atrial enlargement, and left anterior fascicular block. A chest radiography showed an increased cardiothoracic ratio and early signs of pulmonary congestion. A transthoracic echocardiogram showed segmental changes in left ventricular (LV) contractility, due to akinesia of the middle and distal segments of the anteroseptal wall, middle and distal segments of the lower lateral wall, and distal and apical segments of the anterior and lateral walls, with moderate overall systolic dysfunction (39%). Moderate aortic valve stenosis (maximum systolic gradient of 52mmHg and mean of 31mmHg) was also observed. Myocardial scintigraphy showed moderate ischemia in the medial and apical anteroseptal wall, as well as severe ischemia in the medial and apical regions of the inferolateral LV wall.
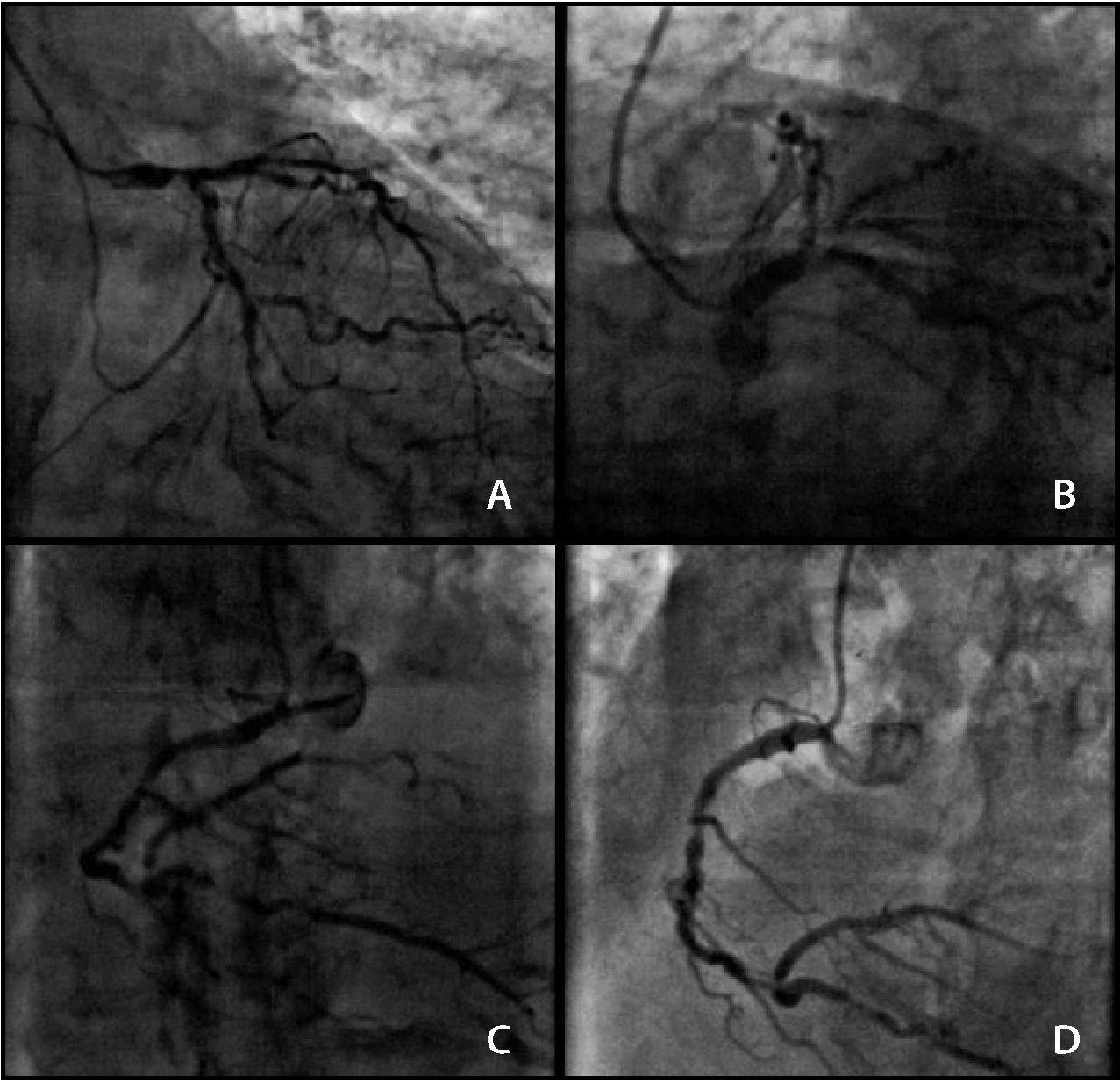
Coronary angiography showed the presence of severe, heavily calcified lesions in the distal segment of the left main coronary artery (LMCA), proximal and middle segments of the left anterior descending (LAD) and left circumflex (LCx) arteries, the full extent of the right coronary artery (RCA), and the proximal segment of the posterior descending (PD) branch (Fig. 1). The calculated SYNTAX score was 56. The calculated Society of Thoracic Surgeons (STS) score was 15.5% for mortality. The case was discussed with the Heart Team, patient, and family; due to the high surgical risk, a staged PCI procedure was chosen.
Presence of severe and calcified lesions in the distal portion of the left main coronary artery, in the proximal and middle segments of the left anterior descending and left circumflex arteries (A and B), and in the entire extent of the right coronary artery and proximal segment of the posterior descending branch (C and D).
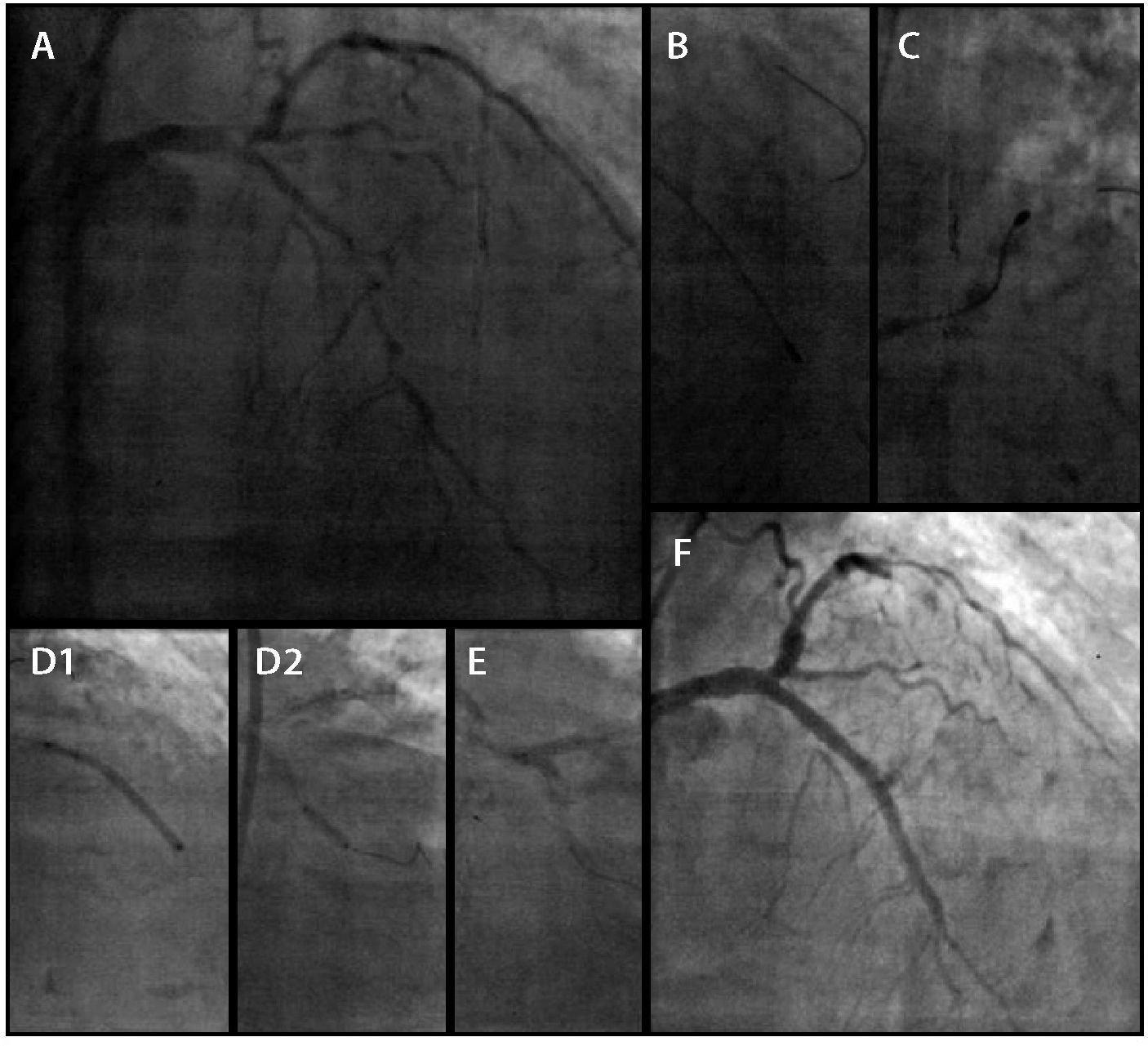
The PCIs were performed during a 15-month interval and the left coronary artery was initially treated (Fig. 2). The RA was performed using a 1.25-mm olive-shaped burr (RotalinkBurr, Boston Scientific, Natick, USA) in the LMCA, LAD, and LCx, with the implantation of five XIENCE PRIME® drug-eluting stents (Abbott, Santa Clara, USA): two in the LMCA (kissing-stent technique), two in the LAD, and one in the LCx, over a total of 91mm. The procedure was guided by an Atlantis SR Pro 3.6 F × 135cm, 40MHz intravascular ultrasound (Boston Scientific, Natick, USA). Post-procedure intravascular ultrasound was not performed after stent implantation, as the procedure duration was too long.
Coronary angiography on the left (A). Rotational atherectomy to the left anterior descending (B) and left circumflex (C) arteries. Stent implantation in the left anterior descending artery (D1 and D2), in the left main coronary artery, and in left circumflex (E). Angiographic result (F).
During hospital stay, although the patient was asymptomatic from a cardiovascular standpoint, there was an increase in markers of myocardial necrosis, which constitutes periprocedural infarction criteria. The patient developed a respiratory infection and was treated with clarithromycin followed by cefepime, with subsequent improvement. The hospital length of stay was 25 days; at hospital discharge she was asymptomatic, on isosorbide mononitrate 40mg/day, ramipril 10mg/day, furosemide 40mg/day, spironolactone 25mg/day, ivabradine 15mg/day, rosuvastatin 20mg/day, acetylsalicylic acid 100mg, clopidogrel 75mg, and pantoprazole 40mg/day. Six months after discharge, she had recurrence of angina, with no improvement after drug optimization and, therefore, a new coronary angiography was scheduled.
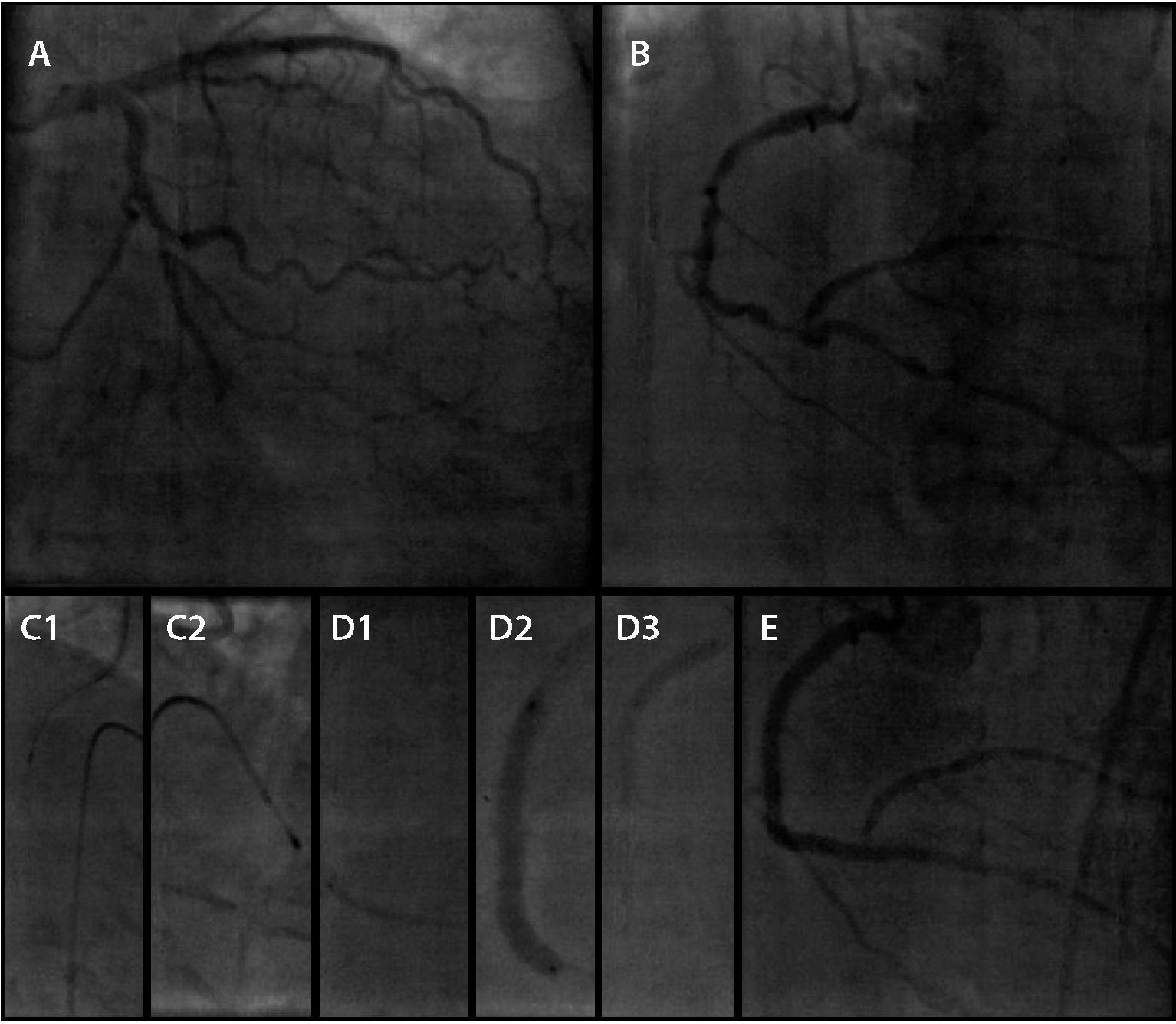
In the second procedure (Fig. 3), after obtaining angiographic evidence of the success of the previous procedure, the RCA was approached by RA using a 1.25-mm olive-shaped burr and four drug-eluting stents of different calibers were implanted, over a total of 108mm (Resolute Integrity, Medtronic Vascular, Santa Rosa, EUA) covering from the proximal segment of the PD branch to the RCA ostium. A temporary transvenous pacemaker was preventively used and triggered during the procedure for bradycardia treatment. The intervention was guided by intravascular ultrasound, which confirmed angiographic success with good stent strut expansion along its entire length. The angiographic result was satisfactory, with Thrombolysis in Myocardial Infarction (TIMI) flow grade 3, with moderate stenosis only in the posterior ventricular branch ostium.
After the procedure, the patient showed a decrease in hemoglobin levels (from 12.7g/dL to 9.5g/dL), remaining stable with no apparent bleeding. An abdominal CT scan was performed without contrast, which showed no retroperitoneal hematoma. Additionally, she had an increase in myocardial necrosis markers without electrocardiographic changes, remaining asymptomatic on the cardiovascular standpoint. She was discharged 3 days after the procedure. She is currently asymptomatic 11 months after the second procedure and 26 months after the first.
A transthoracic echocardiography was performed 75 days after the second procedure, when compared with the previous exam, showed significant improvements in segmental and global LV contractility, with reversal of segmental changes in contractility and ejection fraction of 64%.
DiscussionMarked coronary calcification is a major challenge and a limitation for PCI, as it hinders lesion navigability, approach, and stent expansion. Poor stent expansion may be associated with target lesion restenosis and revascularization, stent thrombosis, and myocardial infarction.3
The rationale for the use of RA in highly calcified lesions before stent implantation is based on the assumption that the following risks will be reduced: (1) risk of acute occlusion, resulting in a smoother lumen, with less barotrauma; (2) risk of in-stent restenosis, increasing luminal gain and decreasing residual plaque; and (3) risk of stent thrombosis through improved stent expansion and apposition.2 Additionally, highly calcified lesions can pose a particular threat to drug-eluting stents, as they could damage the polymeric coating,4 with consequent diffusion of antiproliferative agent to the vessel wall, leading to reduced device efficacy.
RA and drug-eluting stents are complementary techniques in highly calcified lesions. Observational studies have shown the safety and efficacy of RA strategy followed by drug-eluting stents in the treatment of calcified lesions, with good long-term clinical results.5–7 Dardas et al.,8 using this combined strategy in highly calcified stenoses, showed that the mortality and major adverse cardiac events were very low (3.8% and 14.9%, respectively) in a mean follow-up of 49 months.
Conversely, no benefit was demonstrated in the ROTAXUS (Rotational Atherectomy Prior to Taxus Stent Treatment for Complex Native Coronary Artery Disease) study,9 in which the RA before the drug-eluting stent implantation was not superior to stent implantation without previous RA in reducing the primary endpoint of late in-stent lumen loss at 9 months of follow-up. It is noteworthy that, in that study, the mean lesion lenght was 20mm. In the present study, the lesions, in addition to being highly calcified, were very extensive; their anatomical profile was more severe when compared to those in the ROTAXUS study. Furthermore, in the ROTAXUS study, the acute gain that was initially obtained was counterbalanced by the greater late lumen loss at follow-up, with a neutral effect on restenosis. Due to the scarcity of data in the literature, only new randomized clinical trials will be able to properly evaluate these approaches.
Finally, the use of PCI, which was exceptionally performed in this case with adjunctive RA to prepare the lesions for subsequent drug-eluting stent implantation, demonstrates that it is possible to adopt this technique for percutaneous treatment in unfavorable and complex cases.
Funding sourceNone declared.
Conflicts of interestThe authors declare no conflicts of interest.
Peer review under the responsibility of Sociedade Brasileira de Hemodinâmica e Cardiologia Intervencionista.