The second-generation LotusTM transcatheter aortic valve was designed to provide the interventional cardiologist with complete control of its release during the procedure. This study presents the initial experience and in-hospital outcomes of patients treated with this prosthesis in Brazil.
MethodsThis observational and retrospective study included patients with symptomatic severe aortic stenosis considered at high surgical risk, treated in seven centers.
ResultsThe device was used in 31 patients, 61.3% female, aged 82.9 ± 6.9 years, and with STS score of 6.5 ± 4.1%. The aortic valve area was 0.73 ± 0.18cm2 and the mean gradient was 51.7 ± 13.9mmHg. All procedures were performed by the transfemoral access route, and pre-dilation was necessary in 65% of cases. The success rate of the procedure was 96.7%. There were no vascular complications requiring surgical intervention nor cases of stroke. The mean gradient after the procedure was 10.5 ± 5.8mmHg; no cases of moderate to severe aortic regurgitation were observed. The rate of permanent pacemaker implantation was 38.7%, and mean in-hospital length of stay was 8.5 ± 4.8 days.
ConclusionsIn the initial experience with the use of the LotusTM aortic valve, in-hospital results demonstrated the safety and efficacy of the device; no cases of significant aortic regurgitation were observed.
A válvula aórtica transcateter de segunda geração LotusTM foi desenhada para proporcionar ao intervencionista o controle completo de sua liberação durante o procedimento. O presente estudo apresenta a experiência inicial e os desfechos hospitalares de pacientes tratados com essa prótese no Brasil.
MétodosRegistro observacional, retrospectivo, que incluiu pacientes com estenose aórtica grave sintomáticos, considerados de alto risco cirúrgico, tratados em sete centros.
ResultadosReceberam o dispositivo 31 pacientes, sendo 61,3% do sexo feminino, com idade de 82,9 ± 6,9 anos e escore STS de 6,5 ± 4,1%. A área valvar aórtica foi de 0,73 ± 0,18cm2 e o gradiente médio de 51,7 ± 13,9mmHg. Todos os procedimentos foram realizados pela via transfemoral, e a pré-dilatação foi necessária em 65% dos casos. A taxa de sucesso do procedimento foi de 96,7%. Não houve complicação vascular com necessidade de intervenção cirúrgica e nem casos de acidente vascular cerebral. O gradiente médio após o procedimento foi de 10,5 ± 5,8mmHg e não foram observadas regurgitações aórticas de graus moderado/importante. A taxa de implante de marca-passo definitivo foi de 38,7%, e a permanência hospitalar de 8,5 ± 4,8 dias.
ConclusõesNa experiência inicial com o implante da válvula aórtica LotusTM, os resultados hospitalares demostraram a segurança e a eficácia do dispositivo, além de ausência de regurgitação aórtica relevante.
Aortic valve stenosis is a disease with high prevalence in the elderly population, and is associated with high rates of cardiovascular morbidity and mortality, from symptom onset onwards. For these patients, the treatment of choice is surgical valve replacement. However, not all are ideal candidates for this type of procedure, due to the high surgical risk often observed in patients in this age group.1,2
The literature shows favorable results with percutaneous transcatheter aortic valve implantation (TAVI), with reductions in mortality rates and new hospitalizations when compared with patients considered to be inoperable, and similar mortality rates when compared with surgery in high-risk patients.3,4 Therefore, over the past decade, TAVI has established itself as an important therapeutic alternative for the subgroup of patients with degenerative aortic valve stenosis, whose surgical risk is considered high or unacceptable.5–8
Nonetheless, these procedures still have potential limitations and complications, such as stroke, residual paravalvular regurgitation, vascular complications, and need for permanent pacemaker implantation.9 In this context, the second-generation transcatheter valves feature major developments, such as the possibility of repositioning and recapturing the device before its implantation or the use of a seal on its base to reduce the risk of paravalvular regurgitation.
This article presents the initial experience in Brazil with the second-generation LotusTM transcatheter aortic valve (Boston Scientific Corporation, Natick, USA), as well as the in-hospital outcomes.
MethodsThis is an observational, retrospective, multicenter registry. All patients who received the LotusTM valve in the following institutions were included: Instituto do Coração do Hospital das Clínicas da Universidade de São Paulo, Hospital Sírio-Libanês, and Hospital São Luiz, Unidade Morumbi, all located in São Paulo (SP); Hospital e Maternidade Brasil, Santo André (SP); Hospital Quinta D’Or, Rio de Janeiro (RJ); Hospital Esperança, Recife (PE); and Instituto de Cardiologia do Rio Grande do Sul, Porto Alegre (RS). The device was chosen by the operator, based on the characteristics of the aortic valve, the aorta, and iliofemoral route.
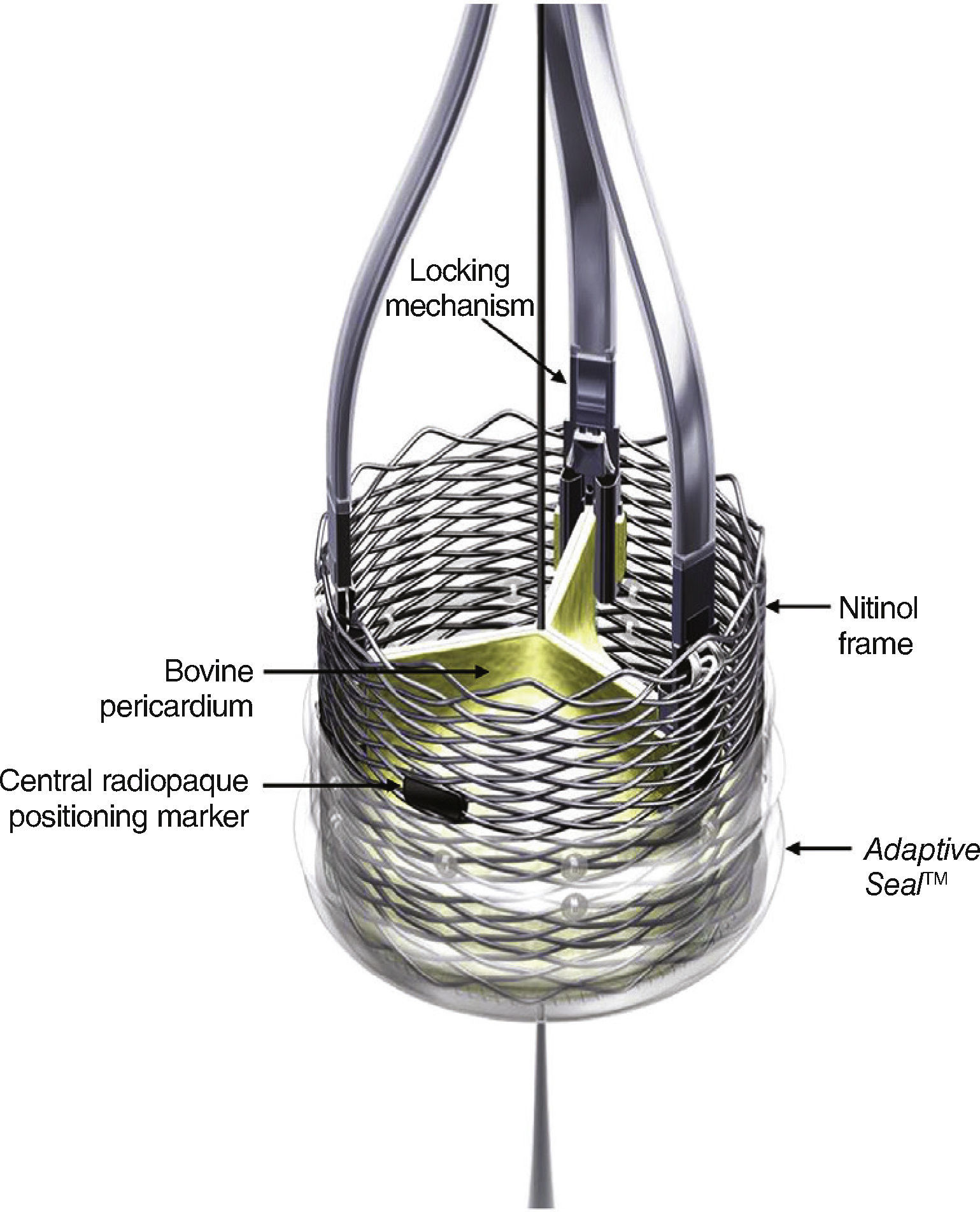
DeviceThe LotusTM transcatheter aortic valve implantation system consists of a bovine pericardium bioprosthesis sutured to a single-wire nitinol frame.
One of the main features of the device is the Adaptive SealTM, which consists of an outer shell, located in the inferior section of the valve, that seals the aortic annulus in order to minimize paravalvular regurgitation.
The valve is available in three different sizes (23, 25, and 27mm), and is pre-mounted on the delivery system (Fig. 1). The device is released and expanded by anti-clockwise rotation through controlled mechanical expansion.
The LotusTM valve is composed of three bovine pericardial leaflets sutured to a nitinol frame. It has a radiopaque mark in the center position, which aids in positioning, and a polyurethane/polycarbonate sealing mechanism, whose function is to conform to the anatomy of the valve annulus and minimize paravalvular regurgitation. The device is mechanically expandable, and is locked in position using locking mechanisms located in each of the leaflets insertion points.
High-frequency ventricular stimulation with transvenous pacemaker is not required during valve implantation. The release can be done slowly, as the prosthetic leaflets start to operate at an early stage of deployment.
The LotusTM valve can be recaptured and repositioned at any stage of the procedure, even when fully expanded, through clockwise rotation of the distal portion of the delivery system. Once a satisfactory final position is reached, the final release occurs in two stages by clockwise rotation of the release ring (Fig. 2).
LotusTM valve premounted delivery system. A counterclockwise rotation of the distal portion of the delivery system (blue knob) results in the release and mechanical expansion of the device; a clockwise rotation stretches and resheaths the valve, if there is need for repositioning. The clockwise rotation of the release ring (black ring) leads to the release of the valve.
Patients were selected by a multidisciplinary team (Heart Team). Briefly, the criteria used were high surgical risk, according to the classification of the Society of Thoracic Surgeons (STS), when the predicted risk of mortality was ≥ 8%, and/or when the Heart Team considered that there were comorbidities that would prohibitively increase the surgical risk. Patients had to present severe aortic stenosis documented by transthoracic or transesophageal echocardiography (aortic valve area < 1.0cm2, and/or mean transvalvular gradient > 40mmHg, and/or peak velocity > 4 m/s) and New York Heart Association (NYHA) congestive heart failure functional class 2 or higher.
ProcedureThe main method used for anatomical screening of patients was computed tomographic angiography of the heart and aorta. These images were used to measure structures such as the annulus (diameter, perimeter, and area), the left ventricular outflow tract, and the sinotubular junction, as well as the height of the coronary ostia and the diameters of the vascular access routes (iliofemoral system). Thus, it was possible to choose between the available sizes of the prosthesis (23, 25, or 27mm), as well as to determine the best access route and the entire anatomy to be covered by the device.
The procedures were performed in accordance with the practices of each hospital, and hospital outcomes were assessed by the Valve Academic Research Consortium (VARC)-2 criteria.10 Patients received acetylsalicylic acid and clopidogrel before the procedure; clopidogrel was maintained in the first 3 months after device implantation, whereas acetylsalicylic acid was maintained indefinitely.
Clinical, laboratory, electrocardiographic, and imaging data were tabulated in a single spreadsheet for further analysis. The qualitative variables were described as absolute numbers and percentages, and the quantitative variables as means and standard deviations.
ResultsThirty-one consecutive patients were treated in seven different hospitals, from October 2015 onwards, corresponding to 80% of the number of LotusTM devices implanted in Brazil.
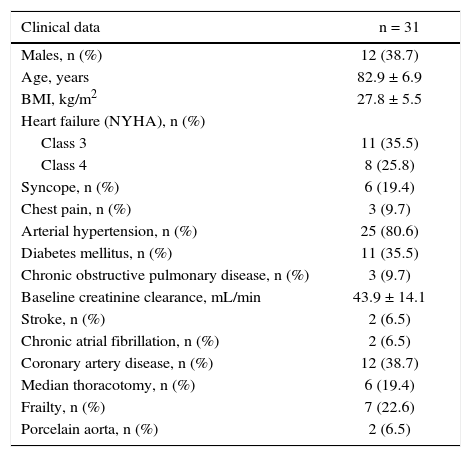
The population was predominantly female (61.3%); the absolute majority of patients presented signs and symptoms of congestive heart failure classes 3 or 4 according to the NYHA, or syncope (Table 1).
Clinical characteristics.
| Clinical data | n = 31 |
|---|---|
| Males, n (%) | 12 (38.7) |
| Age, years | 82.9 ± 6.9 |
| BMI, kg/m2 | 27.8 ± 5.5 |
| Heart failure (NYHA), n (%) | |
| Class 3 | 11 (35.5) |
| Class 4 | 8 (25.8) |
| Syncope, n (%) | 6 (19.4) |
| Chest pain, n (%) | 3 (9.7) |
| Arterial hypertension, n (%) | 25 (80.6) |
| Diabetes mellitus, n (%) | 11 (35.5) |
| Chronic obstructive pulmonary disease, n (%) | 3 (9.7) |
| Baseline creatinine clearance, mL/min | 43.9 ± 14.1 |
| Stroke, n (%) | 2 (6.5) |
| Chronic atrial fibrillation, n (%) | 2 (6.5) |
| Coronary artery disease, n (%) | 12 (38.7) |
| Median thoracotomy, n (%) | 6 (19.4) |
| Frailty, n (%) | 7 (22.6) |
| Porcelain aorta, n (%) | 2 (6.5) |
BMI: body mass index; NYHA: New York Heart Association.
The mortality predicted by the STS score was 6.5 ± 4.1%; by EuroSCORE II, 7.6 ± 7.0%; and by the logistic EuroSCORE, 14.0 ± 9.1%. Some patients had features not included in these models, such as weakness, porcelain aorta, or hostile chest, which would considerably increase the surgical risk.
The aortic valve area measured by echocardiography was 0.73 ± 0.18cm2; 70% of patients had some degree of aortic regurgitation and 20% had moderate to severe ventricular dysfunction. Echocardiographic data relating to the maximum and mean gradients, at baseline and after the procedure, are shown in Table 2.
All procedures were performed using the transfemoral access route, 58% percutaneously and 42% by dissection. In 84% of cases, patients underwent general anesthesia and transesophageal echocardiography, while the remaining 16% received conscious sedation and underwent transthoracic echocardiography. The procedures were performed on an elective basis in 87% of cases, and in 13% on an urgent basis. Pre-dilation was required in 65% of procedures. One patient had a bioprosthesis in the aortic position and underwent a valve-in-valve procedure.
The 23-mm valve was used in 45% of cases, followed by the 25-mm, in 39%, and the 27-mm, in 16%. Post-dilation was needed in only one case (3%).
According to the VARC-2 criteria, the device was successfully implanted in 96.7% of cases, with one death (3.2%) caused by cardiac tamponade after right ventricle perforation by the electrode of the temporary pacemaker.
There was substantial reduction in the left ventricular-aortic pressure gradient after the intervention. In 83% of cases, there was complete absence of aortic regurgitation; in 17% (five cases), the observed regurgitation was minimal and, in one case (3%), mild. No cases of moderate or severe regurgitation were observed.
There were no vascular complications requiring surgical intervention, nor cases of stroke. In accordance with the VARC-2 classification, acute kidney injury stage 1 was observed in 32% of patients (ten cases), and stage 2, in 3% of patients (one case). There were no cases of acute kidney injury stage 3.
The permanent pacemaker implantation rate after valve implantation was 38.7% and the in-hospital length of stay was 8.5 ± 4.8 days.
DiscussionThe main finding of this study was that the transcatheter aortic valve implantation with the second-generation LotusTM device was associated with a high success rate, a low incidence of residual periprosthetic regurgitation, and relatively high rates of post-procedure permanent pacemaker implantation. The results of the present study reflect the initial experience with this prosthesis in Brazil, and are similar to results from previously conducted international studies (Table 3).11,12
Percutaneous post-implantation results of aortic valve bioprosthesis with LotusTM system in the literature and in the present study.
| Variable | REPRISE I (n = 11) | REPRISE II (n = 250) | RESPOND (n = 500) | Present study (n = 31) |
|---|---|---|---|---|
| Mean gradient, mmHg | 13.7 ± 3.7 | 12.05 ± 4.35 | 10.4 ± 4.3 | 10.5 ± 5.8 |
| Paravalvular leak ≥ moderate, % | 0 | 1.8 | 0.4 | 0 |
| Permanent pacemaker implantation, % | 36 | 28.6 | 30.6 | 38.7 |
The first-generation prostheses that are commercialized in Brazil, which have been widely studied in the literature, have different characteristics: the first is made of porcine pericardium and is self-expanding (CoreValve, Medtronic Inc., Minneapolis, USA); the second is made of bovine pericardium and is balloon-expandable (Edwards SAPIEN, Edwards Lifesciences, Irvine, USA). Both prostheses have demonstrated efficacy and safety, but still have limitations when compared with conventional surgery, especially in the incidence of stroke, paravalvular aortic regurgitation, and need for permanent pacemaker. Another important aspect to be considered is the relative difficulty of implanting these devices in the ideal position. Once the valve is positioned and the balloon is inflated, the balloon-expandable valve cannot be repositioned, while the self-expanding valve has a small possibility of being repositioned, after a certain point in the release. These aspects become extremely relevant in patients with severely calcified valve annulus (one of the predictors of paravalvular regurgitation), bicuspid valves, and horizontal aortas, as well as those with low coronary ostia and, consequently, greater predisposition to coronary occlusion. It is extremely important to emphasize that the new generations of these two devices already feature technical improvements; nonetheless, they still are in the registration phase with the regulatory agencies and are not commercially available in Brazil.
The second-generation LotusTM aortic valve bioprosthesis has unique characteristics that can overcome the limitations of the aforementioned devices, such as the ability to be 100% repositionable and recapturable, in addition to the seal at its base, which minimizes the chances of paravalvular regurgitation. These features, coupled with a mechanism that permits early operation before its release, allow for a greater hemodynamic stability during the procedure, with less need for vasoactive amines. In the present study, this profile allowed for the performance of 16% of the procedures with a minimally invasive strategy, with local anesthesia and conscious sedation, and without the use of transesophageal echocardiography, bringing more comfort to patients and early hospital discharge.
Naturally, this valve has limitations, such as requiring a large-diameter arterial vascular access. Its delivery device is also more rigid and somewhat unfavorable to tortuous anatomies. Additionally, the LotusTM device presents higher rates of permanent pacemaker implantation when compared with other valves. There is debate about the possible causes of increased incidence of advanced atrioventricular blocks, but the most likely cause is the larger device profile and its radial strength.
ConclusionsThe initial experience in Brazil with the LotusTM device presented data consistent with the world literature, offering operators the possibility of using a second-generation aortic valve with excellent safety and efficacy results.
Funding sourcesNone declared.
Conflicts of interestThe authors declare no conflicts of interest.
Peer review under the responsibility of Sociedade Brasileira de Hemodinâmica e Cardiologia Intervencionista.