Reoperation is the method of choice for correcting prosthetic paravalvular leaks. The percutaneous repair of mitral paravalvular leaks might represent an interesting alternative in select cases. The present study reports two cases of mitral paravalvular leaks that were treated with Amplatzer™ Vascular Plug III guided by three-dimensional (3D) transesophageal echocardiography, using the percutaneous approach. The first case involved a 55-year-old male who had undergone five cardiac surgeries (aortic and mitral valve replacements) and presented with New York Heart Association (NYHA) class III heart failure and severe mitral regurgitation. The second case was a 76-year-old male who had undergone two aortic valve replacements; the last procedure was associated with mitral valve replacement, which developed into NYHA class IV heart failure and severe mitral regurgitation. This patient had several comorbidities that increased his surgical risk. The procedures were performed percutaneously under general anaesthesia, using the transseptal approach to deploy the plugs, which occluded the paravalvular defects successfully in both cases.
Correção de Regurgitação Paravalvar Mitral por Via Percutânea Guiada por Ecocardiograma Transesofágico Tridimensional
A reoperação é o método de escolha para a correção de defeitos protéticos paravalvulares. A correção da regurgitação paravalvar mitral por via percutânea constitui uma alternativa interessante para casos selecionados. Relatamos dois casos com regurgitação paravalvar mitral, tratados por via percutânea, com prótese AmplatzerTM Vascular Plug III, guiados por ecocardiograma transesofágico tridimensional. No primeiro caso, o paciente, com 55 anos de idade, já havia sido submetido a tratamento cirúrgico por cinco vezes (trocas valvares aórtica e mitral) e evoluía com insuficiência cardíaca classe III da New York Heart Association (NYHA) e insuficiência mitral grave. No segundo caso, o paciente, com 76 anos de idade, tinha sido submetido a troca valvar aórtica por duas vezes, a última associada a troca valvar mitral, e evoluía em insuficiência cardíaca classe IV (NYHA) e regurgitação mitral grave, e várias comorbidades aumentavam seu risco cirúrgico. Os procedimentos foram realizados por via percutânea, sob anestesia geral, utilizando-se a via transeptal para o implante dos plugs, que ocluíram os defeitos paravalvares com sucesso em ambos os casos.
Although surgical repair or replacement are the reference standards for treating paravalvular mitral regurgitation (mitral paravalvular leak), the high rates of operative morbidity and mortality are still a cause of great concern. The percutaneous closure of paravalvular leaks is an attractive method for interventional cardiologists and patients; the procedure eliminates the need for repeat surgeries. However, interventional cardiologists are reluctant to choose this method due to the lack of a specific device to perform the percutaneous closure, which is caused by the lack of sufficient experience of the surgical centres, the scarcity of data in the literature about the procedure because of its complexity and the relatively high rates of occluder displacement. This study reports two cases of successful prosthesis implant using Amplatzer ™ Vascular Plug III (AGA Medical – Plymouth, USA) guided by three-dimensional (3D) transesophageal echocardiography.
CASE REPORTClinical case 1A 55-year-old male patient presented with rheumatic valvular disease. The patient underwent five cardiac surgeries (the first in 1974, at age 19) with replacement of the mitral and aortic valves with a mechanical Starr-Edwards prosthesis. In May of 1975, he underwent new mitral and aortic replacement, and another mitral prosthesis replacement was necessary in the same year because of prosthesis dysfunction. In 1979, he underwent a new mitral and aortic replacement with a mechanical Starr-Edwards prosthesis because of the dysfunction of the previous device.
He remained oligosymptomatic and underwent clinical follow-up until 2001, when he presented dyspnea on moderate exertion in atrial fibrillation, with severe stenosis in both prostheses, in addition to ascending aortic aneurysm (60mm in diameter). In 2001, at age 46, he underwent his fifth heart surgery with implantation of mitral and aortic double-disc mechanical prostheses in conjunction with a replacement of the ascending aorta segment with a tubular prosthesis and reimplantation of the coronary arteries.
In 2006, during the clinical follow-up, the patient underwent a two-dimensional (2D) transthoracic echocardiography, which showed severe mitral paravalvular regurgitation, but the patient refused further surgical treatment. Since then, he has had several hospitalisations for heart failure, aggravated by anemia (hemolysis). The patient, however, maintained his decision not to undergo another cardiac surgery and was always discharged with optimised drug therapy.
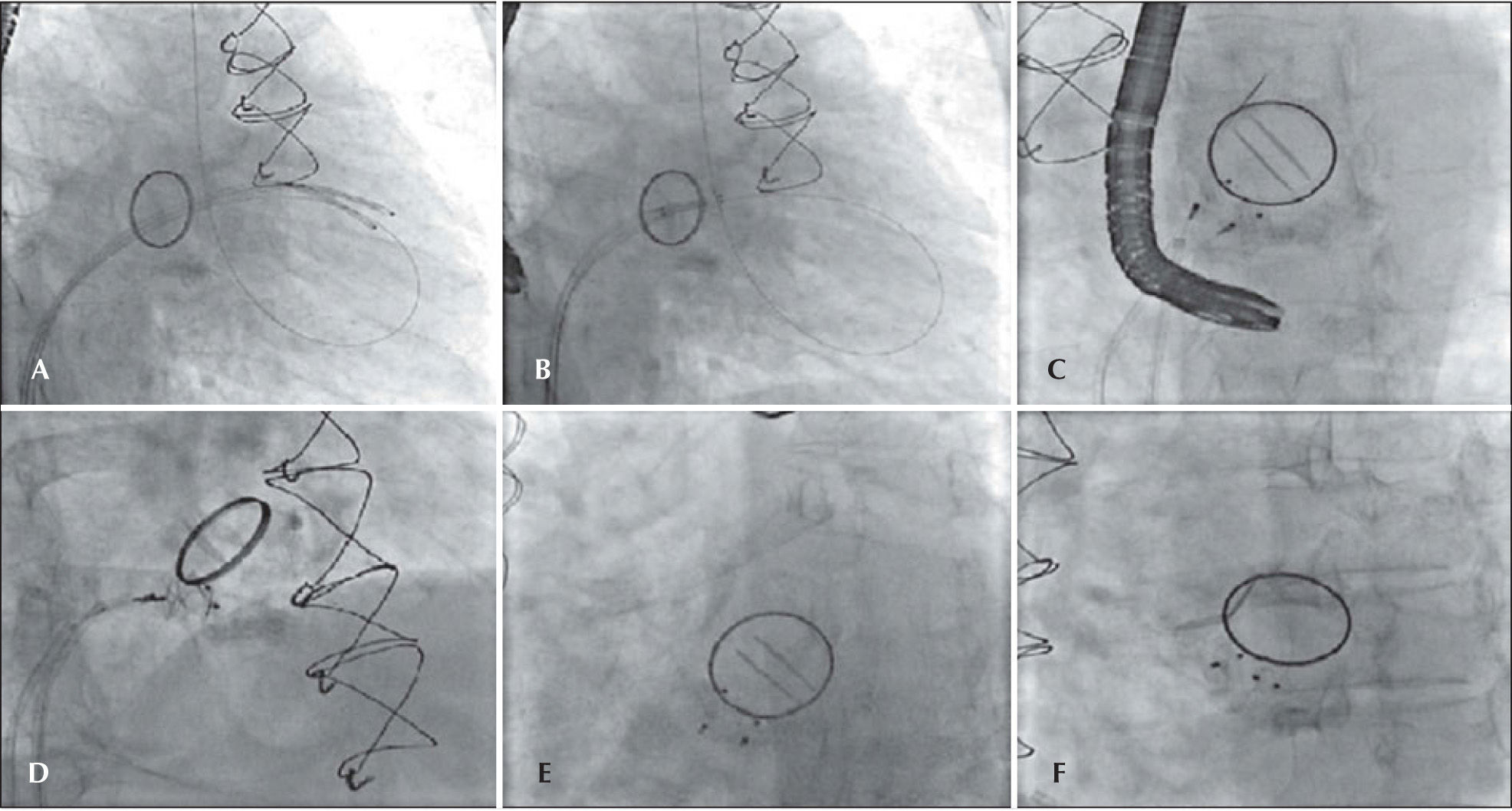
In 2011, the 2D transthoracic echocardiography showed mechanical prostheses with normal disc excursion and preserved left ventricular systolic function despite the severe paravalvular mitral regurgitation, moderate right ventricular dysfunction, and pulmonary artery systolic pressure estimated at 90mmHg. In that same year, the team proposed to the patient a percutaneous correction of the periprosthetic defect, which was performed on July 6, 2011 (Figure 1). The procedure was performed under general anaesthesia and was initiated by puncturing the right femoral vein and the left femoral artery with 7F and 6F introducers, respectively, followed by the administration of a 100 U/kg dose of unfractionated heparin. Subsequently, a transseptal puncture was performed using a Brockenbrough needle and an 8F Mullins sheath. A hydrophilic guidewire was introduced through the Mullins sheath with a 0.035-inch Radiofocus Terumo® 260cm angle tip (Terumo Medical Co. – Tokyo, Japan), maintained in the left atrium. Using this guidewire, the Mullins sheath was exchanged for a Fu Star® sheath (Lifetech Scientific – Shenzhen, China), through which the paravalvular defect was found and the 0.035-inch Radiofocus Terumo® 260cm angle tip guide wire was steered past the defect with the aid of 3D transesophageal echocardiography. The Fu Star® Lifetech sheath was exchanged with a therapeutic Multipurpose 7F catheter (Johnson & Johnson Co. – Miami, FL, USA), which was guided past the paravalvular defect and through which another 0.035-inch Radiofocus Terumo® 260cm angle tip guide wire was introduced. This catheter was removed and two Multipurpose 7F catheters were introduced separately through the two guide wires that were guided past the paravalvular orifice. Then, two Amplatzer™ Vascular Plug III (numbers 10 and 8) prostheses were implanted through the 7F multipurpose catheters with continuous monitoring by 3D transesophageal echocardiography, which guided the correct prosthesis positioning and release. At the end, the disappearance of the paravalvular defect was demonstrated. There were no complications during the procedure. The patient was extubated in the catheterisation laboratory and observed for 2 hours before discharged to the infirmary.
– In A, catheters in the left ventricle after moving past the mitral paravalvular orifice. In B, the positioning of the prostheses, with guidewire in the left ventricle. In C, placement of the prostheses, starting their release. In D, the release of the prostheses. In E and F, the final result.
The second case was a 76-year-old male patient with a history of systemic arterial hypertension, chronic renal failure, and stroke. He was diagnosed with hepatitis B in the late postoperative period of biological aortic valve replacement number 23, which was performed in January of 2011. In April of 2011, he developed infective endocarditis from coagulase-negative Staphylococcus aureus and was treated with vancomycin, gentamicin, and rifampicin. The biological aortic prosthesis was exchanged for a new bioprosthesis (number 27) in conjunction with a mitral valve replacement and tricuspid plasty, in addition to reducing the aortoplasty of the ascending portion of its calibre. He was discharged after two months of hospitalisation.
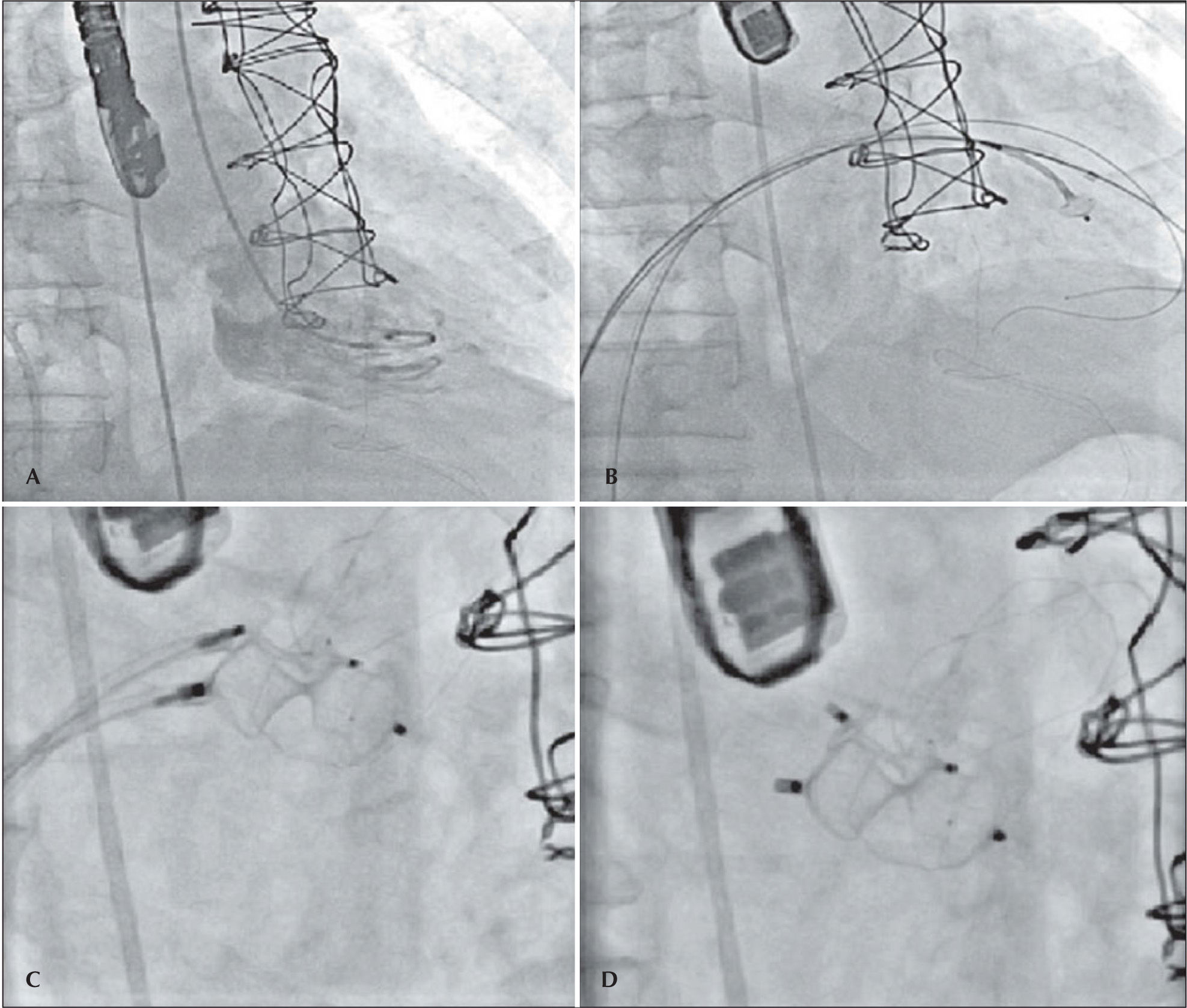
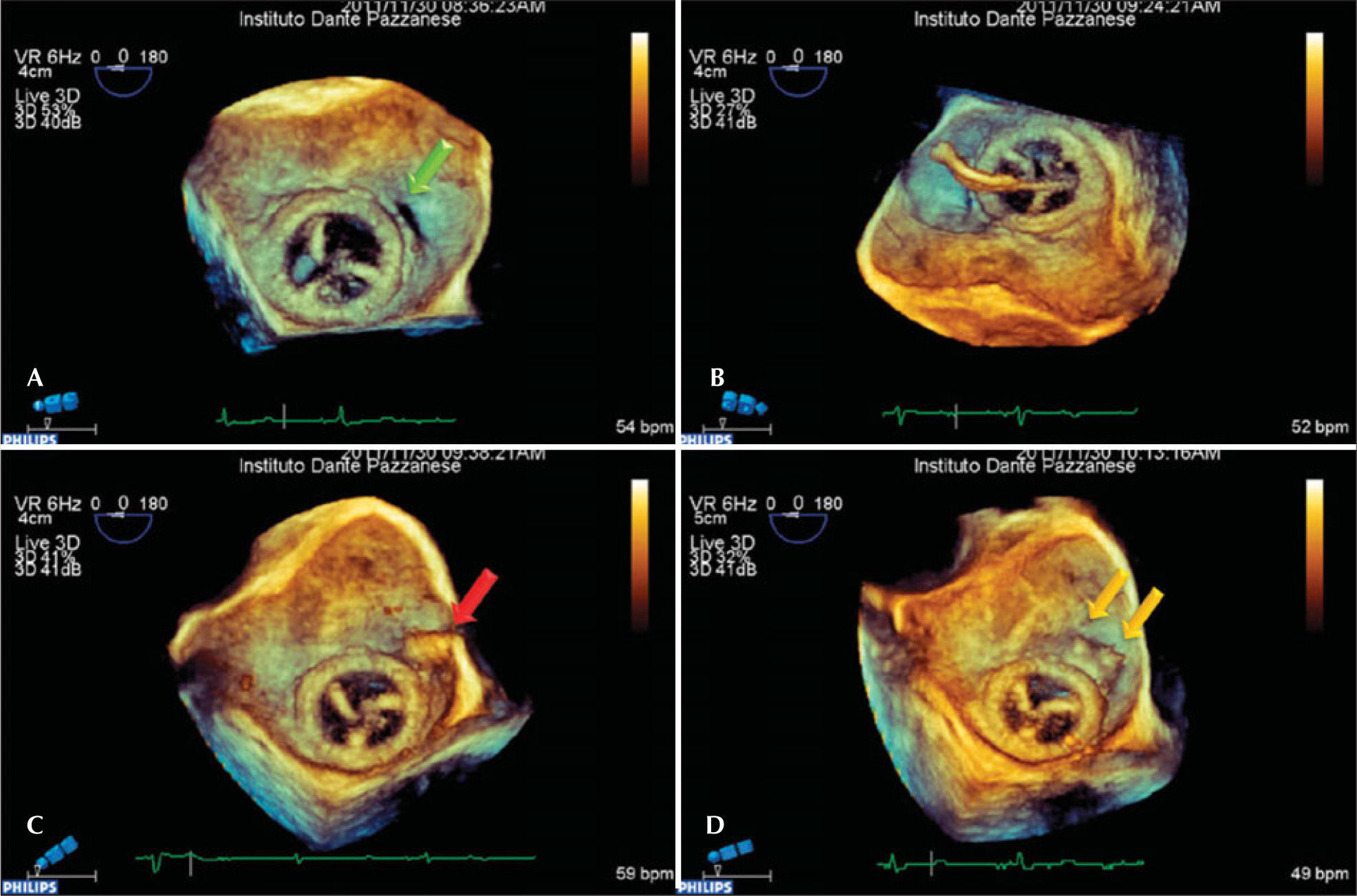
The patient developed dyspnea upon exertion, and a 2D transthoracic echocardiography was performed on November 18, 2011, which showed an ejection fraction of 60%, aortic bioprosthesis with thin leaflets without regurgitation (valve area of 1.7cm2 and maximum systolic gradients of 18mmHg, mean 10mmHg), mitral bioprosthesis with thin leaflets, preserved opening and mobility, and severe anterior paravalvular regurgitation, with maximum diastolic gradients of 13mmHg (mean 7mmHg) and slight tricuspid regurgitation. As a result of relevant symptoms and high surgical risk, a percutaneous closure of the mitral paravalvular defect was indicated and performed on November 30, 2011 (Figure 2). The procedure was performed under general anaesthesia and was initiated by puncturing the right femoral vein and the left femoral artery with 7F and 6F introducers, respectively, followed by administering a dose of 100 U/kg of unfractionated heparin. Left ventriculography was performed in the right anterior oblique projection, which showed increased end-diastolic volume and moderate inferobasal hypokinesia, as well as mild hypokinesia in the other left ventricular walls. Subsequently, a transseptal puncture was performed with a Brockenbrough needle and an 8F Mullins sheath. The paravalvular orifice was located and a 260-cm, 0.035-inch extra-stiff guidewire was introduced past it together with a 3.5, 6F Judkins right catheter curve (Johnson & Johnson Co. – Miami, FL, USA) and another similar guide with a Multipurpose 7F catheter, with the aid of 3D transesophageal echocardiography. Then, two Amplatzer™ Vascular Plug III (numbers 10–5 and 4–2) prostheses were implanted with the help of 3D transesophageal echocardiography, which was used to guide the correct positioning of the prosthesis (Figure 3). At the end of procedure, it was demonstrated that the paravalvular defect had completely disappeared (Figure 4). There were no complications during the procedure. The patient was extubated in the catheterisation laboratory and kept under observation for two hours, before discharge to the infirmary.
– In A, ventriculography in right anterior oblique view, showing mitral paravalvular regurgitation. In B, catheters in the left ventricle after moving past the mitral paravalvular orifice to start the prosthesis positioning. In C, the positioning of the prostheses and their release. In D, the final result.
– Three-dimensional transesophageal echocardiogram images obtained sequentially during the procedure. In A, the periprosthetic defect (green arrow), which is located at the 3 o’clock position with the heart in anatomical position. In B, the unsuccessful attempt to go past the periprosthetic defect with the catheter, which actually passed through the central orifice of the prosthesis. In C, the catheter after going past the periprosthetic defect (red arrow). In D, two Amplatzer™ Vascular Plug III devices are already released, closing the periprosthetic defect (yellow arrows).
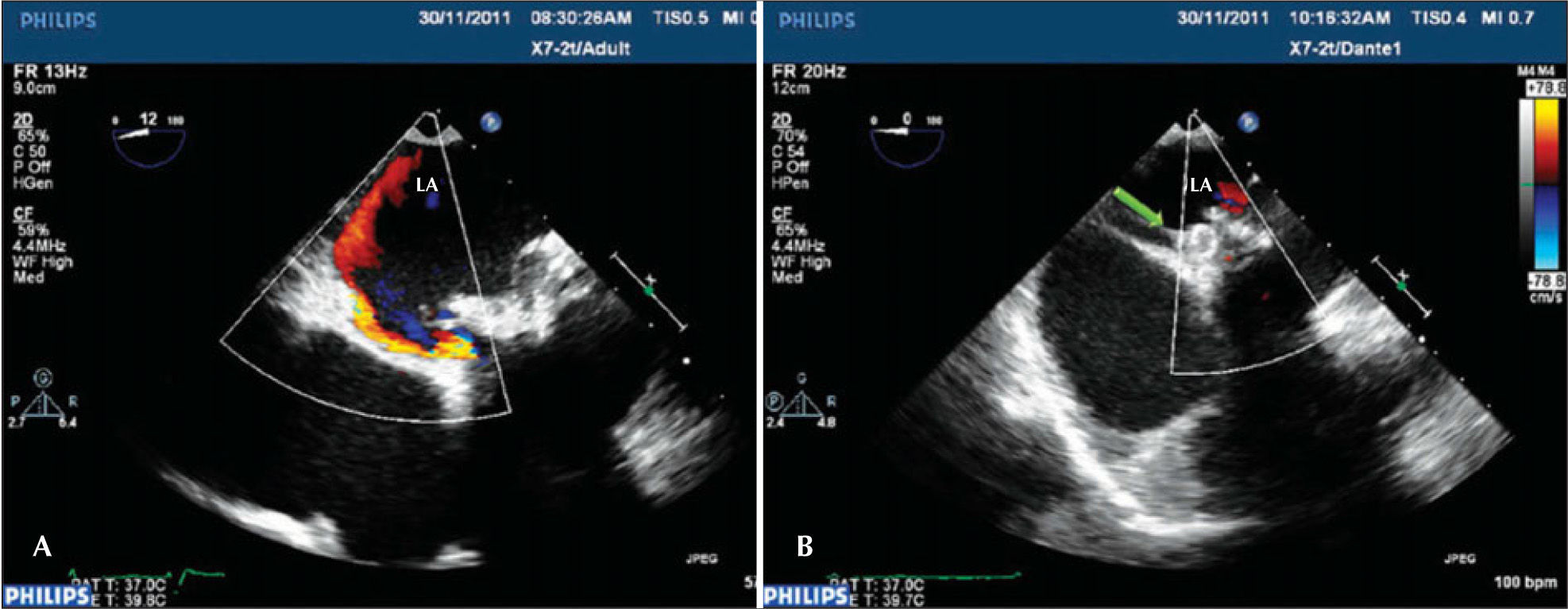
– Conventional transesophageal echocardiography images. In A, the periprosthetic defect with regurgitation into the left atrium. In B, after the Amplatzer™ Vascular Plug III (green arrow) device implantation, which is located between the prosthetic ring and the atrial wall, defect occlusion with the disappearance of periprosthetic regurgitation. LA=left atrium.
The transesophageal echocardiography, which was performed two days after the procedure, showed mitral bioprosthesis with thin leaflets, preserved opening and mobility, and no paravalvular regurgitation. The puncture site showed no bleeding or bruising. The patient was asymptomatic upon discharge and was asked to return for follow-up at the outpatient valve disease clinic.
DISCUSSIONParavalvular regurgitation after prosthetic valve implantation is a complication that occurs in 2.5% to 5% of patients who are referred for surgical valve replacement.1
These regurgitations occur as a result of prosthesisannulus suture dehiscence and vary in size. A small regurgitation may be asymptomatic or cause hemolysis, whereas large regurgitations can cause severe hemodynamic alterations and heart failure.2 In significant paravalvular regurgitations, percutaneous treatment can constitute an alternative to surgery for patients with high perioperative risk, particularly considering that a new surgery is associated with mortality rates of approximately 16%.3,4 However, significant regurgitation after percutaneous treatment is sometimes observed, and further intervention is required in up to 40% of cases.4,5
Recently, new techniques and devices for percutaneous closure of paravalvular regurgitations have been developed, although most do not specifically address the anatomical peculiarities of these regurgitations. Based on this discussion, it is necessary to develop specific devices for closing paravalvular regurgitations.4 Coils are usually used in small defects, patent ductus arteriosus occlusion devices for moderate defects, and septal defect occlusion devices for large defects.1,3 The ideal device should be suitable to seal the defect without causing hemolysis and thrombus formation and their consequent embolic complications.4
The 3D transesophageal echocardiography performed before the procedure is essential to evaluate the size, location, and shape of the regurgitation. All such information is essential for choosing the most appropriate model of the device and to better plan the occlusion strategy. The 3D transesophageal echocardiography, which is also performed during the procedure, helps to obtain spatial resolution; therefore, it contributes to improved success rates and provides better positioning of the guide wires and devices related to paravalvular regurgitation. This improvement is attributed to the possibility of obtaining images of the prosthesis through the left atrium, which decreases the acoustic shadow generated by the prosthesis.1
The percutaneous closure of paravalvular regurgitations is one of the most challenging procedures currently performed by interventional cardiologists. Experience suggests that using 3D echocardiography can improve the indices of immediate procedural technical success. Clinical success in the long term, however, is influenced by the limitations associated with the use of currently existing devices, which justifies the need to develop specific devices to close paravalvular regurgitations.4
CONFLICTS OF INTERESTSThe authors declare no conflicts of interest.