Transcatheter aortic valve implantation (TAVI) has emerged as an alternative treatment for those patients with severe aortic stenosis considered to be at high or prohibitive surgical risk. 1 While transfemoral approach has been considered as the primary approach in the vast majority of centers and studies, non-optimal iliofemoral vessel characteristics preclude the safe placement of the sheaths in a high number of patients. In addition to the small size of iliofemoral arteries, about one third of the TAVI candidates present significant peripheral vascular disease. 1
See page 247
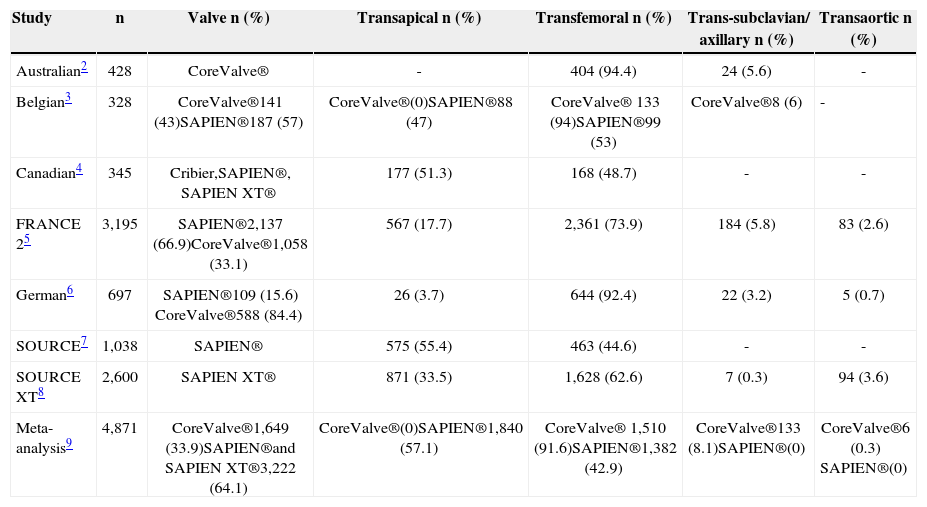
Accordingly, other alternative approaches such as transapical, subclavian/axillary, and transaortic have been developed in the recent years. The transapical approach has been the most frequent alternative to the transfemoral approach when using the balloon-expandable Edwards SAPIEN valve (Edwards Lifesciences, Irvine, USA) (> 50% of cases in the SOURCE registry; Table 1). More recently, and even with the use of lower profile sheaths, transapical TAVI is still performed in more than 30% of the patients. 4,7,10,11 However, the transapical approach is not an option for patients receiving the CoreValve® system (Medtronic, Inc., Minneapolis, USA), so that other approaches such as the subclavian/ axillary and the transaortic approaches have also been developed. As highlighted in Table 1, more than 90% of the procedures with the CoreValve® system have been performed transfemorally and around 5-8% were performed through the subclavian/axillary approach.
Rates of different approaches in transcatheter aortic valve implantation registries
| Study | n | Valve n (%) | Transapical n (%) | Transfemoral n (%) | Trans-subclavian/ axillary n (%) | Transaortic n (%) |
|---|---|---|---|---|---|---|
| Australian2 | 428 | CoreValve® | - | 404 (94.4) | 24 (5.6) | - |
| Belgian3 | 328 | CoreValve®141 (43)SAPIEN®187 (57) | CoreValve®(0)SAPIEN®88 (47) | CoreValve® 133 (94)SAPIEN®99 (53) | CoreValve®8 (6) | - |
| Canadian4 | 345 | Cribier,SAPIEN®, SAPIEN XT® | 177 (51.3) | 168 (48.7) | - | - |
| FRANCE 25 | 3,195 | SAPIEN®2,137 (66.9)CoreValve®1,058 (33.1) | 567 (17.7) | 2,361 (73.9) | 184 (5.8) | 83 (2.6) |
| German6 | 697 | SAPIEN®109 (15.6) CoreValve®588 (84.4) | 26 (3.7) | 644 (92.4) | 22 (3.2) | 5 (0.7) |
| SOURCE7 | 1,038 | SAPIEN® | 575 (55.4) | 463 (44.6) | - | - |
| SOURCE XT8 | 2,600 | SAPIEN XT® | 871 (33.5) | 1,628 (62.6) | 7 (0.3) | 94 (3.6) |
| Meta-analysis9 | 4,871 | CoreValve®1,649 (33.9)SAPIEN®and SAPIEN XT®3,222 (64.1) | CoreValve®(0)SAPIEN®1,840 (57.1) | CoreValve® 1,510 (91.6)SAPIEN®1,382 (42.9) | CoreValve®133 (8.1)SAPIEN®(0) | CoreValve®6 (0.3) SAPIEN®(0) |
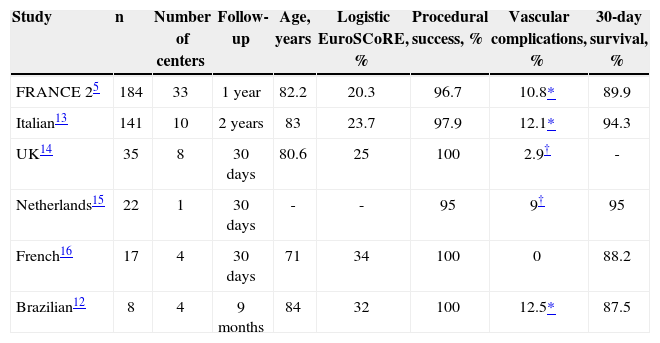
In this issue of the Revista Brasileira de Cardiologia Invasiva, Brito Júnior et al. 12 reported the subclavian experience from the Brazilian Registry of TAVI. Among 277 patients included in this registry, 8 patients (2.9%) were treated by subclavian approach with the CoreValve® system, all of them performed under general anesthesia and dissection. Even though being a small series, it was shown that device success was achieved in all cases, and there was only one major access site complication, also related to one death, and no cerebral event at 30-day follow-up. Interestingly, these results are comparable with other CoreValve® sub clavian registries (Table 2).
Main results of the subclavian approach cohort from transcatheter aortic valve implantation registries
| Study | n | Number of centers | Follow-up | Age, years | Logistic EuroSCoRE, % | Procedural success, % | Vascular complications, % | 30-day survival, % |
|---|---|---|---|---|---|---|---|---|
| FRANCE 25 | 184 | 33 | 1year | 82.2 | 20.3 | 96.7 | 10.8* | 89.9 |
| Italian13 | 141 | 10 | 2years | 83 | 23.7 | 97.9 | 12.1* | 94.3 |
| UK14 | 35 | 8 | 30days | 80.6 | 25 | 100 | 2.9† | - |
| Netherlands15 | 22 | 1 | 30days | - | - | 95 | 9† | 95 |
| French16 | 17 | 4 | 30days | 71 | 34 | 100 | 0 | 88.2 |
| Brazilian12 | 8 | 4 | 9months | 84 | 32 | 100 | 12.5* | 87.5 |
Apart from registries and small series, there is no randomized data to date comparing transfemoral or transapical approach with the subclavian approach. However a recent propensity-matched analysis compared the procedural and 2-year results of the subclavian (n=141) with those of the femoral approach (n=141).13 This study included all consecutive patients undergoing TAVI by the subclavian approach with the 18F CoreValve® prosthesis, and these patients were matched on the basis of baseline clinical characteristics (except peripheral vascular disease) with a transfemoral cohort. Both groups showed similar procedural success rates (97.9% for subclavian vs. 96.5% for transfemoral; P=0.47), major vascular complications (5% vs. 7.8%, respectively; P=0.33), life-threatening bleeding events (7.8% vs. 5.7%, respectively; P=0.48), and combi ned safety endpoints (19.9% vs. 25.5%, respectively; P=0.26). Nonetheless, the subclavian group had lower rates of acute kidney injury/stage 3 (4.3% vs. 9.9%, respectively; P=0.02), minor vascular complications (2.1% vs. 11.3%, respectively; P=0.003), and of all types of bleeding events related to vascular complications. Survival at 2 years was similar in both groups (74+4% vs. 73.7+3.9%, respectively; P=0.78). It should be highlighted that the subclavian approach was related to longer procedure times, though with similar fluoroscopy times.
Overall, these data including registries, small series and propensity-matched analysis suggest that the subclavian approach is an interesting alternative to the transfemoral route for TAVI, and should be considered, especially in patients where clinical situations such as severe calcification, severe tortuosity, small vessels size diameter, preclude the femoral approach. Another important aspect about the subclavian access is that a careful analysis of the subclavian anatomy has to be performed, seeking for minimal diameter > 6mm, avoidance of circumferential calcifications and severe tortuosity, and also the presence of previous ipsilateral pacemaker. Lastly, while the presence of previous coronary artery bypass graft (CABG), including the left internal mammary artery (LIMA), that has usually been considered a formal contraindication, Modine et al. 17 recently showed the feasibility and safety of the subclavian approach even in 19 patients with prior CABG and a patent LIMA. However, a minimal subclavian diameter of 7mm and sheath removal during valve placement is recommended in these cases to avoid the occurrence of myocardial ischemia.
In conclusion, the transfemoral approach is not possible in a high proportion of TAVI candidates. Several prior studies and the work from Brito Junior et al. 12 in this issue showed that the subclavian approach using the CoreValve® system is a good alternative for those TAVI candidates with non-appropriate iliofemoral arteries. Future randomized studies are needed to evaluate the potential superiority of this approach compared to other alternative approaches as well as to the transfemoral access.
CONFLICT OF INTERESTJosep Rodés-Cabau is consultant for Edwards Lifesciences (Irvine, USA), and St. Jude Medical (St Paul, USA). Henrique Barbosa Ribeiro has no conflict of interest to declare.