Thrombelastography (TEG) is a method to assess clot formation and destruction. Various applications have been suggested in the literature.
ObjectiveTo provide an overview of the current knowledge about TEG applications.
MethodsA database search in PubMed was performed up to July 2012 using the term “Thrombelastography [MeSH Terms]”. We analysed retrospective and prospective studies, reviews and guidelines with information about the applications of TEG written in English and Spanish.
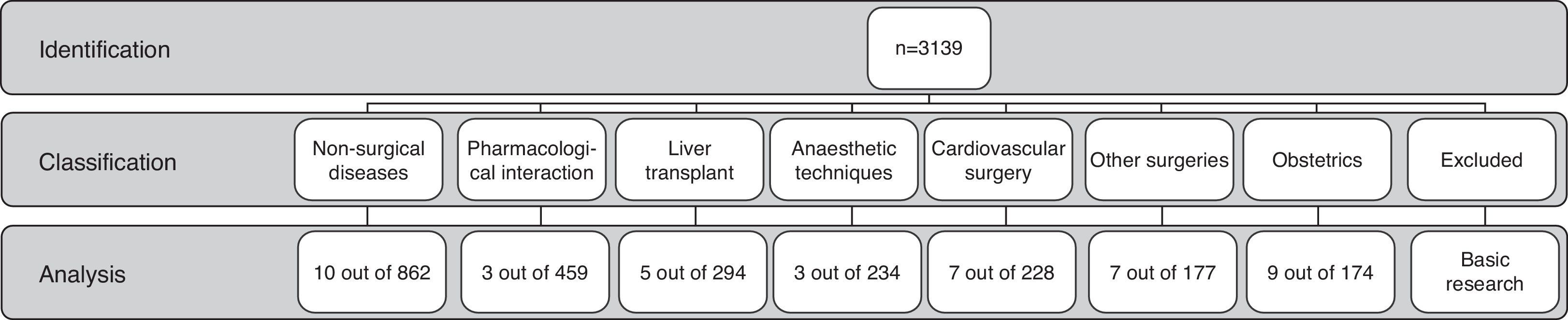
ResultsThe search resulted in 3139 papers since 1962. These were classified in 8 categories: 862 (27.6%) in non-surgical diseases, 294 (9.4%) in liver transplant, 711 (22.6%) in basic research, 174 (5.5%) in obstetrics, 228 (7.3%) in cardiovascular surgery, 177 (5.6%) in other types of surgery, 234 (7.4%) in anaesthetic techniques, and 459 (14.6%) in relation with medications.
ConclusionThe application of TEG as a diagnostic tool and as a guide in transfusion therapy is increasing. Its use is still in development in different clinical fields and the advantages and limitations of this technique still have to be defined. It is evident that thrombelastography should be used with caution, and its strengths and weaknesses as well as new applications must continue to be explored.
La tromboelastografía (TEG) es un método para valorar las características de la formación y destrucción del coágulo. Una variedad de aplicaciones han sido sugeridas en la literatura.
ObjetivoProporcionar un resumen acerca del conocimiento actual de las aplicaciones de la TEG.
MétodosSe realizó una búsqueda en la base de datos PubMed hasta julio de 2012 con el término «Thrombelastography [MeSH Terms]». Se analizaron artículos de estudios retrospectivos y prospectivos, revisiones y guías conteniendo información acerca de las aplicaciones de la TEG escritos en inglés y español.
ResultadosLa búsqueda arrojó 3.139 artículos desde 1962. Se clasificaron en 8 categorías: 862 (27,6%) asociados a enfermedades no quirúrgicas, 294 (9,4%) a trasplante hepático, 711(22,6%) a investigación básica, 174 (5,5%) a obstetricia, 228 (7,3%) a cirugía cardiovascular,177 (5,6%) a otras cirugías, 234 (7,4%) a técnicas anestésicas y 459 (14,6%) a fármacos.
ConclusiónLa TEG como herramienta diagnóstica y para guiar terapia transfusional está en aumento. La TEG aún continúa en estudio en diferentes áreas del conocimiento clínico y aún falta definir adecuadamente los alcances de esta técnica diagnóstica. Es evidente que se debe hacer un uso racional de la TEG, conocer a fondo sus fortalezas y debilidades y continuar explorando nuevas aplicaciones.
Thrombelastography (TEG) provides a graphic representation of blood clot formation and destruction, as well as of clot viscosity and elasticity.1 It has been used in clinical practice to detect and quantify hypercoagulability, hypocoagulability, fibrinolysis, clot strength, and anticoagulant therapy effects.2 Recently, this diagnostic method has also been used in cardiovascular surgery with bypass circulation, in neurosurgery, trauma and other surgical interventions involving the haematological system.3 There are several methods available in the market for assessing the viscoelastic properties of blood, besides conventional TEG (Haemoscope Corporation, Niles, Illinois E.U.) which is the most widely referenced method in national publications1,4–6 and is the subject of this paper. The other systems available include ROTEM (Pentapharm GmBH, Munich, Germany) and Sonoclot Analyser (Sienco Inc., Arvada, Colorado, USA). In the first system, fibrin polymerization is detected by the restricted oscillation of the cup where the sample is placed; in the second, by the restricted oscillation of a pin submerged inside the sample; and in the third, by the restricted vertical oscillation of a probe.7
In our setting, the clinical and surgical uses of TEG are growing, as evidenced by the publications in Colombian Journal of Anesthesiology1,4,5 related to the understanding of the physiology of coagulation and the principles of TEG.
Despite increasing access to publications describing new implementations of TEG, there is no review in our literature that encompasses them from the beginning to the present. The objective of this article is to provide a summary of the current knowledge on the clinical applications of TEG.
MethodsAn electronic search was conducted in the PubMed database on all studies published on the subject between January 1962 and July 2012. In order to increase the sensitivity of the search as much as possible, the Medical Subject Heading (MeSH term) “Thrombelastography” was used. No language restriction was applied. The references included in the articles that were downloaded were all reviewed by three researchers (OMS, CCC and GAP) in order to identify additional articles. Titles, abstracts and/or full texts of the articles in English or Spanish (n=3139) were read in order to classify them according to the type of use suggested by the study (Fig. 1).
The classification categories emerged as the review proceeded, in accordance with the main study objective in each article. For the purpose of this study, the description of one use for TEG was accepted when the authors of the article described it as such, in relation to medical disease, a surgical disease, a diagnostic procedure or a therapeutic procedure. One category emerged in the classification that did not correspond to the use of TEG in human beings, mainly comprising articles that reported basic science research8,9; these were excluded from the analysis.
The most relevant publications (n=44) were selected for the analysis of each category, in accordance with the following criteria: systematic reviews and meta-analyses, original papers (experimental or descriptive), and reviews of the literature. Conclusions related to thrombelastography use and areas of research were derived from this selection; these conclusions are summarized by category. Trends were analysed based on the number of publications per year.
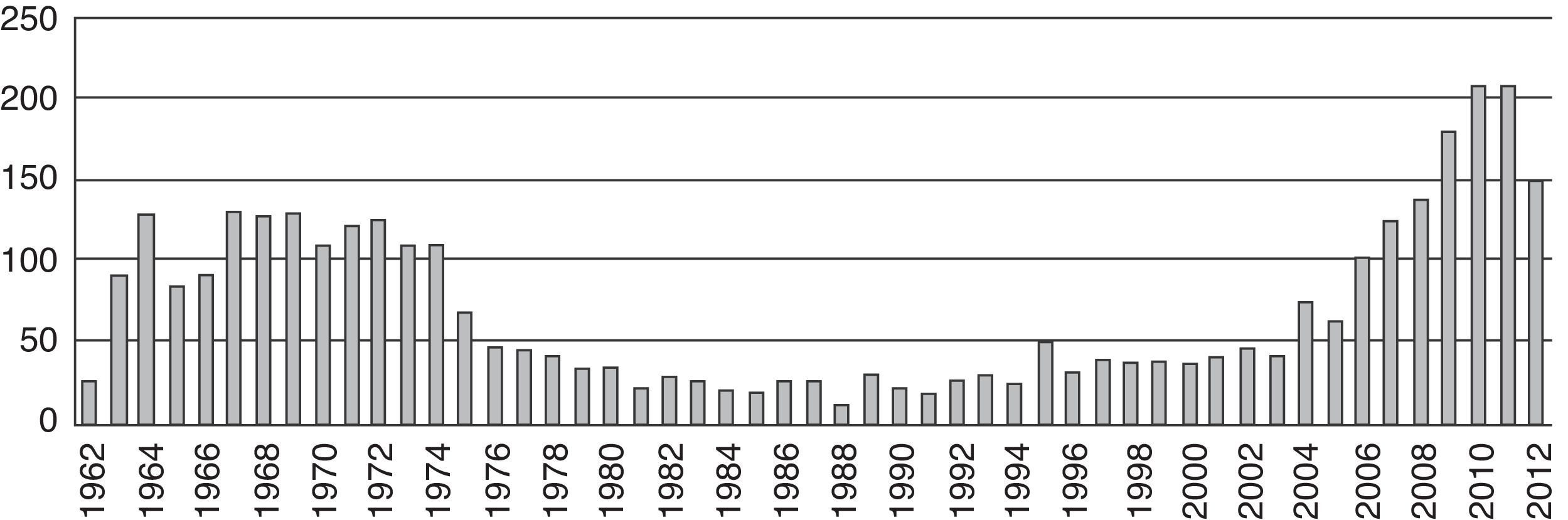
ResultsThe first articles identified were published in 1963 (n=84). As of that date, we observed a trend towards an increase in the number of publications per year until 1975 (n=62). Afterwards there was a steady decline in the number of publications down to a minimum in 1988 (n=7). No important changes were observed in the number of publications until 2004, when the numbers picked up and reached a peak finally in 2010 (n=174) (Fig. 2).
Uses of thrombelastographyNon-surgical diseasesTEG reflects the clinical efficacy of the use of prothrombin complex concentrate and recombinant factor VIIa7,10 and guides the management with fibrinogen replacement therapy.11 The use of TEG is an alternative for identifying abnormal clotting patterns, blood dyscrasias (in the follow-up of patients suffering from poisonous snake bite or toxins with the potential of causing haemorrhagic events),12 for the detection of hypercoagulability states, for differentiating diseases (ischaemic and haemorrhagic stroke, neoplasms, uraemic syndrome, and pulmonary thromboembolism),10,13–15 and for assessing coagulation status in neonates.16
Pharmacological interactionThe use of enoxaparin prolongs the R time and is correlated with an anti-Xa activity peak.17 Some articles show increased clotting activity measured by TEG occurring at mild and moderate levels of haemodilution with the use of some crystalloids and colloids in in vitro experiments.18 In more than 40% dilution, hypercoagulability diminishes, and when dextran and hydroxyethyl starch are used, a state of hypocoagulation is produced.
Dilution with crystalloids and albumin has to be greater than 50.18 Although low-dose oral contraceptives have been associated with deep vein thrombosis in epidemiological studies, and with changes in several factors of the coagulation cascade, they have not been associated with significant changes in thromboelastographic parameters leading to hypercoagulability in women without other risk factors.19 It is worth highlighting that these findings do not support the routine use of TEG in this type of patients.
Liver transplantThe most marked changes in haemostasis occur during the anhepatic phase and immediately after reperfusion, mainly in the form of hyperfibrinolysis resulting from the accumulation of the tissue plasminogen activator due to inadequate liver clearance and the release of heparin-like substances.20 Kang et al. began to use TEG in this scenario. The basic algorithm they used for replacing blood products is summarized as follows: prolonged R time requires the use of fresh frozen plasma, reduced maximum amplitude (MA) requires the use of platelets, and angle reduction requires the use of cryoprecipitates.21
Hendriks et al. evaluated the use of recombinant factor VII with TEG, and showed association with improved clot formation speed and physical characteristics during liver transplant.
Anaesthetic techniquesKettner et al.22 report changes of the different parameters used to assess coagulation, including thrombelastography in patients with isolated induced hypothermia at 36°C, 34°C, and 32°C. It was found that PTT and haematocrit did not change, while PT and platelet count dropped during cooling. TEG measurements showed a delay in clot formation at the adjusted measurement temperature (during TEG measurement, temperatures were adjusted to patient temperatures at the time of taking the sample), but there was no evidence of change when the test temperature was 37°C. This indicates that hypothermia reduces plasma coagulation and platelet reactivity. However, hypothermia does not affect clot strength. All the coagulation variables remained within normal ranges, showing that hypothermia in the conditions described has only minor adverse effects on healthy human beings, and correlates with other studies of patients who underwent liver and cardiac surgery.23,24
In evaluating the effects of propofol vs. isoflurane on thrombelastography changes, Law et al. did not find significant changes in TEG parameters or differences in blood loss during surgery (head and neck tumour resections).25 Also, no significant changes were found in platelet aggregation with the use of enflurane, opioids and local anaesthetics.26
Cardiovascular surgeryOverall, 228 articles were found on the topic of cardiovascular surgery. They focus on the use of TEG results in different stages of the surgical procedure in order to guide transfusion therapy and reduce the need for blood and blood products.27 They compare TEG with routine coagulation studies in an attempt to identify the best predictor of excess bleeding following bypass circulation and the need for reintervention,28–30 monitoring heparin reversal, and monitoring the effect of anti-platelet aggregation agents and low molecular weight heparins (LMWH) in elective and non-elective surgery requiring cardiovascular intervention.31,32 These studies conclude that TEG is a useful method for predicting post-operative bleeding when compared to routine methods used after bypass circulation.30 Moreover, they suggest the use of TEG for heparin reversal, given that results are available within a short period of time to help guide the definitive therapy and reduce the number of unnecessary transfusions.31
TEG has been used together with platelet mapping as a pre- and post-operative assessment tool in patients scheduled for surgery or invasive cardiovascular procedures and who have been treated previously with anti-platelet aggregation therapy; although the papers reviewed show good results in terms of guidance for transfusion therapy and for predicting complications, these techniques still have some limitations such as their correlation with the pharmacokinetic and pharmacodynamic properties of the drugs. Consequently, it is not possible to provide general recommendations on the routine use of these techniques at the present time.33–35
Other surgical proceduresIn the category of other surgical procedures, a total of 177 articles were found, most of them focused on the management of coagulopathy in neurosurgery, major orthopaedic surgery, renal transplant, and surgery in multiple-trauma patients.36
In neurosurgery, the articles support the use of TEG as a screening technique for the diagnosis of haemostatic disorders in neurosurgical patients with risk factors such as the following: coagulation disorders detected in routine laboratory tests, chronic use of anticoagulants and anti-platelet aggregation agents, use of anticonvulsants leading to abnormalities of the haemostatic system, haematological diseases, and intra-operative interventions such as large volumes of crystalloids, colloids and hypertonic solutions.
Compared to routine laboratory tests, TEG may be useful for fast and accurate coagulation monitoring in neurosurgical patients; for identifying patients at high risk of bleeding or for intra- and post-operative thromboembolic events; and for guiding transfusion therapy in order to help reduce transfusion of blood products with no negative effects on the results of the treatment.37–39
In major orthopaedic surgery of the hip, knee and spine, TEG did not only show coagulopathy abnormalities from significant bleeding and was useful to guide the transfusion therapy, but the results also suggested that total knee and hip arthroplasty leads to a hypercoagulability state in the early post-operative period, increasing morbidity and mortality secondary to thromboembolic events.40
ObstetricsIn this area, TEG is used in studies for the diagnosis of the state of maternal coagulation in order to establish normal ranges in the pregnant population41 and for the assessment of gestational hypertension and post-partum bleeding. Sharma et al. showed that pregnancy is a hypercoagulability state that prevails until 24h after delivery, and even up to 3 weeks in some studies.21,42 This state is correlated with increased levels of procoagulant factors,43 and with patients with recurrent miscarriage, where it is associated with an elevated maximum amplitude (MA) in the TEG.44 In patients with preeclampsia, the degree of thrombocytopenia is correlated with reduced K time and MA.45 Fibrinogen concentration is correlated with the severity of postpartum bleeding.43 Thrombelastography has been useful in guiding fibrinogen transfusion during post-partum bleeding,46 detecting and successfully addressing hyperfibrinolysis states with tranexamic acid.47
DiscussionThis review on TEG applications shows, based on the number of publications found, that there is a focus on non-surgical diseases, in particular haematological diseases and immune disorders, as well as pharmacological interactions. A substantial number of publications have come from the surgical realm, which we divided into three groups: liver transplant, cardiovascular surgery and other surgical interventions (trauma, abdominal surgery, thoracic surgery and neurosurgery). A smaller number of studies on the use of TEG in anaesthetic technique and obstetrics were found.
Publications in the area of non-surgical diseases relate to haematological diseases such as haemophilia, mainly type A (where, occasionally, factor levels do not correlate with the clinical findings) and, apparently, factor absence is not the only element involved in the pathogenesis of the disease.10,48,49 In general terms, the use of TEG in this area is still under exploration and growing at a fast pace. In a meta-analysis performed by Afshari et al. there was no evidence of improvement of morbidity or mortality in patients with severe bleeding of different types, using TEG to guide the treatment.50 Hence the suggested need to standardize the method for the various pathologies and their management on the basis of the TEG results. It is not clear yet which diseases lend themselves to a correct analysis using this method.
Regarding drug interactions, studies have focused on drugs that have a mechanism of action related to coagulation. For example, the use of TEG with the administration of unfractionated heparin (UFH), LMWH and anti-platelet aggregation agents has been evaluated. The results of the articles show that TEG parameters are affected by UFH and LMWH concentrations, whereas they do not have any effect on conventional fibrin-based tests such as PT and PTT. Estimates of the difference between standard tests and TEG show a substantial increase in the sensitivity for assessing the effects of UFH and LMWH.
Bleeding is a frequent complication in liver transplant51 as a result of surgical trauma and because the majority of the coagulation factors are produced in the liver.3 In a meta-analysis by Gurusamy et al.18 and a review by Wikkelsoe et al.52, it was shown that TEG reduces the use of blood products during the procedure.
An important issue in anaesthetic practice is the management of patients with hereditary forms of coagulation disorders. Multiple articles show that prolonged R time is consistent with prolonged PTT observed in patients with known hereditary coagulation disorders, just as clot strength values (K time and α angle) and platelet function assessment with MA are measured in Von Willebrand's disease, and could be useful for intraoperative management. These results show that TEG may serve as a useful coagulation monitor for the anaesthetist in the management of surgical patients with haemophilia, Von Willebrand's disease, or isolated factor deficiencies. Moreover, it has been used to differentiate between bleeding of surgical origin or from coagulopathy.53
In cardiac surgery, attention has been focused on coagulation disorders secondary to intraoperative bleeding, bypass circulation, induced hypothermia, the use of heparin, and post-operative monitoring of the patient's coagulation state.3,31 At the present time, TEG continues to be used as a diagnostic test in cardiovascular surgery, before, during and after the procedure. It has been shown that thrombelastography signs of fibrinolysis are clearly detectable during the important bypass circulation phase in cardiac surgery.29,30,54
In trauma, blood products are used on an empirical basis and “blindly”, particularly in patients requiring massive transfusion.36 It is often said that assessing coagulation status is the most reliable way to improve clinical results in injured patients, and must be used to guide transfusion of blood products.55 Most articles show that RapidTEG provides parameters that may be used as reliable indicators of coagulation status in multiple-trauma patients.56
In obstetrics, TEG is gaining acceptance because of its versatility and the timeliness of the results. The evidence of its application for detecting bleeding associated with abnormal fibrinogen levels is growing in importance. There is still a need to standardize the method by means of additional studies in different clinical scenarios, such as preeclampsia and coagulopathic states, standardize cut-off points,43,57 and provide training to the available staff in order to benefit from the full potential of the technique.
In conclusion, this search shows a growing trend over the past decade in the use of TEG since its introduction into clinical practice. There is still a need to do further studies on some of the topics assessed; additionally, there are other topics that have not been studied in relation with TEG. There is no method available to make a comprehensive assessment of the entire coagulation system, but TEG, combined with other tests, may improve our understanding of the haemostatic system.58 Likewise, there is a need to train staff in how to take samples and perform quality analyses using this tool.43,59 This review points to the important and diverse applications of TEG in all clinical and surgical specialties and in all age groups, and shows its high potential as a diagnostic test which is sometimes underestimated and underutilized.
FundingNone.
Conflict of interestThe author has no conflicts of interest to declare.
Please cite this article as: Sulaiman OM, Pabón GA, Cortés CC, Muñoz LA, Reyes LE, Arevalo JJ. Un resumen de la investigación en tromboelastografía. Rev Colomb Anestesiol. 2014;42:302–308.