Subdural anesthesia is a relatively frequent complication though seldom recognized. It has a broad spectrum of presentations ranging from an unexpectedly high sensory block with limited motor block, to substantial hemodynamic and respiratory involvement.
Case presentationA 22-year old woman undergoing cesarean section under epidural anesthesia with evidence of long-lasting higher than expected sensory block and respiratory distress.
ConclusionNeuraxial anesthesia comprises a number of versatile and safe techniques, though not exempt from complications including subdural anesthesia. We should be aware of this possibility in our clinical practice, know the risk factors and the diagnostic criteria.
La anestesia subdural es una complicación de la anestesia neuroaxial relativamente frecuente pero poco reconocida, tiene un espectro de presentación bastante amplio que va desde un bloqueo sensitivo inesperadamente alto con poco bloqueo motor, hasta compromiso hemodinámico y respiratorio importante.
Presentación del casoMujer de 22 años que es llevada a cesárea con anestesia epidural, con evidencia de bloqueo sensitivo más alto de lo esperado, de larga duración y dificultad respiratoria.
ConclusiónLa anestesia neuroaxial es un conjunto de técnicas versátiles y seguras, aunque no exentas de complicaciones como lo es la anestesia subdural. En la práctica clínica debemos estar atentos a esta posibilidad, conocer los factores de riesgo y los criterios diagnósticos.
Subdural anesthesia is a relatively frequent complication from neuraxial anesthesia though seldom recognized,1 with a spectrum of presentation of variable severity. According to Lubenow's studies the overall incidence among the general population has been estimated at 0.87%2 and in obstetric patients undergoing epidural anesthesia the incidence is estimated at 0.024%.3 However, in studies with contrast medium, values as high as 7–11%1,4–6 have been identified. This case illustrates an unusual presentation, the risk factors involved, and the preventive actions that could be adopted.
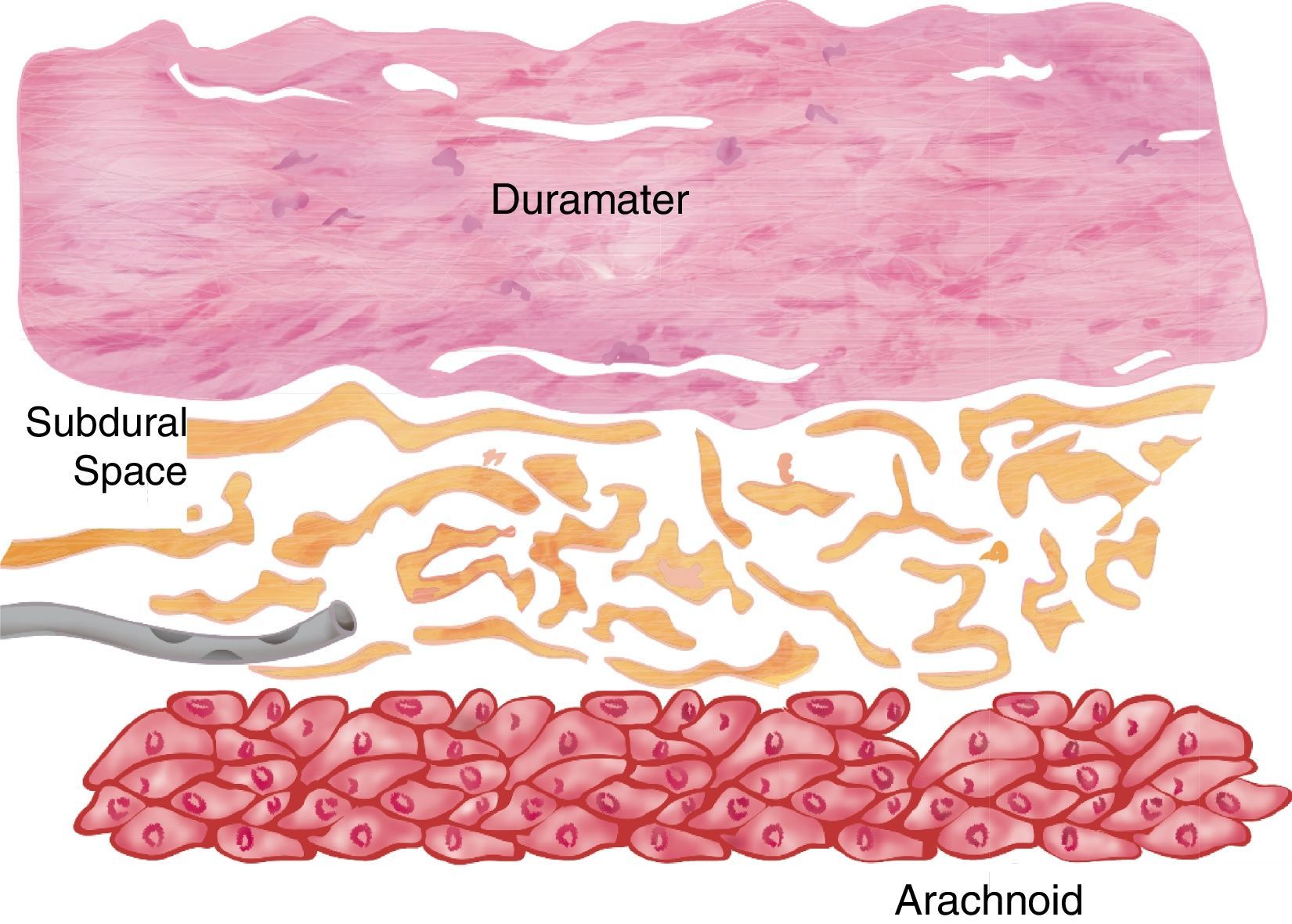
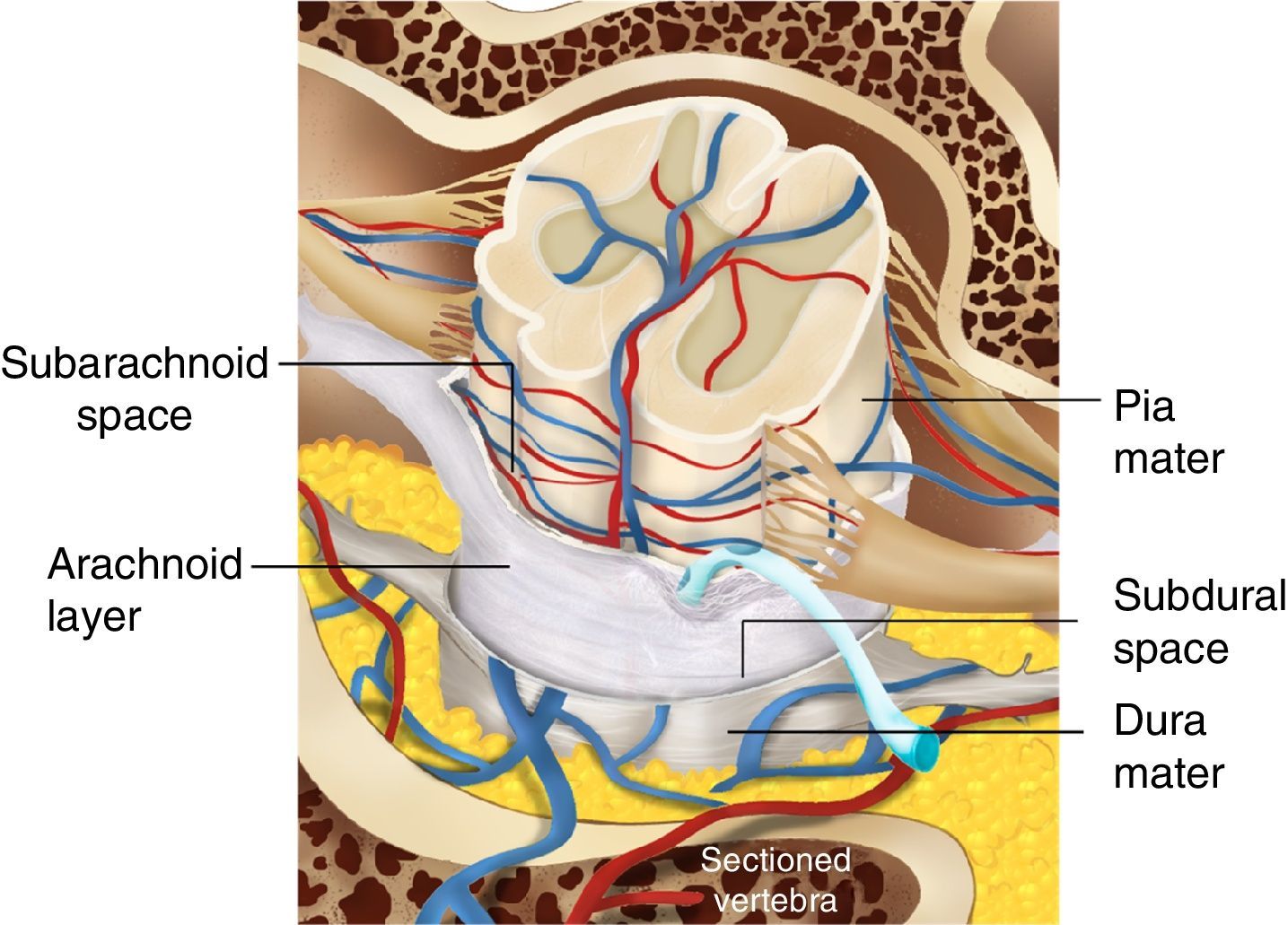
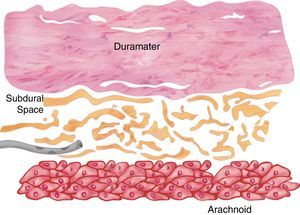
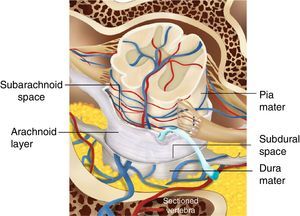
The subdural space has been classically described as a virtual space occupied by serous fluid contained between the dura mater and the arachnoid. Consistent with this anatomical denomination it could be similar to other serous cavities such as the pericardium or the pleurae; this means two layers in contact with a serous structure that promotes friction in the absence of any intercellular bonds. However, recent studies in dead human bodies and using electron microscopy show that this space does not actually exist and if present is the result of pathologic or iatrogenic factors.7–10
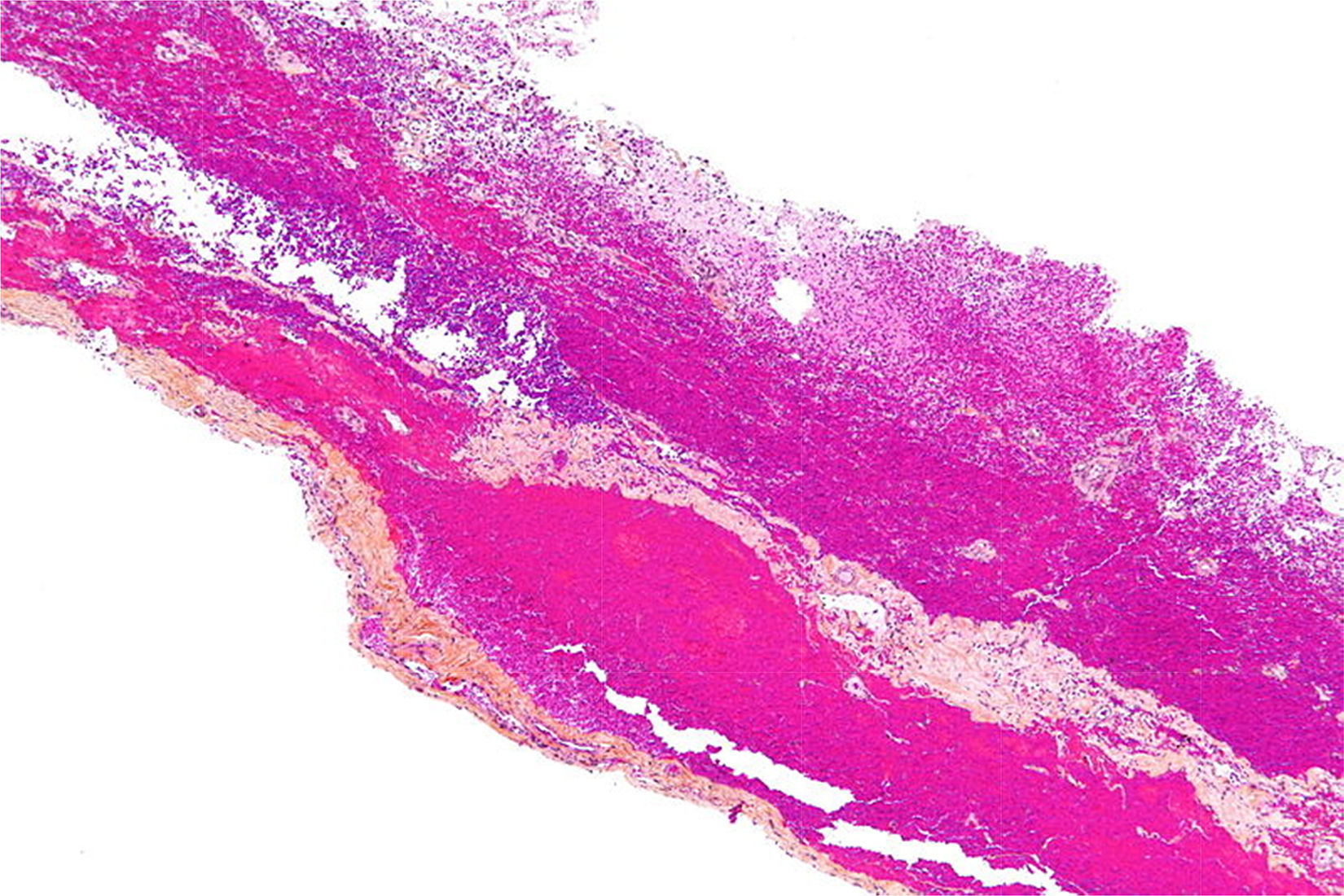
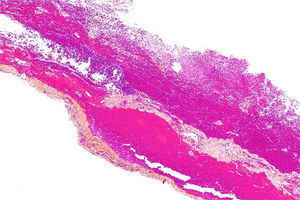
Histologically speaking, the “subdural space” is made up by a neuroepithelium of elongated, spindle-shaped and branched cells with lax intercellular bonds surrounded by few collagen fibers and some blood vessels resulting in low mechanical resistance11,12 (Figs. 1 and 2). The subdural space is localized between the most inner segment of the dura mater – a very tough tissue composed of 80 layers of web-shaped collagen fibers that that run in multiple directions and the arachnoid mater composed of several cellular planes connected by desmosome-type specialized narrow intercellular bonds that make it the primary barrier against the passage of substances.12 The subdural neuroepithelium is concentrically oriented around the dural sac and, in contrast to the epidural space, it is not limited by the foramen magnum. This is the most frail tissue inside the meninges that may sustain injuries and result in a pathological space whose size and shape are determined by the strength of the generating force and represents is a critical factor for the direction and distribution of any substances administered in that area. In the case of local anesthetic agents this accounts for the huge variability of clinical presentations.2,5,6 Finally the clinical presentation of the anesthetic block is determined by the meningeal structures permeated. If the dura mater is not permeated, the characteristics will mimic an epidural anesthesia but if the dura mater is disrupted while the arachnoid is preserved, the clinical presentation will resemble a subdural anesthesia. Lastly, if the arachnoid is disrupted and the anesthetic agent deposits in the subarachnoid cavity (intrathecal), the anesthesia will be spinal or subarachnoid.13,14
Subdural hematoma seen under optic microscopy to identify the plane of separation of the neuroepithelium.
22-year old patient, 39 weeks in her first pregnancy, 80kg of body weight, previously healthy, scheduled for cesarean section due to a breech presentation, negative history, vital signs: Blood pressure: 110/70, heart rate: 80/Min, respiratory rate: 18/Min, body temperature: 37°C, arterial oxygen saturation: 99.
The decision was made to administer epidural anesthesia after obtaining the patient's informed consent and using non-invasive monitoring, following a strict aseptic technique, a single attempt L3–L4 Tuohy # 18 needle puncture was performed. The epidural space was identified using the loss of resistance to air technique, leaving in place a 3cm catheter.
A test dose with 3cc of lidocaine 2%+100mcg fentanyl was administered with no signs of intrathecal or intravenous injection.
7cc of lidocaine 2% and 10cc of bupivacaine 0.5% were added to complete the volume of anesthetic. A T6 sensory level was achieved and the surgery began uneventfully.
A male baby was born 12min later with an Apgar score of 9–10.
20min into the procedure the patient developed upper limb paresthesia and respiratory distress, became anxious and the monitor then indicated the following vital signs: BP 90/60 HR 54min–1 SaO2 94.
Sensory block was evidenced with level C5 upper extremities involvement, with no motor block. The patient was managed with a bolus of lactated Ringer's solution and increased FiO2 and there were no major complications afterwards. The procedure was then completed with no further deterioration of the patient. No additional doses of anesthetic were required via the epidural catheter, which was then removed at the completion of surgery. The sensory block lasted for 6h.
DiscussionDifferent scenarios may evolve in terms of the mechanisms leading to subdural anesthesia:
- -
The spinal or epidural needle may perforate the dura mater and the arachnoid with the orifice located between the two spaces. In this context we must keep in mind the pressure difference since pressure is below the atmospheric pressure in the subdural tissue but positive in the CSF, giving rise to positive aspiration of CSF. However, the anesthetic injection will take the path of less resistance, i.e. the subdural space which is the mechanism accounting for failed subarachnoid anesthesia.8
- -
The spinal or epidural needle may perforate the dura with no involvement of the arachnoid. In this case, the CSF aspiration will be negative, while the loss of resistance in air or water will be positive and a catheter may be easily placed inside this tissue. (Fig. 3) This is the mechanism accounting for the occurrence of subdural anesthesia.8
There are some other less common mechanisms such as the multi-orifice catheters with a subdural placement of the distal end while the proximal orifices that may be placed in the epidural space. This results in a normal presentation with low rate infusions allowing for perfusion through the proximal ends; however, a bolus administration will lead to a clinical presentation of subdural anesthesia.15 Catheter migration is yet another mechanism.8,15
Other subdural anesthesia-associated factors have been identified with the common denominator of physically damaging the dura mater; these are:
Post lumbar puncture, prior subdural injection, rotation of the epidural needle, a history of spine surgery, and repeated attempts at the same site; however, there is no relationship with the level of experience.5,6,8.
The clinical presentation is quite variable, ranging from a normal presentation of epidural anesthesia, unilateral blocks, patchy blocks or involving some dermatomes, or a very high sensory block in contrast with a minimal or non-existent motor block.1,2,14,16
Such heterogeneity results from the above-mentioned characteristics. The prevalence of a sensory over a motor block is due to the fact that the space is created and the access is posterior allowing selectivity of the for dorsal roots, in addition to the fact that the dura mater and the arachnoid tend to be fused on the anterior root, limiting the diffusion at this level.11,12 Furthermore, based on the volume and pressure used, extensive, patchy or unilateral may be expected, depending on the dissection plane that the injected fluid follows into the “subdural space”.11,12
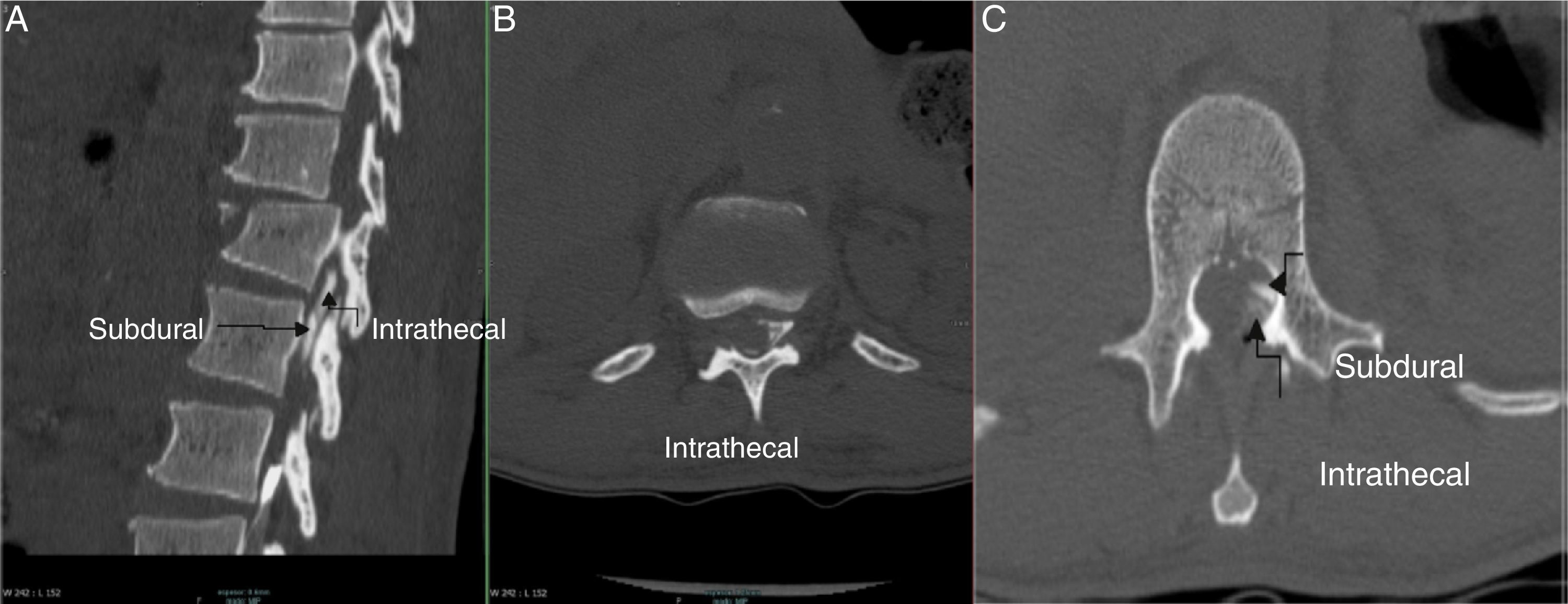
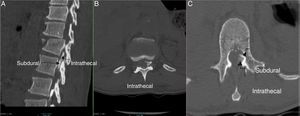
An accurate diagnosis is made by injecting 6cc of contrast medium and obtaining AP and lateral X-rays, or with the use of fluoroscopy or CT, delivering a characteristic subdural distribution pattern1,4 (Fig. 4A–C).
Several signs or symptoms are suspicious of a subdural placement of the catheter or the needle; i.e., the absence or evident loss of resistance; frontal headache or pain following the application. Any of these symptoms call for a radiological confirmation of the position or changing space as a last resort. When neither of the above recommendations is feasible, quit the technique.5,6
In terms of the test dose typically used, such dose is of little use for identifying subdural anesthesia.8,9,17,18 However, the use of the neurostimulator has been considered useful because a response below 1mA when the localization is in the subdural space gives a diffuse response with the involvement of multiple dermatomes, while if the localization is in the epidural space with stimuli between 1 and 10mA, the responses obtained will correspond to the dermatome where the needle is placed.16,19
Hoftman and Ferrante1 recently developed some diagnostic criteria for subdural anesthesia without imaging, using as major criteria for the epidural technique the negative outflow of CSF associated to tactile sensation due to loss of resistance and they suggest two scenarios: excessive or restricted block. In each case, the following minor criteria are considered, though one is enough to make a diagnosis:
For excessive block (93% sensitivity) the criteria are: starts after more than 20min, cardiovascular stability, sensory involvement with minimum or absent motor activity, patchy or asymmetrical distribution, respiratory failure and head or face anesthesia.
In the case of restricted block (sensitivity 100%), the criteria include a start after more than 20min and sensory involvement with minimum or absent motor effect.
In terms of the subarachnoid technique, Hoftman and Ferrante1 say that in the presence of tactile sensation of a subarachnoid puncture and CSF leak as major criterion, one of the following conditions is enough to make the diagnosis: excessive (no cases reported), restricted (sensitivity 100%), start beyond 20min, sensitive involvement with minimal or absent motor effect and failed block.
No deaths have been reported due to subdural block. Management is based on support therapy, atropine for bradycardia control, crystalloids or colloids boluses for hypotension, trendelemburg position, and vasopressors if needed. Ventilation support may be required and although some subdural catheters may behave normally, the recommendation is to remove them due to their unpredictable nature.5
Although the patient did not report any symptoms such as headache or pain upon advancing the catheter, nor was the loss of resistance during the identification of the epidural space reported as “vague”, all of these signs and symptoms are too subtle. Additionally, saline solution instead of air shall be used for the identification of the epidural space, since according to a recent meta-analysis the former has been associated with less post-puncture headaches and fewer attempts.20 It should be noted that the number of attempts and post-puncture headaches are associated with meningeal lesions, a critical event in subdural anesthesia.11,12
ConclusionNotwithstanding the fact that the diagnosis of subdural block is confirmed radiologically using the above-mentioned criteria, there is evidence of good diagnostic sensitivity based on clinical criteria. In our case, according to Hoffman's criteria, the sensitivity was 93%. There were no incidents alerting us about a subdural localization during the catheter insertion. However, we should be attentive to these signs and symptoms and in case of doubt proceed with a radiological verification, use the neurostimulator, and when neither of these options is available, change the intervertebral space or the anesthetic/analgesic technique.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
FundingThe authors did not receive sponsorship to carry out this article.
Conflict of interestThe authors have no conflicts of interest to declare.
Dr. Fabián Valdés radio-diagnostic resident of the Hospital Universitario del Valle Evaristo García.
Stéphany Santanilla Arana, graphic designer for her artwork contribution.
Please cite this article as: Palacio-García CA, Gómez-Menéndez JM. Informe de caso: Anestesia subdural en la paciente obstétrica. Rev Colomb Anestesiol. 2016;44:174–178.