Bedside ultrasound is now more commonly used in anesthesiology and critical care. There are numerous applications beyond its role in regional anesthesia and vascular access.
ObjectiveTo describe how bedside ultrasound can be integrated to current clinical management is dealing with hemodynamically unstable, hypoxemic, oligoanuric patient and in the patient with altered neurological status.
Material and methodsEssay article describing a synthesis of the current literature, expert opinion, current practice and recent clinical trials in the development of proposed algorithm dealing with the use of bedside ultrasound in the management hemodynamic instability and hypoxemia.
ResultsThree algorithms currently used in the hemodynamically unstable and the hypoxemic patient and the patient are described. In addition, a simple bedside ultrasound approach to oligoanuria and altered neurological status is proposed.
ConclusionFurther studies incorporating head-to-toe bedside ultrasound by trained clinicians will need to be validated but are likely to demonstrate the significant advantages of incorporating bedside ultrasound in the practice of anesthesiology and critical care.
El ultrasonido realizado al lado de la cama del paciente se utiliza cada vez con más frecuencia en anestesiología y cuidado crítico. Son muchas sus aplicaciones aparte de la anestesia regional y el acceso vascular.
ObjetivoDescribir la forma de integrar el ultrasonido al lado de la cama del paciente en el actual manejo clínico del paciente hemodinámicamente inestable, hipoxémico y oligoanúrico y del paciente con estado neurológico alterado.
Materiales y métodosEnsayo que describe una síntesis de la literatura actual, las opiniones de expertos, la práctica corriente y los experimentos clínicos recientes para el desarrollo de la propuesta de un algoritmo relativo al uso del ultrasonido al lado de la cama del paciente en el manejo de la inestabilidad hemodinámica y la hipoxemia.
ResultadosSe describen tres algoritmos utilizados actualmente en el paciente hemodinámicamente inestable e hipoxémico. Adicionalmente se propone un enfoque simple de ultrasonido a la cabecera del paciente para la oligoanuria y el estado neurológico alterado.
ConclusiónSerá necesario validar estudios ulteriores que incorporen la realización de ultrasonido de la cabeza a los pies por parte de clínicos entrenados, pero es probable que demuestren las ventajas importantes de incorporar el ultrasonido a la cabecera del paciente en la práctica de la anestesiología y el cuidado crítico.
The role of ultrasound (US) is already well established in the operating room in cardiac anesthesia, for regional nerve block and vascular access. Articles and guidelines demonstrating the advantages of US guided decisions, interventions and procedures have been published.1–4 Resuscitation guidelines embraced US as a key resource to make differential diagnosis during cardio circulatory arrest, as well as an alternative tool for endotracheal tube placement confirmation, or, in other words, to exclude esophageal intubation.5,6 In a recent analysis on advances on anesthesia monitoring, transesophageal echocardiography was found to be the second most frequently reported topic with 141 papers on high impact journals for the years 2009–2013. The use by anesthesiologists of transthoracic echography for cardiac and lung assessment in the perioperative setting has been found to be both reliable and of clinical impact. It is a matter of time when anesthesiologist will embrace the use of US for relatively common situations in the operating room as emergency physician have done it for decades now in the emergency room. It is of the most importance that the widespread use of echocardiography by anesthesiologists in the perioperative setting follows a rigorous process of training and obeys well-founded regulations to bring the most positive impact on patient care. In the following pages we will describe the critical role of bedside US for the management of the acutely hypoxemic patient with or without hemodynamic instability. Other potential applications such as the oligo-anuric patient and the patient with altered neurological status and compromised brain perfusion will be discussed briefly.
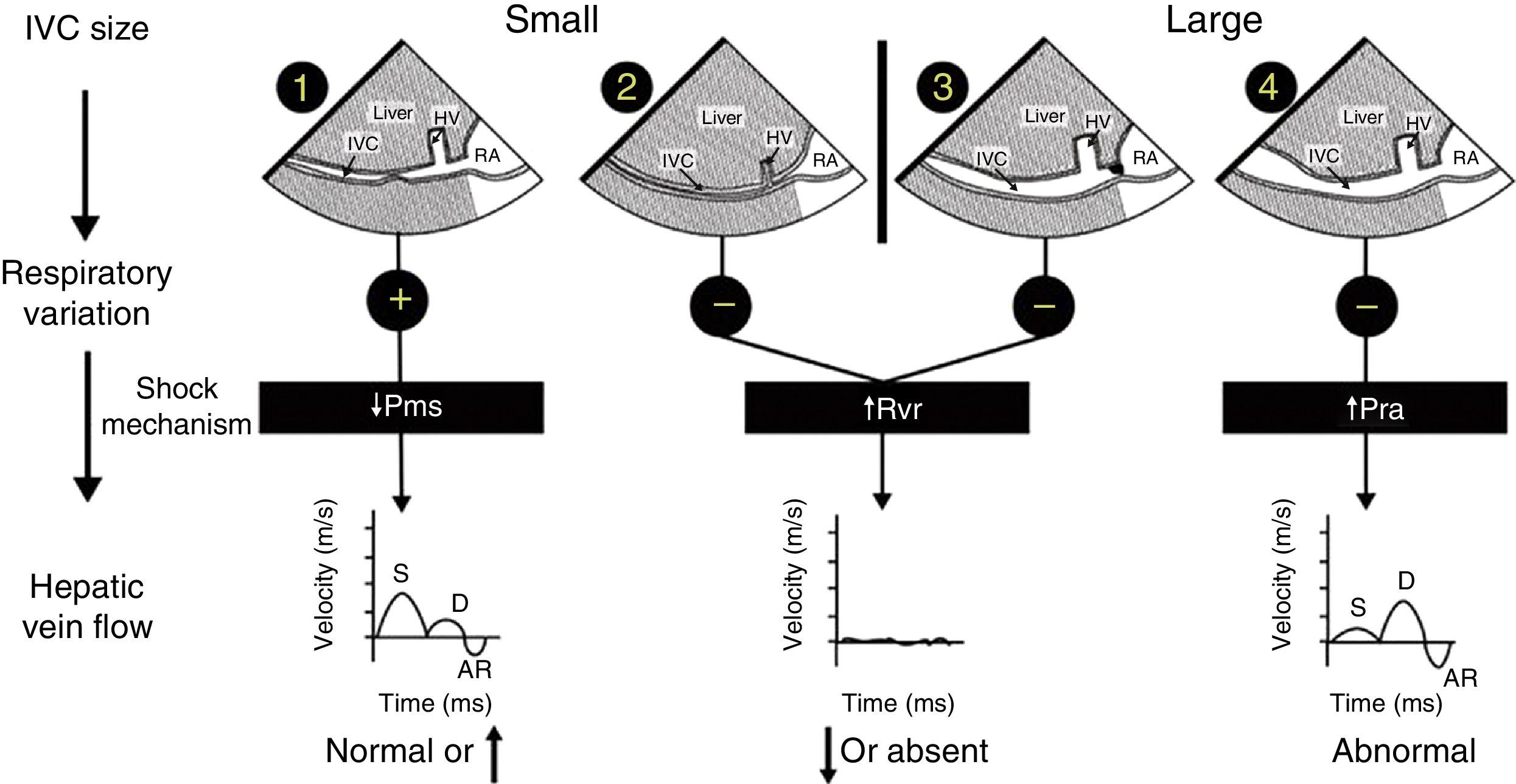
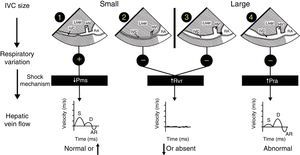
Mechanism of hemodynamic instability and hypoxemiaHemodynamic instability and hypoxemia are critical and time-sensitive situations. Time-sensitive implies that the longer it takes to re-establish perfusion and oxygenation, the worse the outcome. This has been clearly shown in emergency medicine,7 in septic shock8 and probably in any situation where oxygen transport is compromised. Prolonged hypoxemia will lead to neurological damage. If in addition hypotension is associated with reduced brain perfusion then the outcome may be worse. The critical role of US in hemodynamically unstable patients using a physiological approach based on Guyton's concept on venous return has been reported in 2014.9,10 In simple words, venous return which is normally equal to cardiac output will be determined by 3 variables: right atrial pressure, mean venous systemic pressure and resistance to venous return. Bedside US can rapidly identify the mechanism of shock by interrogation of the inferior vena cava (IVC) and the hepatic venous flow (HVF) using pulsed-wave Doppler (Fig. 1). Once the mechanism is identified, the next step is to determine the etiology of shock.
Shock mechanism. Algorithm to determine shock mechanism by using inferior vena cava (IVC) size, respiratory variation during spontaneous ventilation and hepatic venous flow (HVF) is shown (see text for details). AR, atrial reversal velocity of the HVF; D, diastolic HVF velocity; RA, right atrium; S, systolic HVF velocity; HV, hepatic vein; Pms, mean systemic venous pressure; Pra, right atrial presssure; Rvr, resistance to venous return.
Cardiogenic shock will be associated with increased right atrial pressure which can result from left or right systolic dysfunction but also diastolic dysfunction, left or right ventricular outflow tract obstruction, valvular disease and pulmonary embolism. In addition, uncorrected hypoxemia or hypercapnia will increase pulmonary hypertension and consequently right atrial pressure. In cardiogenic shock, a dilated IVC and abnormal HVF will be observed. The relationship between abnormal HVF and progressive increase in RAP and its clinical relevance is increasingly recognized.12,13 Examination of the heart will be important in order to determine the etiology and the most appropriate treatment. For instance, inotropes will be indicated in systolic dysfunction, not useful in diastolic dysfunction but contra-indicated if there is left or right outflow tract obstruction.14,15
Reduced mean venous systemic pressure can result from loss of volume or hypovolemic shock or through an increase in venous compliance or a distributive shock. Hypovolemic shock can result from external hemorrhage or blood losses in the thorax, the abdomen, the gastrointestinal tract or the retroperitoneum. It can also result from fluid losses such as severe diarrhea. Venous vasodilatation can result from several processes. The most common are drug related such as those used during anesthesia induction. Other conditions such as septic shock, anaphylaxis, neurogenic shock and Addison disease are typically associated with vasodilatation. In both volume losses and vasodilatation, the IVC will be small and the HVF with normal or elevated velocity.10 Ultrasound will then be used to identify a source a bleeding or a source of sepsis. The treatment will be completely different in either case.
Resistance to venous return is uncommon. In simple words, it results from the inability of the blood to reach the heart. This can occur if there is an intrathoracic or intra-abdominal obstruction to venous return. For instance, a tension pneumothorax, dynamic hyperinflation, pericardial or mediastinal tamponade will typically increase right atrial pressure from external compression. Abdominal compartment syndrome will do the same through the diaphragm. The presence of an IVC stenosis which can occur after certain types of procedure like liver transplantation, Fontan procedure or from a large thrombus will also increase resistance to venous return.16 In these conditions the aspect of the IVC will be variable. If the resistance is above the diaphragm, the IVC will be dilated. Is the obstruction is below the diaphragm, the IVC will be small and often difficult to visualize. If the IVC is dilated, careful examination of the IVC close to the junction of the right atrium can reveal stenosis. The clue to diagnose resistance to venous return is that there will be a significant reduction, absence or significant abnormal HVF.16 In addition the heart will look normal with preserved or elevated pulmonary venous flow in comparison to the HVF which will be significantly reduced. In these patients once the obstruction is relieved, there typically is a sudden increase in venous return which can lead to right ventricular failure with transient left ventricular diastolic function.17,18
Unfortunately, several mechanisms of shock can be present simultaneously. For example in septic shock from an abdominal source requiring surgery, initially reduced mean systemic venous pressure will be present. If fluid resuscitation is excessive, abdominal hypertension and compartment syndrome can occur which will increase resistance to venous return. Finally in septic shock, inflammatory mediators will often result in myocardial depression.19
Hypoxemia can result from five specific mechanisms. Those are ventilation perfusion-mismatch, shunt, diffusion abnormality, hypoventilation and reduced mixed venous oxygenation. Ventilation perfusion abnormalities are the most common cause of desaturation. The hallmark of this condition is that it responds to oxygen therapy. Typical condition is decompensated chronic obstructive pulmonary disease, most cardiogenic pulmonary edema and atelectasis. The characteristic of a shunt is that it does not respond to oxygen. The shunt can be pulmonary such as a pneumonia or cardiac such as a patent foramen ovale (PFO).20 Pulmonary embolism can be initially associated with a ventilation-perfusion abnormality but if significant vascular occlusion is present the term dead-space will be more appropriate to describe the mechanism. Reduced mixed venous oxygen saturation is associated with severe shock for which the mechanism has already been discussed. Hypoventilation as a cause of hypoxemia is typical of drug overdose and associated with a normal alveolar-to-arterial gradient. Diffusion abnormalities are rare and mostly chronic. Another mechanism of hypoxemia, to consider particularly in environmental medicine, would be cellular hypoxemia from carbon monoxide and cyanide intoxication particularly in burn victims.
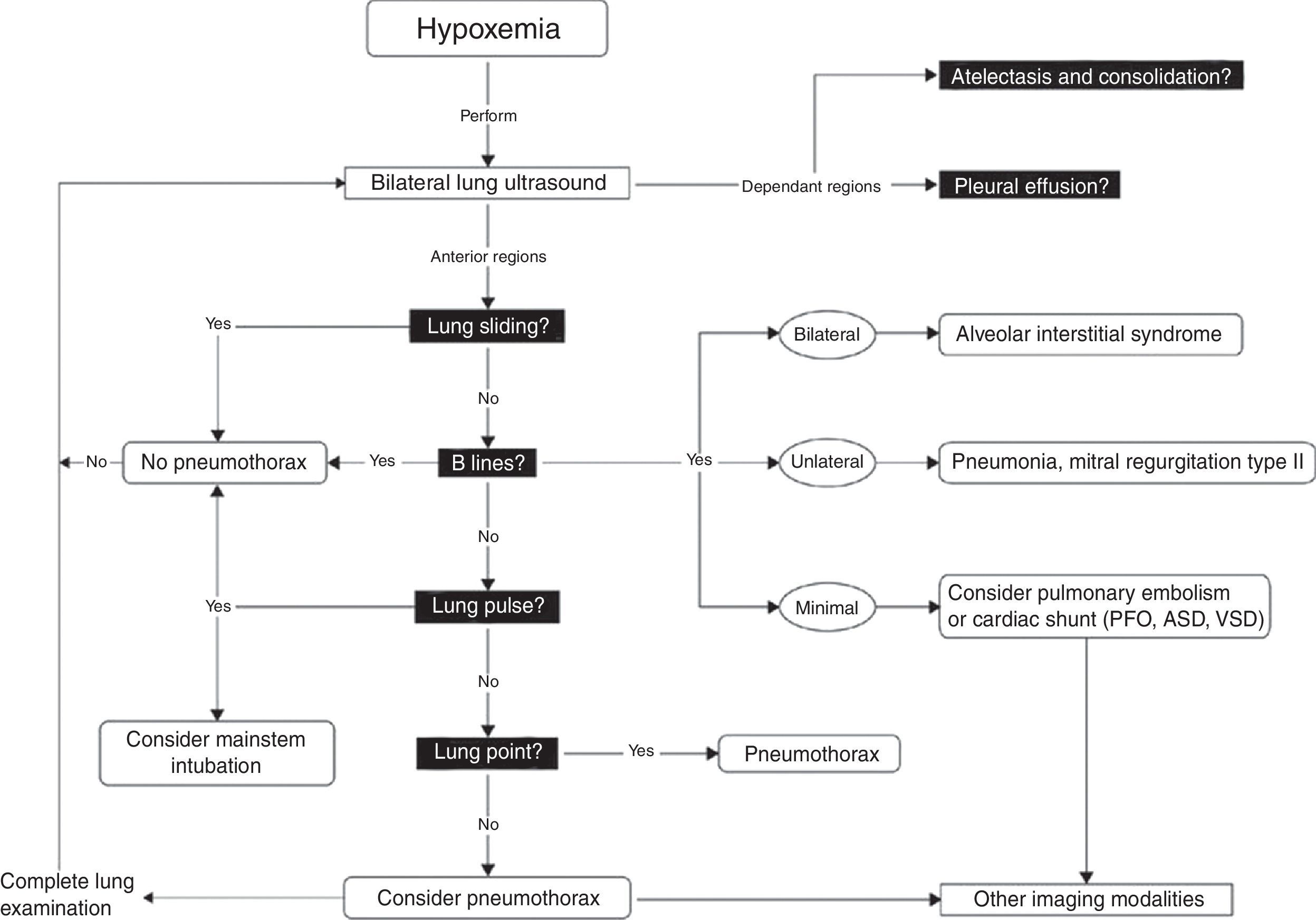
Bedside US in the presence of hypoxemia is very useful. Algorithms such as the “Blue protocol” have been developed and validated in order to approach patients with hypoxemia.21 Physic principles dictate that air is an enemy to any US examination. Indeed, air acts as a mirror to completely reflect US beams. It seems logical to think the lung would not be the right candidate for bedside US. However, lungs are not only made of air, but from various combinations of lung parenchyma and physiologic or pathological fluids. The passage of US beams through normal or pathologic lung creates artifacts. Lung US relies on the interpretation of these artifacts in combination with the clinical setting to make a diagnosis. By following a simple algorithm, it is easy to diagnose the most common thoracic and lung pathologies such as pleural effusion, pulmonary edema, acute respiratory distress syndrome, pneumonia and pneumothorax. Lung US is also useful to exclude mainstem bronchial intubation and diagnose diaphragmatic paralysis. Several protocols using lung US have concentrated their attention to the pulmonary artifacts, but there are certain situations in which examination of the heart is essential, such as intracardiac shunt and pulmonary embolism. In the case of desaturation that results from pulmonary edema or atelectasis, applying positive end-expiratory pressure (PEEP) will improve the patient's condition. However, PEEP will worsen an intracardiac shunt such as a PFO. This is commonly present in up to 20% of adult patients.22
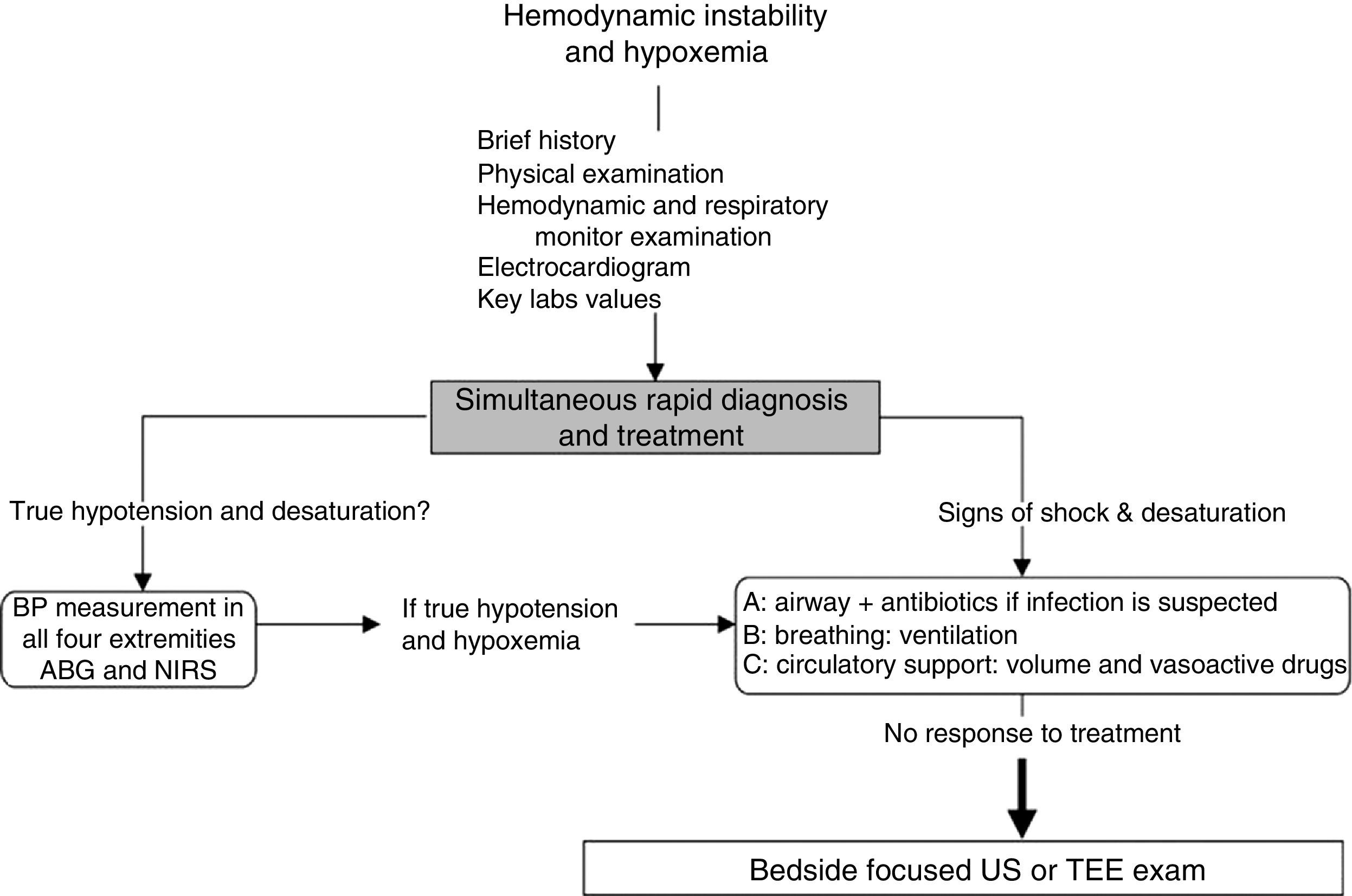
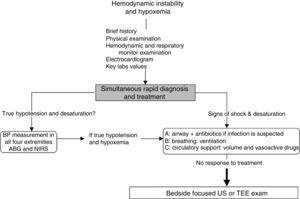
Approach to hemodynamically unstable and hypoxemic patientThe approach to hypotension and desaturation using bedside US is summarized in Fig. 2. This approach is based on our clinical experience where US is seen as a complement or a confirmatory element of the clinical differential diagnosis. Ultrasound does not replace clinical judgment and its usefulness is proportional to the clinician competency in recognizing specific US signs and patterns.
General approach to hemodynamic instability and hypoxemia. In the presence of shock, a brief focused history, examination of the patient and the monitors is performed. In addition key laboratory values and an electrocardiogram are obtained. Once true hypotension and hypoxemia are confirmed, an ABC approach is proposed as described in the text. If these initial steps do not work, bedside surface or transesophageal echocardiography (TEE) focused ultrasound (US) examination of the unstable and hypoxemic patient should be considered. ABG, arterial blood gas; BP, blood pressure; NIRS, near-infrared spectroscopy.
In hypotensive or hypoxemic patient, diagnosis and treatment will be initiated simultaneously. Important clues on the history, physical examination and key laboratory data will orient toward the diagnosis. They are summarized in Table 1.
Key elements in assessment of an hemodynamically unstable patient.
| Reduced Pms | Increased Pra | Increased Rvr | |
|---|---|---|---|
| History | Drugs (antihypertensive, steroids, antibiotics, anticoagulants) Co-morbidities (cirrhosis) Past and recent medical history (abdominal or thoracic procedures) Neuroaxial blockade | Drugs (anti-arrhythmic; B-block) Coronary artery disease Hypertension Acute stroke Prolonged immobilization thrombophilia | Recent abdominal procedure Central venous access Surgery involving the IVC |
| Exam | Warm skin temperature Grey Turner's sign or flank bruising Cirrhosis stigmatas Bronchial breath sound | Cold skin temperature Focal or global neurologic deficits Clubbing Heart murmur | Cold skin temperature Tense abdomen Distant cardiac sounds Unilateral reduction in breath sound |
| Laboratory | CBC K, glucose Blood culture Coagulation profile Amylase Pro-calcitonin | CK, troponin ECG | CXR Arterial blood gas |
Other useful global indicators of shock severity: vital signs, creatinine, liver enzymes, lactate, mixed venous saturation, veno-arterial partial pressure of carbon dioxide (PCO2).
CBC, complete blood count; CK, creatinine kinase; CXR, chest radiograph; ECG, electrocardiogram; IVC, inferior vena cava; Pms, systemic venous pressure; Pra, right atrial pressure; Rvr, resistance to venous return.
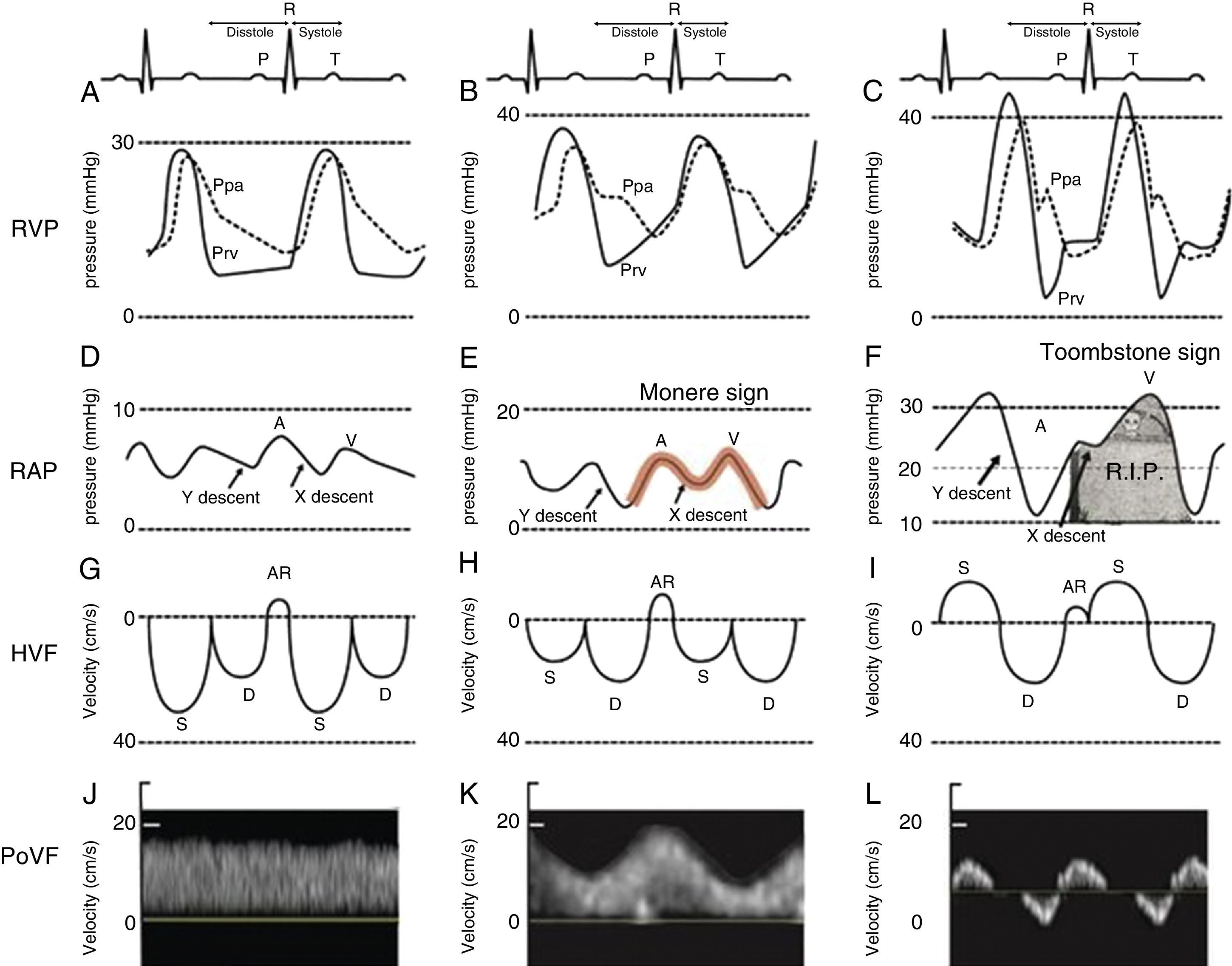
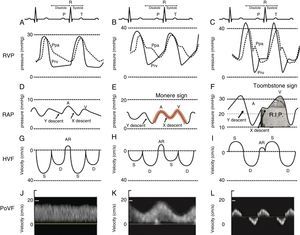
Close examination of the monitor is very important as it may give clues to the mechanism of shock or desaturation. Findings such as electrocardiographic ST changes with cardiogenic shock, prolonged QT interval from massive transfusion with resulting hypocalcemia and microvoltage from cardiac tamponade are important clues. The hemodynamic waveforms are also important to analyze. For instance, cardiogenic shock can be associated with pulsus alternans or pulsus tardus. Pulsus paradoxus is commonly used and a marker of reduced mean systemic venous pressure however it can also be present in dynamic hyperinflation, tamponade and severe right ventricular (RV) dysfunction. Central venous pressure or right atrial pressure (RAP) can provide clue on the presence or the absence of right ventricular dysfunction. At our Institute, we have been using RV pressure waveform monitoring for several years.15 This has allowed us to establish the link between RV pressure waveform, HVF and more recently portal venous flow velocity.23 As the RV function deteriorates, the Y descent predominates over the X descent and it creates a M or W sign. We call it the “monere” or “warning” sign. Monere is the origin of the word monitoring and monument. It means “warning”. As RV function deteriorates, the X descent disappear and only a Y descent and a CV wave are present. We call it the “tombstone” sign because its persistent will be associated with increased mortality from RV failure. As can be shown in Fig. 3, HVF is the equivalent of a CVP but expressed as a velocity in cm/s instead of a pressure in mmHg. In addition, there is significant interest in the current literature on the use of portal venous flow as indirect and simpler method to evaluate the extra-cardiac impact of right ventricular dysfunction. Finally, the fourth line of Fig. 3 is the portal vein venous flow which correlates with the severity of right heart failure.23–25 A normal portal vein is monophasic and it becomes pulsatile and even reversed as right atrial compliance is reduced typically from right heart failure. The association between portal hypertension and cardiac failure has been known to radiologist and cardiologist for several years.26–28 The signal is relatively simple to obtain and was recently described in Anesthesia & Analgesia.29 Tremblay et al. recently reported that the use of bedside ultrasound in order to detect portal hypertension was critical in the management of two patients with right heart failure.30
Right ventricular dysfunction classification. Right ventricular pressure (RVP), right atrial pressure (RAP), hepatic venous flow (HVF) and portal venous flow (PoVF) in normal patients (A,D,G,J) and those commonly observed in patients with mild (B,E,H,K) and severe (C,F,I,L) right ventricular dysfunction. The “Monere” sign (Latin word for warning) is a sign of early right ventricular dysfunction. It occurs when the “V” wave is higher than the “A” wave and the “Y” descent equal or deeper than the “X” descent. The “Tombstone” sign indicates right heart failure. It occurs when no “X” descent is present and only a “V” is seen. AR, atrial reversal HVF velocity; D, diastolic HVF Doppler velocity; Ppa, pulmonary artery pressure; Prv, right ventricular pressure, S, systolic HVF velocity.
Therefore, in absence of pericardial and valvular disease, non-invasive bedside evaluation of the HVF allows rapid diagnosis of RV dysfunction. Respiratory waveforms are important to analyze if hypoxemia is associated with increased peak airway pressure in the intubated patient.9
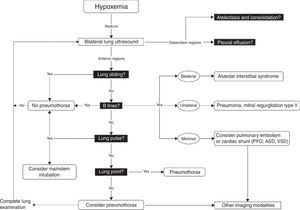
Once these initial steps taken, make sure that you are dealing with true hypotension and/or true desaturation. It is not infrequent to observe a discrepancy between the severity of the hypotension and the corresponding signs and symptoms. Pseudo-hypotension and pseudo-desaturation is probably underestimated particularly in patients under significant vasoactive agents with a radial catheter.31 In the operating room significant radial to central or femoral gradients can occur in up to 45% of patients.32 This is why we recommend to take the blood pressure in all four extremities before progressing in the treatment of hypotension if the clinical signs do not fit the clinical picture. The same can occur with desaturation particularly in patients who have peripheral vasoconstriction. In the operating room and in the intensive care unit we find cerebral near-infrared spectroscopy (NIRS) useful for that purpose. In true shock state and desaturation, NIRS is invariably reduced. In pseudo hypotension and desaturation NIRS will be normal.33 Once true hypotension and desaturation are confirmed, then the ABC will be undertaken. A stands for airway but also antibiotics because if septic shock is suspected antibiotics have to be given as soon as possible.8 B stands for breathing or ventilation and volume up to 15ml/kg which would be sufficient to determine fluid responsiveness. A leg raising maneuver can also be used for that purpose avoiding unnecessary fluid challenge.34 Finally C stands for circulation and we recommend noradrenaline as an initial agent.35 Once these initial steps are taken, then a focus US should be considered. The goal of the US exam will be to determine first the mechanism of shock (Fig. 1) followed by investigating the etiology. Then as hypoxemia is in more than 90% associated with a pulmonary problem, lung US will be performed first to rule-out a pneumothorax and then to determine the other causes of hypoxemia (Fig. 4).36
Lung ultrasound algorithm. Bedside ultrasound algorithm in the presence of hypoxemia. A total of 6 abnormalities will be looked for: those include pleural effusion, atelectasis in the dependant regions and in the non-dependant regions, lung sliding, B lines lung point and lung pulse will be looked for. Once those artifacts are identified or ruled out, specific diagnosis can be ruled-in or ruled out. ASD, atrial septal defect; PFO, patent foramen ovale; VSD, ventricular septal defect. For more detail, the reader is referred to Piette et al.36 and Denault et al.11
The first step in managing a patient with reduced urine output or elevated creatinine is to rule-out a post-renal cause. Examination of the bladder and the kidney using US is mandatory before any intervention. Obstructed Fowley catheter particularly in patients with traumatic or post-op hematuria is very common. Hydronephrosis is also important to rule-out. Once this initial step taken, then volume status is evaluated as described before in Fig. 1. There is a significant interest recently in the bedside US identification of renal congestion as a cause of renal failure. A recent study in patients with heart failure has identified 3 patterns of venous congestion which are similar to those described previously with HVF.13 In our practice we include bladder and renal 2D and Doppler examination in the routine evaluations of patients with heart failure.
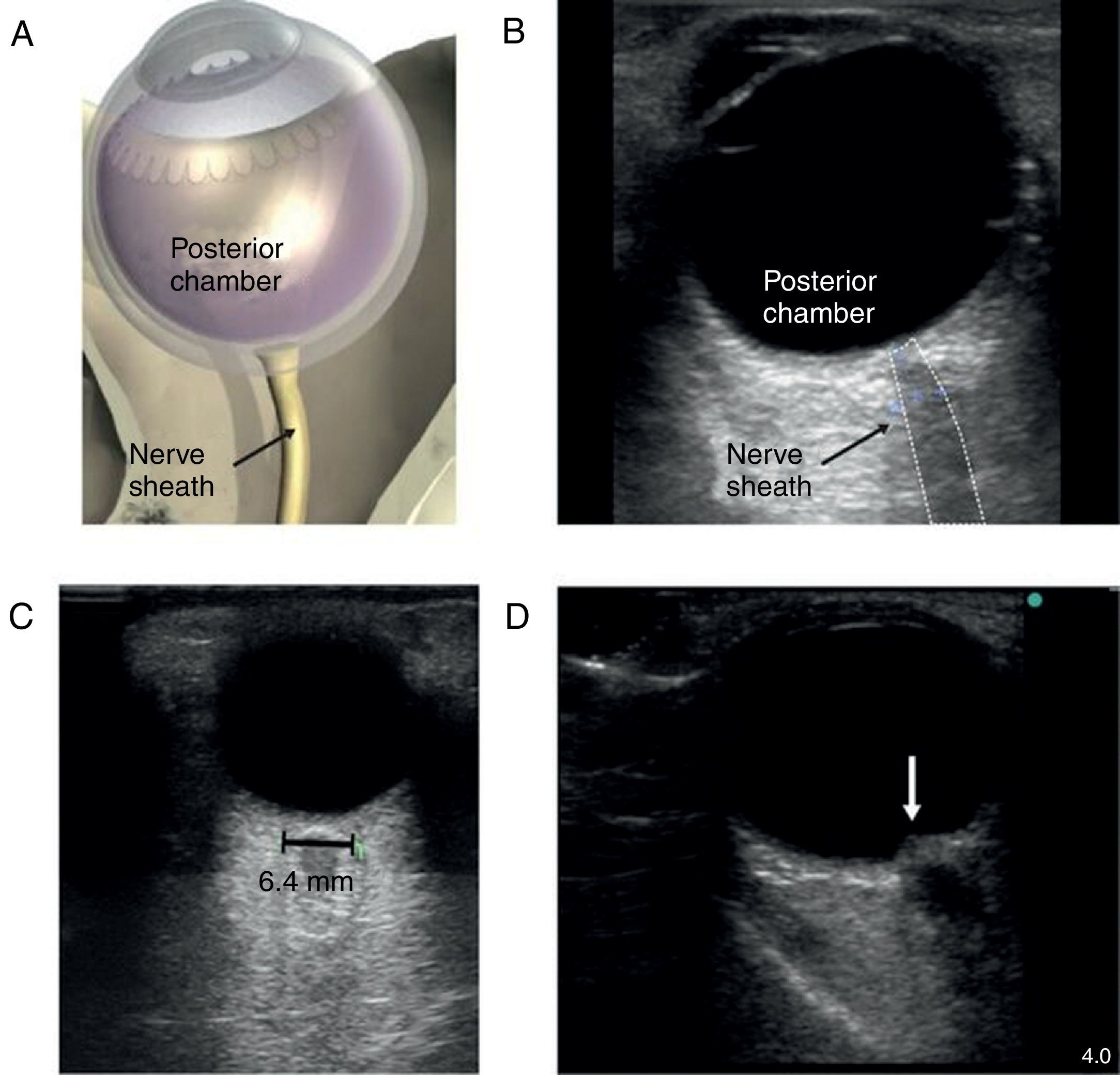
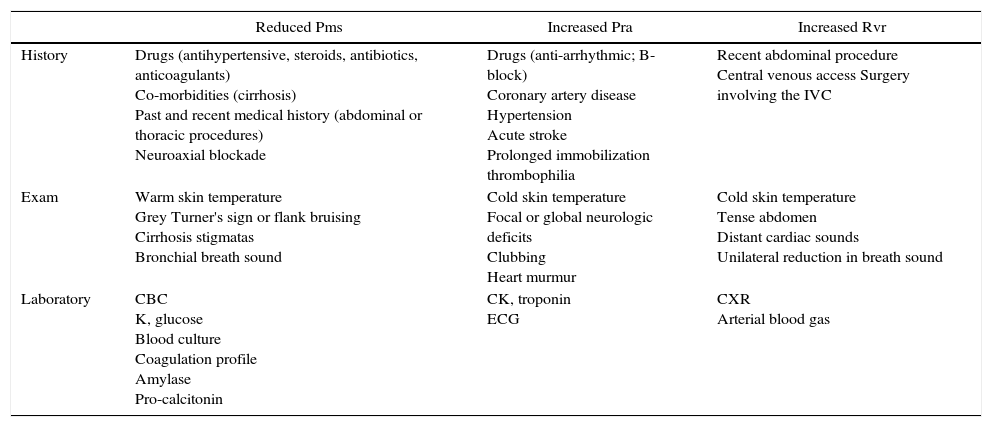
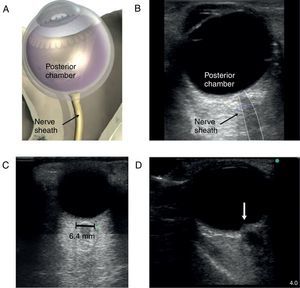
The patient with altered neurological statusBedside ultrasound can be useful in determining the presence of intracranial hypertension. This can be done with the use of transcranial Doppler (TCD). As intracranial pressure increase, the diastolic velocity will be reduced and absent when the intracranial pressure reaches the arterial diastolic pressure. However, TCD is relatively operator dependant and requires training. A much simpler approach is the use of optic nerve sheath (ONS) measurement.37 This is a very simple technique with rapid learning curve. The technique is presented in Fig. 5. The ONS diameter threshold of 5.2–5.9mm has been used to detect increased ICP. The diagnostic accuracy for the detection of intracranial hypertension has been assessed in multiples observational studies showing good diagnostic accuracy compared with invasive monitoring.38
Optic nerve sheath measurement. (A) The site for measuring the diameter of the optic nerve sheath is shown. A 3mm perpendicular line is drawn from the middle of the optic nerve, at which point the transverse measurement of the optic sheath is performed. Note that the measurement includes the sheath and stops at the transition contrast between the optic nerve and surrounding tissue. (B) A 2D image displays the ocular structures and optic nerve sheath. (C) Ultrasound of the eye orbit from an 85-year-old patient after aortic valve replacement with significant post-operative fluid balance. The optic nerve diameter was 6.4mm (normal 3.5–4.5mm). (D) Optic nerve 2D images of papilledema (arrow) in a brain dead patient for organ donation.
It is important however to realize that US does not replace clinical judgment and has to be used as a complementary tool to what is currently accepted as a standard of care. In clinical medicine, US should be performed following history taking and physical examination. Furthermore, US is a poor monitor as it was mentioned before the term monitor comes from the Latin word “monere” meaning warning. Alarms are not part of any US equipment. Nevertheless, ultrasound is the ideal complement of our monitoring devices. When for instance blood pressure falls, pulse oximetry or NIRS is reduced, there is a wide differential diagnosis. Ultrasound can narrow it rapidly, excluding within seconds life-threatening conditions. The approach we proposed have been published in peer-reviewed journal,9,10,16,36 PhD thesis39 and in textbook.11 The importance and relevance of interrogating venous flow has recently been the subject of an editorial12 and several recent articles.13,23,25,28–30 However, to use bedside ultrasound successfully, adequate training is required and essential.
Ultrasound is now incorporated in the curriculum of several medical schools. At the University of Montreal and University of Western Ontario, it is now part of the training program in critical care and anesthesiology. The objectives are based on current Canadian and American guidelines.40,41 Finally, does bedside US change outcome? The authors of these papers do not think that bedside US changes outcome. So far no technique or even monitor have been shown to change outcome yet, is the competence of the individual in bedside US and its ability to recognize the “warning sign” on the monitor that has been shown to change management. This impact of bedside US is well summarized by Royse et al.42
The approach that we are proposing has not been formally validated in clinical trials. However there are now four clinical trials demonstrating the superiority of bedside ultrasound over conventional assessment in shock state43,44 and respiratory distress45 Zanobeth. A Canadian study performed in 220 critically ill patients has shown that bedside ultrasound performed by competent clinicians and limited only to cardiac and volume assessment was associated with improve outcome and reduction in 28 day mortality.44 A study performed in the United States in 240 trauma patients showed also reduced mortality particularity in neurotrauma patients managed using bedside ultrasound.43 A study from Finland in 320 patients with respiratory symptoms admitted in the emergency room demonstrated that bedside ultrasound was superior than standard diagnostic test alone in establishing a correct diagnosis. Those findings were reconfirmed by an Italian study on 2683 patients.46 Further studies incorporating head-to-toe bedside ultrasound by trained clinicians are likely to demonstrate the significant advantages of incorporating bedside ultrasound in the practice of medicine. Finally, despite all efforts, in some patients, adequate acoustic window will not be obtainable. In such patients, transesophageal echocardiography will be required to rule in or out conditions which could not be approach by surface ultrasound. Basic transesophageal ultrasound is now part of training in several anesthesia program. Guidelines for training have been published47 and a certification examination by the National Board of Echocardiography has been in place now for non-cardiac anesthesiologists and critical care physician.
ConclusionThere is growing interest and evidence for the significant role of bedside ultrasound in managing the hemodynamically unstable, hypoxemic, oligo-anuric patient and the patient with altered neurologic status. Approaches using bedside ultrasound have being developed in order to integrate this important diagnostic modality as a complement to our current approach.
FundingSupported by the Richard I. Kaufman Endowment Fund in Anesthesia and Critical Care and the Montreal Heart Institute Foundation.
Conflicts of interestDr. Denault is a Consultant for CAE Healthcare and on the speaker bureau for Medtronic, Masimo and Edwards.
Please cite this article as: Denault A, Casas C, Puentes W, Eljaiek R, Iglesias I. Ultrasonido de la cabeza a los pies: opinión actual sobre su utilidad en inestabilidad hemodinámica, hipoxemia, oligoanuria y en el paciente con estado neurológico alterado. Rev Colomb Anestesiol. 2017;45:317–326.