Several analgesic modalities – pharmacological and non-pharmacological – may be used during the cesarean section postoperative period. This document focuses on the different pharmacological strategies available.
ObjectivesTo establish the advantages and disadvantages of the various pharmacological options used to control pain following a C-section, improving safety and patient satisfaction.
MethodsA search was done in Medline, Embase, Lilacs, and The Cochrane Library using the terms “Cesarean section”, “Cesarean pain”, “Maternal risk”, and “Analgesia for cesarean”, reviewing articles published in both English and Spanish during the last twenty years. Duplicated articles, redundant or irrelevant content, and articles with methodological flaws were excluded.
ResultsNeuraxial opioids are widely used in postoperative cesarean section analgesia. However, they have to administered at low doses to ensure the best risk-benefit profile. The use of systemic opioids is also appropriate in these patients, reducing the occurrence of some adverse events associated with intrathecal administration. Multimodal analgesia has proven its effectiveness in postoperative pain control after cesarean delivery, significantly reducing the use of opioids and their associated adverse effects.
ConclusionsNotwithstanding the adverse effects described in the literature, the cornerstone of analgesia therapy after cesarean section are opioids, both neuraxial or parenteral administration. Multimodal management using NSAIDs or paracetamol, improves the safety profile and the quality of analgesia, reducing the opioid requirements.
Durante el postoperatorio de cesárea se pueden utilizar diversas modalidades analgésicas, tanto farmacológicas como no farmacológicas. Este documento se centra en las diferentes estrategias farmacológicas disponibles.
ObjetivosEstablecer las ventajas y desventajas de las diferentes opciones farmacológicas usadas después de la cesárea para el control del dolor, mejorando la seguridad y satisfacción de las pacientes.
MétodosSe realizó una búsqueda en Medline, Embase, Lilacs y The Cochrane Library con los términos “Cesarean section”, “Cesarean pain”, “Maternal risk” y “Analgesia for cesarean”. Se revisaron artículos publicados en inglés y español en los últimos veinte años. Se excluyeron artículos duplicados, con contenido redundante o impertinente, y aquellos con defectos metodológicos.
ResultadosLos opioides neuroaxiales son ampliamente utilizados para la analgesia postoperatoria en cesárea; sin embargo, deben usarse a dosis bajas para obtener el mejor perfil riesgo-beneficio. El uso de opioides sistémicos también es válido en estos pacientes, reduciendo la ocurrencia de algunos efectos adversos asociados a la administración intratecal. La analgesia multimodal ha demostrado ser efectiva para el control del dolor postoperatorio de cesárea, disminuyendo significativamente el consumo de opioides y los efectos adversos asociados.
ConclusionesEl pilar terapéutico analgésico en el postoperatorio de cesárea son los opioides, tanto en su administración neuroaxial como por vía parenteral, a pesar de los efectos adversos descritos en la literatura. El manejo multimodal con AINEs o acetaminofén mejora el perfil de seguridad y la calidad de la analgesia, disminuyendo el requerimiento de opioides.
The World Health Organization (WHO) suggests and that the ideal C-section rate should be less than 15%1; however, the number of cesarean sections has been recently increasing and is now the most frequent abdominal surgery performed in the United States.2,3 In 2008 the rate if C-sections in the US exceeded 32%, and in Colombia increased from 24.9% in 1988 to 45.7% in 2013.4,5 In Latin America and the Caribbean the rates experienced a significant increase from 1990 to 2014 and remained above the level recorded in any other region.6 In Brazil, in the private clinics, the rate of cesarean deliveries may reach 80–90%.7
Postoperative pain relief following a cesarean section is extremely important to optimize maternal and neonate wellbeing.8 The following review presents the current evidence with regards to the tools available for a pharmacological analgesic approach during the cesarean section postoperative period.
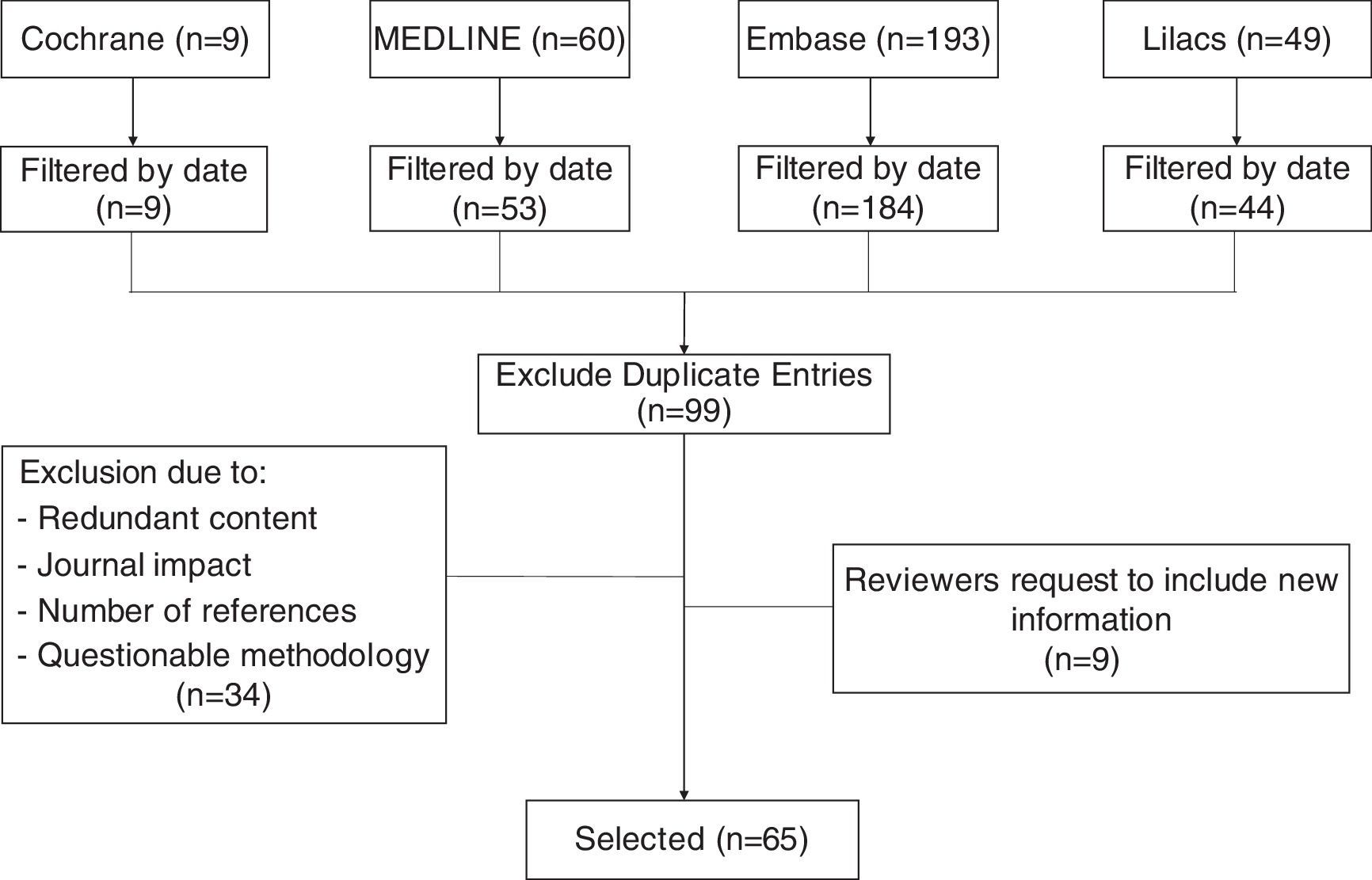
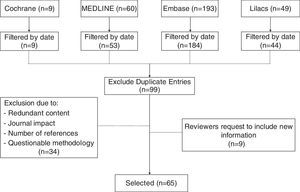
MethodsThis non-systematic review searched databases including Medline via PubMed, The Cochrane Library, Embase, and Lilacs using the terms “Cesarean section”, “Cesarean pain”, “Maternal risk”, and “Analgesia for cesarean”. Clinical trials, cohort studies, cases and controls, in addition to integrative documents published in English and Spanish in the last 15 years were reviewed. Duplicate articles, articles with a primary approach to the management of analgesia during the pre and post-operative period, and articles with significant methodological flaws (Fig. 1), were excluded. The review discusses pharmacological strategies with intrathecal and systemic opioids, as well as multimodal analgesia, describing the advantages and disadvantages of each option.
Neuraxial opioidsThe use of neuraxial anesthetic techniques has been associated with a decrease in anesthesia-associated maternal mortality.9 However, the use of intrathecal morphine may result in side effects such as nausea, vomiting, pruritus, sedation, and respiratory depression.10 The guidelines of obstetric anesthesia of the American Society of Anesthesia (ASA) recommend considering the use of neuraxial opioids instead of intermittent parenteral boluses, with or without salvage doses.11 Although neuraxial administration is not exempt from the occurrence of side affects, these are usually mild and self-limiting, considering that the benefits of analgesia and anesthesia through this route of administration outweigh the risks.12,13 Carvalho et al. recommend that the initial management for moderate pain be based on oral opioids (oxycodone, hydrocodone, and tramadol) and to reserve the use of IV opioids only for severe pain cases or for patients who are intolerant to the oral route.14 Although IV opioids are no better in controlling pain than the oral opioids, they do have a higher incidence of adverse effects.14,15
The evidence about the relationship between intrathecal morphine and the analgesic effect is contradictory, since the dose needed to provide optimal postoperative analgesia following C-section, with the lowest possible incidence of side effects, is yet to be established.16 Wong et al., showed that 200μg of intrathecal morphine provide better postoperative analgesia than 100μg. However, the patients that received the higher dose experienced a higher incidence of nausea.17
Sultan et al. showed that in patients undergoing cesarean section under spinal anesthesia with low (50–100μg) and high (>100–250μg) doses of morphine, the higher dose extended the time of analgesia after the procedure, versus the low dose, with no significant differences in the neonatal outcomes as measured with Apgar or in the incidence of maternal nausea and vomiting.18
Another trial suggested that a 50μg dose of intrathecal morphine provided the same quality of analgesia as a 100μg, but with lower incidence of side effects. Furthermore, it was suggested that all patients undergoing cesarean section should have access to supplementary systemic analgesia, since a considerable number of patients reported moderate to severe pain, regardless of the dose of morphine used.19
Side effects of neuraxial opioidsWhilst intrathecal morphine is considered the “Gold Standard”20 among neuroaxial opioids, numerous dose-associated adverse effects are still reported.
One of the most severe adverse events associated with the use of neuraxial opioids is respiratory depression. Obstetric patients with high BMIs, prior use of opioids, magnesium sulfate infusion, and respiratory comorbidities are at higher risk of developing respiratory depression under these conditions. Likewise, high progesterone concentrations during pregnancy offer some protection against respiratory depression, since progesterone is a respiratory stimulant.21 ASA published an update on the approach to neuraxial opioid-associated respiratory depression22:
- 1.
Monitor patients ensuring adequate ventilation, oxygenation, and awareness.
- 2.
Ensure additional monitoring to patients at higher risk of respiratory depression (unstable medical condition, obesity, obstructive sleep apnea, concomitant use of opioid or hypnotic analgesics via other routes, extreme ages).
- 3.
Fentanyl:
- a.
Monitor for at least 2h following the administration
- b.
Continuous monitoring during the first 20min and then at least once every hour for 2h.
- c.
After 2h, the monitoring frequency depends on the patient's clinical condition and any additional medicines administered.
- a.
- 4.
Morphine:
- a.
Monitor for a minimum of 24h following administration.
- b.
Monitor at least once every hour for the first 12h, then at least once every 2h for the following 12h.
- c.
After 24h, the monitoring frequency depends on the patient's clinical condition and any additional medicines administered.
In case of respiratory depression, the following is recommended:
- 1.
Administer supplementary oxygen if the state of consciousness is altered, the respiratory rate is below 10/min, or the oxygen saturation is below 90%. Continue until the patient is alert, with no signs of respiratory depression or hypoxemia.
- 2.
Maintain the venous accesses.
- 3.
Have reversal agents immediately available.
- 4.
Consider positive pressure non-invasive ventilation.
- a.
In contrast to other analgesic options such as systemic opioids, neuraxial administration opioids have a higher incidence of adverse effects. With regards to postoperative nausea, the risk of presenting adverse events is significantly higher when using neuraxial opioids (RR=1.95 95% CI, 1.17–3.26; P=0.01), and the same goes for the risk of pruritus (RR, 2.71; 95% CI, 2.05–3.58; P<0.00001).23
Neuraxial administration of non-opioid analgesicsThe use of neuraxial adjuvant non-opioid medications has been a topic of great interest when reducing the dose of the opioid, and hence, its side effects. It has been demonstrated that the analgesic effect thereof may be extended. However, the associated side effects are significant, which limits their routine use.24 Clonidine is associated with considerable sedation and hemodynamic lability,25 while neostigmine produces severe nausea and vomiting when administered intrathecally. In the case of epidural administration, in addition to reducing the local anesthetic dose required, pruritus diminishes and there is no significant increase in the risk of hypotension, sedation or adverse fetal effects.26
Khezri et al. observed that the administration of Ketamine in combination with bupivacaine at a dose of 0.1mg/kg in elective cesarean section delayed the request for analgesics and reduced total use over the first 24h.27
Intrathecal administration of dexmedetomidine extended the duration of the motor and sensitive block, reducing the analgesic requirements.28 Dexmedetomidine showed superiority versus fentanyl, since the former facilitates propagation and extends the duration of analgesia, with lower incidence of nausea and vomiting.29,30 Magdy et al. studied its effect in elective cesarean section and described adequate postoperative analgesia without any maternal or neonate adverse effects.31,32 The recommended dose is between 5 and 10 mcg, since doses above 15 mcg are associated with hypotension and bradycardia.28,33,34
Gabapentin reduces the adverse effects of opioids such as vomiting and pruritus, but at the expense of more sedation and potential breast milk transfer. This is why its use is only recommended as the last line of therapy or in patients experiencing chronic pain.14
Systemic opioidPatient Controlled Analgesia (PCA) is a system for self administration of the medication, bypassing the patient-nurse-injection loop, saving valuable time in acute pain control and reducing the peaks and troughs of plasma drug concentrations, achieving enhanced patient satisfaction.35 However, the level of satisfaction seems to be better with PCA.36
ASA recommends the use of neuraxial opioids instead of intermittent parenteral boluses for postoperative analgesia in cesarean section, but its routine use is frequently limited because of shortage of staff to monitor any potential side effects.37
Epidural analgesia provides better pain control than PCA, but is more expensive and hence the latter is considered the most cost-effective option.38 Patel et al. showed a cost reduction against PCA using neuraxial opioids, with higher cost-effectiveness associated with the use of intrathecal morphine.39
In terms of the adverse events of opioids using PCA, apparently the most common are excessive sedation – which is usually transient and at the onset of labor, and mild desaturation episodes that resolve on their own in patients who did not receive supplemental oxygen. Though these effects do occur, they usually do not interfere with labor or maternal satisfaction.40,41
There is little information regarding the safety profile of oxycodone during lactation, although it is commonly used in this context.15 Lam et al. concluded that oxycodone does not offer additional safety as compared against codeine.42 Seaton et al. studied the oral administration of oxycodone, obtaining adequate pain control with a low risk for lactating infants. However, this trial was limited to 72h after delivery.43 The use of codeine is not recommended, because the metabolic and pharmacogenomics variability may impact the drug efficacy and the neonatal side effects.44
Edwards et al. showed the benefits of hydromorphone, which is seven fold more potent than morphine and may be administered through multiple routes. Its use has not been studied at length following cesarean section, but apparently low doses do not affect the infant. Although the passage of hydromorphone into maternal milk is less than other narcotics, caution is advised.45,46
Morphine is the opioid that passes into breast milk in larger volumes, reaching relative doses that may be harmful for the newborn. Oxycodone is second, with doses close to harmful levels. Fentanyl is the agent with the lowest rates of passage. Furthermore, during the first few days post-partum, the amount of colostrum that the mother produces is very small, thus limiting the potential passage of opioids into the neonate.14 Although adverse neonatal events secondary to opiate use are rare, Hendrickson et al. recommend using them rationally during lactation, administering the safest possible drug.47 Anderson et al. conclude that although opioids are among the drugs with the highest rates of adverse events for the lactating infant (25%), if used at low doses, for short time periods, and avoiding combinations with other central nervous system depressants, adverse events are unlikely to occur.48 WHO recommends using low doses when administering morphine and codeine, avoiding repeated administrations and monitoring for any side effects in the infant (apnea, bradycardia, and cyanosis), and removing them as soon as any side effects arise.49
Other systemic analgesicsNSAIDs used for multimodal analgesia reduce the intake of opioids and their associated side effects; in some cases, it has been shown that NSAIDs lower the pain score in the visual analogue scale (VAS).50,51
Berger et al. compared three doses of intrathecal morphine (50μg–100μg–150μg) combined with ketorolac per schedule, to determine the dose-response ratio, the analgesic efficacy, and side effects. There were no differences in the use of morphine over the first 24h or in the pain and nausea outcomes. There was a higher incidence of pruritus among the high-dose groups as compared against the 50μg dose. There were no significant respiratory depression or sedation. The results suggest that 50μg of intrathecal morphine produces a similar level of analgesia as that produced by 100μg or 150μg doses when used concomitantly with IV ketorolac per schedule.52
Boskurt et al. compared diclofenac and meperidine for postoperative cesarean section analgesia. The patients receiving diclofenac only did not experience adequate pain control. However, when combined with meperidin, the VAS pain score was similar to the group treated with higher doses of meperidine only, showing the efficacy of diclofenac as part of a multimodal analgesia regime.53
Hyllested et al. described the use of acetaminophen in high risk patients, due to its low incidence of adverse effects.54 The combined use of acetaminophen with diclofenac resulted in a 38% reduction in the use of morphine, as compared against patients receiving acetaminophen only.55 In another trial, the administration of acetaminophen per schedule, resulted in a decrease in the use of opioids with PCA.56 Ozmete et al. showed that 1g of preoperative acetaminophen was effective to reduce pain and lower the opioid requirements.57
Multimodal analgesia is beneficial for the infant, since both acetaminophen and ibuprofen are considered safe and compatible with breast feeding; by reducing the opioid requirements, the adverse effects resulting from passage into breast milk decrease.42,43,58,59
Fosbøl et al. found increased cardiovascular-associated morbidity and mortality with the use of NSAIDs, particularly diclofenac and rofecoxib.60 Fernández-Liz et al. suggest using low-dose naproxene and ibuprofen (p to 1.200mg/day) as the safest alternative.61 Olsen et al. described a statistically significant increase in mortality with the use of all NSAIDs, regardless of treatment duration. A follow-up study of this cohort described that the cardiovascular risk continuous to be high when reintroducing NSAIDs management, even after 5 years of experiencing a cardiovascular event.62 Several meta-analyses concluded that naproxen is the safest, even at high doses.63,64 It should be said that the risk is not limited to patients with a history of cardiovascular disease, since previously healthy individuals may be affected by the indiscriminate use of NSAIDs.65
Selective NSAIDs, in particular parecoxib, are useful in pain management, resulting in less GI ulcers than the non-selective agents. Neither do these affect platelet aggregation, reducing post-op bleeding. NSAIDs do not induce bronchospasm in sensitive patients, nor do they rise the incidence of thromboembolic events. However, there are no differences in terms of renal adverse events.66 Inthigood et al. studied the effects of parecoxib as a supplement to the neuroaxial opioid and found that the group receiving parecoxib had lower pain scores, with no difference in the requirement of postoperative opioids.67 Passage into maternal milk of the active metabolite of parecoxib is negligible, with a remote probability of affecting the infant.68
The American Society of Pain developed management guidelines for postoperative pain, emphasizing the importance of using IV ketamine for multimodal analgesic management during the most frequently performed surgeries. However, there is not enough evidence on the use of ketamine in postoperative cesarean section management.69 Laskowski et al. described a reduced use of opioids and longer times in between breakthrough doses during chest, upper abdomen, and orthopedic surgeries.70 However, Han et al. did not find a reduced use of opioids when using IV ketamine as adjuvant to the use of fentanyl PCA.71 Senepathi et al. found no significant differences in emergency cesarean section patients in terms of the pain VAS score, but did observe that the use of low-dose IV ketamine, prior to the administration of spinal anesthesia, reduced the inflammatory response to surgical stress, as evidenced by the C-reactive protein levels.72 Rahmanian et al. described reduced pain, less use of opioids, and fewer adverse effects when using low-dose ketamine (0.2mg/kg).73 It should be noted that the newborn Apgar score is not affected by the use of low-dose ketamine.72
A systematic Cochrane review on oral analgesia over the cesarean section postoperative period concluded that the available studies were scarce and with a limited population; thus it was impossible to determine the most effective oral analgesic agent to relief pain, with the lowest incidence of adverse effects. Studies fail to consistently report neonatal findings, length of hospital stay, or treatment costs, so no conclusions can be made in this regard.74
ConclusionsThere are multiple modalities for postoperative cesarean delivery pain management. The cornerstone of therapy are opioids. The frequency of adverse events following opioid administration, and the severity of these events, requires that any patient receiving opioid analgesic management be adequately monitored with easy access to oxygen and reversal medications.22 In the absence of appropriate means to safely administer neuraxial opioids, parenteral administration is possible, achieving adequate pain control and patient satisfaction.36–38
The current recommendations include multimodal therapy that improves the quality of analgesia, reduces the opioid requirements, reduces any adverse effects, and enhances maternal and neonatal safety.51,52,55–57
FundingThis review was financed with resources of the Department of Anesthesia of the Fundación Santa Fe de Bogotá.
Conflicts of interestThe authors have no conflicts of interest to disclose for the publication of this article.
Please cite this article as: Ramos-Rangel GE, Ferrer-Zaccaro LE, Mojica-Manrique VL, La Rotta MG. Manejo analgésico durante el postoperatorio de cesárea: estrategias farmacologicas. Rev Colomb Anestesiol. 2017;45:327–334.