Limb trauma and surgery are frequent causes of complex regional pain syndrome (CRPS). Some cases are very difficult to manage despite the use of high-dose analgesics, anti-inflammatory agents and physical therapy; hence the need to look for interventional therapies to slow its progression.
Case description and resultsWe present the case of a patient diagnosed with CRPS type I with severe trophic changes and marked functional limitation, managed with multiple pharmacological therapies and nerve blocks without apparent improvement. The patient decided to try neurostimulation with favourable results and substantial improvement months later reported as “100% improvement” of pain; reduced oedema; progressive recovery of the normal appearance of the hand; partial recovery of strength, and improved function attributable to the use of this additional therapy.
ConclusionNeurostimulation has a noticeable impact on the course of the complex regional pain syndrome accompanied by trophic changes in patients with a poor response to the recommended pharmacological management. Early initiation of this intervention facilitates functional and psychological recovery of the patients, who are usually in the productive stage of their lives.
Causas frecuentes de síndrome doloroso regional complejo (SDRC) son el trauma y la cirugía en extremidades. Algunos casos resultan de muy difícil manejo a pesar de la utilización de altas dosis de analgésicos, antiinflamatorios y terapia física lo cual motiva la búsqueda de terapias intervencionistas que frenen su progresión.
Descripción del caso y resultadosPresentamos el caso de un paciente a quien se le diagnosticó SDRC tipo i con cambios tróficos severos y marcada limitación funcional, que fue manejado con múltiples terapias farmacológicas y bloqueos nerviosos sin mejoría evidente, a quien posteriormente se decidió realizar una prueba con neuroestimulación, la cual fue favorable. Meses después se obtuvo una mejoría sustancial ya que presentó mejoría del dolor «de un100%»; disminución del edema y retorno progresivo al aspecto normal de la mano, recuperación parcial de la fuerza y disminución de su limitación funcional, explicable por la adición de esta terapia de manera definitiva.
ConclusiónLa neuroestimulación ejerce un impacto notable sobre la evolución del SDRC con cambios tróficos en pacientes con pobre respuesta al manejo farmacológico indicado. El inicio temprano de esta intervención facilita la recuperación funcional y psicológica al paciente que, usualmente, se encuentra en una etapa productiva de su vida.
The Complex Regional Pain Syndrome (CRPS) is a chronic progressive disease characterized by intense pain, oedema and trophic skin changes occurring more frequently after limb trauma or surgery. It may be classified as type I (with no evidence of nerve injury) and type 2 (with proven nerve injury),1 and the reported incidence ranges from 5.6 to 26.2 cases for every 100,000 inhabitants.2,3 It is more frequent in females than in males with a 4:1 ratio4,5 and there is a high association with a triggering event such as trauma, bone fractures being the most frequently associated with it.6 The pathophysiology of CRPS is still not fully understood. There are multiple mechanisms that play an important role in its onset and maintenance.7 Several treatments have been proposed for pain relief, functional recovery and psychological improvement, but management is often difficult and the functionality of the affected limb may be compromised if not treated early on.8
Patient informationA 55-year-old male patient, street vendor, with no important medical history, who fell from a 1-m height and sustained trauma to the right hand 8 years before. He then developed oedema and pain in the limb. When seen by the orthopaedic service, he was diagnosed with a “sprained wrist” and was ordered splinting, anti-inflammatory drugs and physical therapy, with no improvement. The patient came back, complaining of persistent pain and progressive oedema that required splint removal.
Clinical findingsThe patient reported hyperesthesia and persistent hyperalgesia in dermatomes C5–C8, 5/10 on the visual analogue scale, increased oedema involving the hand, the forearm and the arm, and functional limitation of the distal portion of the right upper limb, with discoloration of the surrounding skin. These findings led to the diagnosis of CRPS type I and treatment was initiated with amitriptyline 35mg/night, carbamazepine 800mg/day, acetaminophen 1g every 6h, and tramadol 4 capsules/day without significant improvement. The patient was then lost to follow-up.
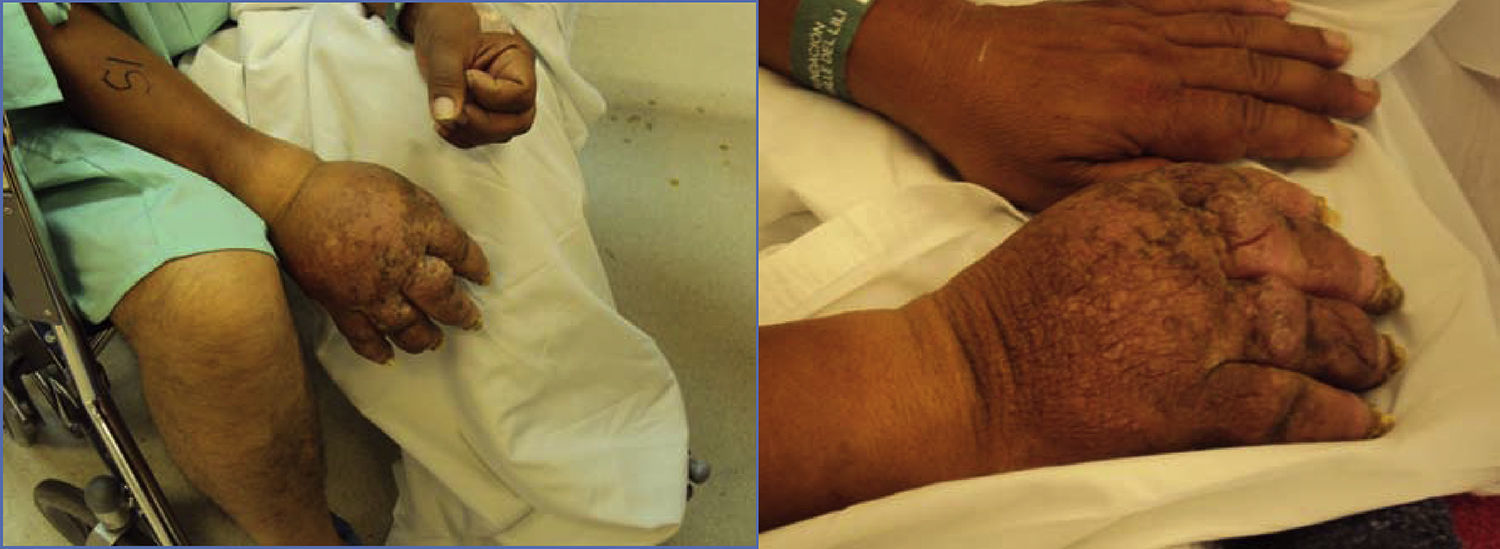
The patient returned three years later with worsened symptoms after sustaining new trauma. The patient showed nail atrophy, absence of hair (Fig. 1), diaphoresis in the involved area, and marked allodynia besides the symptoms described above.
Therapeutic interventionFor this relapse, the regimen selected was prednisolone for 10 days, hydrocodone 10mg every 8h, and a cervical sympathetic block (CSB). Two ipsilateral cervical sympathetic blocks were performed and the patient continued on oral medication, with a 5% subjective improvement. However, the patient recurred after some months and, at this point, it was decided to try neurostimulation and continue the management only with hydrocodone, gabapentin and acetaminophen.
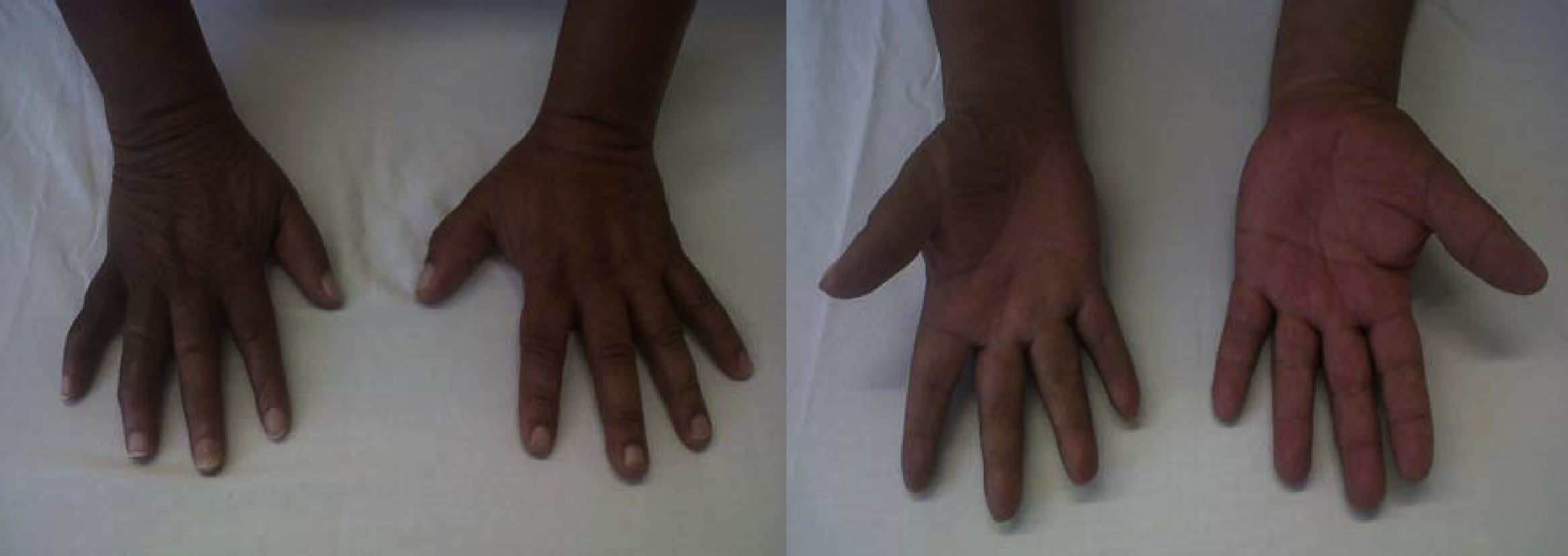
The neurostimulation (NS) trial was performed with a 100% improvement and marked oedema reduction (Fig. 2.)
Results and follow-upAfter completing the trial, the patient again reported a pain score of 10/10 and all the symptoms returned. This finding led to the prescription of definitive implantation of the neurostimulator.
After one month of definitive NS implantation, the patient reported “100% improvement of pain”. There was marked oedema reduction and progressive return to the normal appearance of the hand and reduced functional impairment. With physical therapy, the patient was able to take up work slowly after one year and the affected area acquired almost normal characteristics, with only mild residual limitation of finger flexion (Fig. 2).
DiscussionSeveral theories have been proposed to explain the cellular and biochemical events that might give rise to CRPS. These include peripheral mechanisms such as axonal and tissue hypoxia secondary to vasoconstriction associated with a nitric oxide synthesis imbalance and increased endothelin-1 and pro-inflammatory interleukin (IL6) production.9,10 The diagnosis of CRPS is based on clinical signs and symptoms found in the initial assessment. Since 1994, the International Association for the Study of Pain (IASP)11 has proposed diagnostic criteria to provide very simple and accurate parameters that guide clinicians in the diagnosis of this disorder. More recently, Harden12 proposed some diagnostic criteria based specifically on the symptomatology of this complex syndrome.
CRPS shares treatment and management similarities with neuropathic pain. The usual treatments are tricyclic antidepressants, serotonin and noradrenalin reuptake inhibitors, first and second generation anticonvulsants and, occasionally, muscle relaxants.13,14 This was the first line of treatment used in our patient, with a very poor response. Opioids may play a limited role in the management of the intractable pain, which is characteristic of this disorder. Concomitant management with steroids may be effective given their anti-inflammatory effect, particularly during the early phase. Our patient received opioids initially with no improvement, and steroids were given in a later stage, which might explain the therapeutic failure of medication use. Interventional treatments are usually indicated when conventional therapy fails to control pain (persistence of pain >4/10 on the visual analogue scale) and the associated symptoms.15 It has been suggested that limb immobilization may be associated with the onset of CRPS as happened in this case, since it increases pressure, and that early complaints of compression are predictive factors for CRPS.
The initial work on electrical stimulation for the management of CRPS was based on the gating theory described by Melzak and Wall,15,16 which suggests that intermittent stimulation of A-alpha and beta fibres localized in the spinal dorsal columns would break the vicious circle of central retransmission from peripheral nociceptors in C fibres. To this date, there is no strong evidence regarding the usefulness of spinal NS for the management of CRPS. Considering that some of the NS procedures are relatively new, there is still little evidence in relation to their use and cost- effectiveness.17 Our patient's symptoms are consistent with the classical CRPS type 1 presentation where the management with multiple drugs and physical therapy did not lead to significant improvement, prompting the use of CSB with partial improvement. The decision to use a NS in this case was based on the transient success with this intervention followed by relapse.17,18 There are case reports that may support the use of this interventional treatment when medical therapies fail.
Several sympathetic, regional intravenous and epidural blocks may be given on an outpatient basis. However, responses to sympathetic blocks vary and they appear to be more effective than placebo in terms of duration but not so of the degree of pain relief.19 NS has been considered the definitive treatment of CRPS both through spinal stimulation for CRPS type I as well as through peripheral nerve stimulation for CRPS type II.20 Forouzanfar studied the long-term effects of cervical and lumbar NS in patients with CRPS I and found that pain intensity was reduced after six months, one and two years after the implantation.21 Kemler conducted a two-year study to assess the impact of NS on CRPS and found pain reduction and improved quality of life.22 Other favourable outcomes have been observed with the use of NS in CRPS including the absence of hyperpathia, normalized temperature sustainability, improved functionality when combine with physical therapy, and a noticeable reduction in the use of analgesics.23,24 Our patient showed improvement of all his symptoms after the interventional procedure, with just a mild reduction of finger mobility on flexion and extension.
In conclusion, we suggest that NS may have a noticeable impact on the course of CRPS in patients with recurrent symptoms despite medical management and even despite the use of classical interventional strategies. The rapid onset and development of associated trophic changes could be an indication to intervene early on because of the functional and psychological repercussions for these patients who are usually in the productive stage of their lives. Our patient improved of his symptoms thanks to his therapeutic management, and was able to return to work. He still attends the pain clinic and gave his consent to the publication of his case.
FundingNone.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Villegas Pineda MH, Herrera C, Martínez TL, Fernández VO. Impacto del manejo con neuroestimulación en un paciente con síndrome doloroso complejo y cambios tróficos severos. Reporte de caso. Rev Colomb Anestesiol. 2014;42:321–324.