The intracranial hypotension syndrome (IHS) is a disorder caused by brain descent due to a CSF leak resulting from diagnostic, therapeutic or spontaneous lesions. The pathophysiology, the clinical and the therapeutic approach are similar as in post dural puncture headache, the latter being considered a mild form of IHS. This paper describes two patients with orthostatic headache and severe neurological involvement after epidural and spinal anesthesia, diagnosed and treated as post dural puncture headache, but who required additional care because of their abnormal course. IHS is a serious complication that may result in clinical decline and death; consequently, it requires a comprehensive approach to the various triggering factors, the clinical picture, diagnostic methods, pathophysiology and management.
El síndrome de hipotensión endocraneana (SHE) es una patología causada por el descenso del cerebro debido a fuga de líquido cefalorraquídeo a partir de lesiones durales diagnósticas, terapéuticas o espontáneas. Tanto la fisiopatología como el enfoque clínico y terapéutico son similares a la cefalea pospunción dural, siendo esta ultima considerada como una forma leve del SHE. Se describen 2 pacientes con cefalea ortostática y alteraciones neurológicas severas luego de anestesia epidural y espinal que fueron diagnosticados y tratados como cefalea pospunción dural, pero que por su evolución anormal debieron recibir atención adicional. El SHE constituye una complicación seria que puede llevar al deterioro clínico y a lamuerte, motivo por el cual requiere de un abordaje integral sobre sus factores desencadenantes, cuadro clínico, métodos diagnósticos, fisiopatología y manejo.
The first case was a 24-year-old female with mammary hypoplasia, ASA I, who underwent augmentation mammoplasty under epidural anesthesia and sedation. An epidural catheter was placed at the level of T3–T4, using an 18-gauge needle. Multiple attempts were required for its placement, but without evident dural lesion; the test with 3ml of 0.5% bupivacaine plus epinephrine 1:200,000 was negative for subarachnoidal and intravenous administration; the anesthetic mix was then administered. The procedure was completed with no complications and the patient then continued under ambulatory management.
Three days after surgery, the patient presented with dizziness, vomiting, orthostatic headache and pre-syncopal episodes. Management was started with an NSAID and acetaminophen plus codeine, bed rest, oral and intravenous hydration. On the following day, she presented with horizontal nystagmus and diplopia, and was treated with a blood patch.
On day five, the clinical picture still persisted and a space-occupying lesion was suspected. A gadolinium-enhanced magnetic resonance imaging (MRI) of the brain was normal. Oral alprazolam and dimenhydrinate were added.
On the tenth day, symptoms improved, although there was slight persistence of dizziness and vertigo on ambulation. Four weeks later, the patient went back to her normal activity, with no sequelae.
Case 2The second case was a 24-year-old male patient who underwent knee surgery under spinal anesthesia. Over the following days, there was gradual development of orthostatic headache and horizontal diplopia. A gadolinium-enhanced brain MRI revealed diffuse meningeal thickening, and outpatient management with analgesics was started.
The intensity of the headache increased and was associated with nausea and vomiting, which worsened with ambulation.
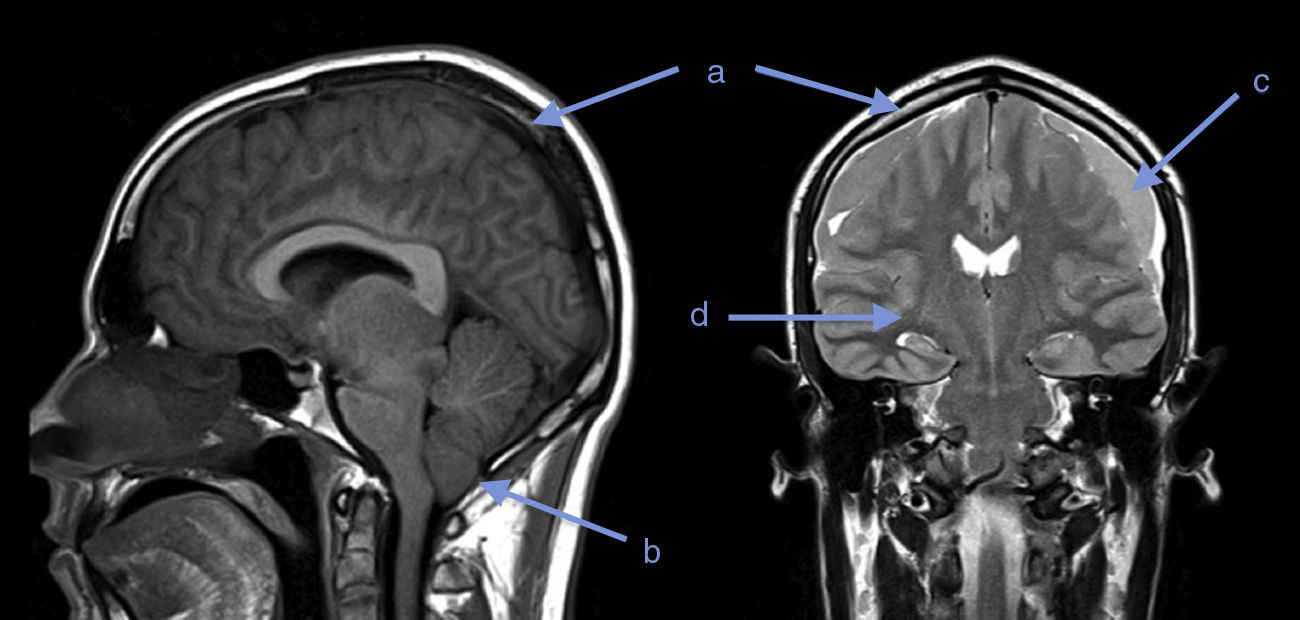
Twenty days later, the patient presented with somnolence, episodes of disorientation and sixth cranial nerve involvement. A new MRI revealed diffuse cerebral edema, herniation of cerebellar amygdalae, diffuse meningeal inflammation, bilateral subdural fronto-temporal collections, and a few signs of acute phase bleeding (Fig. 1).
The patient was taken to drainage of the subdural hematoma and postoperative management in the hospital for two days, with absolute bed rest and hydration that resulted in total resolution of the symptoms.
DiscussionBoth patients developed orthostatic headache and neurological changes that were initially evaluated and managed as post dural puncture headache of unusual presentation or additional complications. However, the clinical course of these patients has a more important background, where the IHS emerges as the most accurate diagnosis, as will be discussed below.
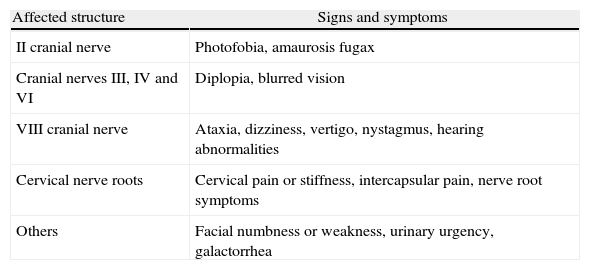
What are the signs and symptoms of IHS?The clinical picture is commonly characterized by orthostatic headache of variable characteristics and localization, which becomes exacerbated with coughing, jugular compression and Valsalva Maneuvers. Additionally, there may be involvement of cranial nerves II, III, IV, VI and VIII, and of the cervical nerve roots, always of orthostatic nature. In severe cases, it may be associated with deterioration of consciousness, and death (Table 1).1,2
Signs and symptoms depending on the affected structures.
| Affected structure | Signs and symptoms |
| II cranial nerve | Photofobia, amaurosis fugax |
| Cranial nerves III, IV and VI | Diplopia, blurred vision |
| VIII cranial nerve | Ataxia, dizziness, vertigo, nystagmus, hearing abnormalities |
| Cervical nerve roots | Cervical pain or stiffness, intercapsular pain, nerve root symptoms |
| Others | Facial numbness or weakness, urinary urgency, galactorrhea |
The diagnosis of this syndrome is mainly clinical and also includes recognition of the triggering factor. Diagnostic tools can be of use, including cerebral and spinal MRI. Routine lumbar puncture is not recommended because of the risk of greater neurological deterioration as a result of increased CSF deficit.3
What does diagnostic imaging show?Although gadolinium-enhanced brain MRI has not been validated as a diagnostic test in IHS, it may show diffuse dural thickening with gadolinium uptake, increase in blood volume, descent of the brain, subdural collections, subdural hematoma or cerebellar amygdalae herniation. There is a clinical correlation with these findings: they are more frequent and intense when the severity of the symptoms is greater, and they disappear as the symptoms improve.4,5
Gadolinium-enhanced spinal MRI may show extra-arachnoidal fluid collections, thickening of the spinal pachymeninges and epidural venous dilatations. On occasions, it picks up the CSF leak, and even if the latter is not active, it may show CSF accumulation.6
Brain MRI was performed in both our patients. In the first case it was normal, perhaps due to low sensitivity for diagnosing IHS; however, a spinal MRI could have helped to clarify the diagnosis or location of the potential leak.7 In the second case, the first MRI showed a finding suggestive of this syndrome. Twenty days later, as a result of worsening of the symptoms, a follow-up MRI showed evidence of progression of the imaging findings and onset of complications: cerebellar amygdalae herniation and subdural collections.
What are the causes of IHS?The most frequent cause is a persistent CSF leak, due to diagnostic or therapeutic spinal punctures. It has also been discussed following craniotomy, spinal surgery, cranio-spinal injury, or placement of a ventricular-peritoneal catheter. In some cases, it may appear spontaneously.8–10
In our two patients, there is a common history of the application of conductive anesthesia, and although in the first case there was no evidence of dural lesion, it cannot be actually ruled out.
What is the pathophysiology?Descent of the brain due to CSF leak is the corner stone of the pathophysiology and explains the orthostatic nature of the syndrome. Traction exerted on the meninges, the cerebral and cerebellar veins, the cranial nerves, and the cervical nerve roots, explains most of the symptoms. This may cause rupture of the cerebral veins, creating subdural hematomas, as was the case with our second patient.11–13
Dilatation of intra-cerebral vascular structures is another mechanism that may explain headaches due to changes in vascular tone, pituitary hyperemic galactorrhea and CSF leaks due to cell diapedesis and protein passage into the subarachnoidal space.13,14
Is post-puncture headache an IHS?Dural post-puncture headache may be considered a mild form of IHS in which orthostatic headache is the predominant symptom. This and other signs and symptoms are part of IHS. On MRI, there are occasional signs of dural thickening. Likewise, pathophysiological mechanisms and treatment strategies are similar.3 However, the boundary between the definition of post-puncture headache and the degree of severity leading to the categorization of this entity as IHS is blurred, creating a discussion where there are still many questions to be answered.
What is the course?Most case reports and literature reviews point to the fact that the clinical course of IHS is benign, with full recovery and no sequelae, when adequate treatment is provided.15 There is little information about the time of onset and duration, but its similarity with post-puncture headache leads to believe that it starts within three days following a dural lesion; it rarely occurs immediately, and duration varies significantly from patient to patient.16–18
What are the differential diagnoses?Meningitis is a differential diagnosis due to the presence of headache, neurologic changes and a history of disrupted meningeal integrity. However, meningitis may be ruled out by means of a lumbar tap and measurement of the opening pressure and CSF analysis. An opening pressure under 6cmH2O is highly suggestive of IHS,3 unlike meningitis, where it is usually elevated.
CSF analysis may be normal in IHS, but there may be findings of high protein levels in the CSF, lymphocytic pleocytosis, erythrocytic increase and/or xantochromia, normal glucose and culture levels, findings which are not consistent with meningitis.3 For these reasons, a lumbar tap is recommended when there is a high suspicion of meningitis.
Another differential diagnosis is intracerebral neoplasm, which presents with non-orthostatic headache, and localized clinical involvement. In these cases, cerebral MRI shows a space-occupying lesion.
What should be the treatment?Initially, treatment is conservative, with bed rest in order not to trigger headache episodes and diminish the pressure on the site of the dural rupture, and to facilitate healing. The administration of caffeine or oral or intravenous theophylline, steroid therapy, the restoration of CSF volume with oral or intravenous hydration, an increase in salt intake, and CO2 inhalation, are all measures that can improve IHS. However, few studies have been conducted to assess these strategies adequately.3,12,14–16
When these measures fail, there is a need to consider applying a dural blood patch, which is 85–90% effective. In the event symptoms do not improve, this procedure may be repeated with a 98% probability of success.19 Continuous epidural infusion of saline solution for a period of 2 or 3 days is an alternative therapeutic option.1
Another option that needs to be considered is surgical correction, in particular if symptoms persist despite using the strategies described above and if there is evidence of a meningeal defect or a subdural hematoma with significant clinical effects.3,1
In our first case, we could have tried a second blood patch given the persistence of the symptoms, but the patient improved progressively with bed rest and pharmacological treatment.
In the second case, the clinical picture became complicated with the development of an intracranial subdural hematoma, perhaps because of non-compliance with the required treatment plan. Improvement after drainage of the subdural hematoma was striking, probably because the descent of the brain was diminished. Moreover, post-operative bed rest contributed to the correction of the probable spinal dural defect.
In conclusion, IHS is an infrequent occurrence, probably underdiagnosed, which complicates the clinical condition in some patients undergoing conductive neuroaxial anesthesia. Post-puncture headache may be considered a benign form of IHS. Adequate diagnosis and management lead to a favorable course and reduce associated morbidity.
FundingAuthor's own resources.
Conflict of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Quintero IF, et al. Síndrome de hipotensión endocraneana: ¿una cefalea pospunción dural? Rev Colomb Anestesiol. 2013;41:57–60.