The tool most widely used for measuring the intensity of pain in children is the Faces Pain Score – Revised (FPS-R). Pain management depends on the level of care and the knowledge of the physician regarding dosing, indications and side effects of the medications available for use.
ObjectiveTo assess pain management in patients 3–17 years of age with limb fractures using the FPS-R within the first 6h.
Materials and methodsObservational cohort of patients 3–17 years of age presenting with limb fractures between October 2013 and January 2014. Patients with comorbidities associated with chronic pain were excluded. The tool was administered four times in accordance with the validated instructions – on admission, at first hour, at three hours and at six hours.
ResultsOverall, 60 patients were assessed and 4 pharmacological regimens were identified: dipyrone alone (63.3%), combined therapy with dipyrone plus tramadol (10%), tramadol alone (8.3%), acetaminophen alone (6.6%).
The mean pain intensity reduction with the use of dipyrone was 1.7 points on the FPS-R within the first hour, with a mean reduction of 4 points by the end of the six hours of follow-up. With tramadol, pain reduction was 1.6 points and 4.6 points, respectively. The combined use of dipyrone plus tramadol did not result in significant pain reduction within the first hour.
ConclusionFracture immobilization is the mainstay for analgesia but it does not suffice as a form of pain management. Monotherapy with dipyrone or tramadol resulted in the best pain reduction, whereas the combined use of dipyrone plus tramadol was not better than the use of either medication alone.
El parámetro de medición de la intensidad del dolor en niños más usado es la escala revisada de caras de dolor (FPS-R). El manejo del dolor depende del nivel de atención y al conocimiento que el médico tenga respecto a la dosificación, indicación y efectos secundarios de los medicamentos disponibles.
Objetivoevaluar el manejo de dolor en pacientes entre 3 y 17 años con fracturas de extremidades mediante escala (FPS-R) en las primeras 6 horas.
Materiales y métodosEs una cohorte observacional, en pacientes entre 3 y 17 años con fracturas en extremidades, entre Octubre de 2013 y Enero de 2014. Se excluyeron pacientes con comorbilidades que impliquen presencia de dolor crónico. La escala se aplicó en cuatro ocasiones según las instrucciones validadas, al ingreso, a la hora, a las tres horas y a las seis horas.
ResultadosSe evaluaron 60 pacientes, identificando 4 esquemas farmacológicos: monoterapia con dipirona (63.3%), terapia conjugada de dipirona más tramadol (10%), monoterapia con tramadol (8.3%), monoterapia con acetaminofén (6.6%).
La dipirona logró en promedio disminuir 1.7 puntos en la FPS-R durante la primera hora, para el final del seguimiento a las 6 horas disminuyó en promedio 4 puntos en la escala; el Tramadol logró disminuir 1.6 puntos y 4.6 puntos respectivamente. La asociación Dipirona+Tramadol no logró disminución significativa de dolor en la primera hora.
ConclusiónLa inmovilización de la fractura es el pilar de la analgesia pero no suficiente para el manejo del dolor. La monoterapia con dipirona o tramadol tuvieron los mejores resultados en cuanto a disminución de la intensidad de dolor, la combinación dipirona – tramadol no es mejor que la monoterapia.
The National Association for the Study of Pain defines pain as “every unpleasant sensitive and emotional experience associated with real or potential tissue damage”; however, pain has not been studied at length in the paediatric population, not only because of the many baseless myths regarding painful sensations and neurological maturity of children or the types of medications used, but also because of the challenges associated with maturity of verbal expression and associative thinking.
The most widely used parameter for pain assessment in children, both in clinical practice as well as in research, is the measurement of pain intensity, validated by the Paediatric Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (Ped-IMMPACT).1
The various studies on pain measurements in children have been assessed by the Ped-IMMPACT group and by the working group of the Society of Paediatric Psychology based on empirical evidence and expert consensus. These groups have summarized the most relevant and valid techniques for measuring pain in children between 3 and 18 years of age, and have identified well-established pain intensity self-reporting2 and psychometric-based observational measurements.3
Stinson et al.2 identified 34 self-reporting measurements of pain intensity for children 3–18 years of age, six of which meet the criteria of a “well-established measurement” in accordance with the evaluation criteria developed by Cohen et al.4 The latter reviewed a total of 8 self-reporting measurements commonly used for assessing pain intensity in children. There was agreement between the two groups regarding the fact that the Pieces of Hurt tool, the Faces Pain Scale – Revised, the Oucher scale and the Visual Analogue Scale were the best measurements available for clinical practice and research.
The Faces Pain Scale – Revised (FPS-R) uses facial expressions to assess pain intensity. From a set of faces representing different levels of intensity on a horizontal layout, the child is asked to select the face that best reflects his/her pain intensity.5 This measurement is an adaptation of the Faces Pain Scale, which was revised in order to make it consistent with the common score from 0 to 10.
The original version consisted of seven faces and the new revised version consists of six, allowing a numerical value from 0 to 10 (0–2–4–6–8–10) to be assigned to each face. The scores on the two ends are explained as “no pain” or “a lot of pain”.
There are several advantages of this scale over others that use faces. First, it does not include smiling and/or crying faces, which is relevant considering that scales ranging from smiling faces (no pain) to a crying face (a lot of pain) may be confusing in terms of the affective and sensitive components of pain.6 Second, the scale intervals are equivalent and, thirdly, instructions have been translated to more than 32 languages.
The psychometric properties of this scale have been explored in depth, and it has been shown to be reliable and valid for use in the paediatric population between 4 and 16 years of age.7 Perrot reported stability coefficients that remained quite high for one or two days. Additionally, it also correlates with the Visual Analogue Scale and with observational and clinical scores.8
The Visual Analogue Scale (VAS) consists of a horizontal, 10mm-long, horizontal line marked at each end by non-standardized verbal descriptors that represent the extremes of pain intensity. The child is asked to mark a point on the line that represents their pain intensity. The VAS is scored by measuring from the “no pain” anchor to the selected point on the scale.9 Due to the conceptual complexity required for understanding the tool, this measurement is recommended for children over 8 years of age. Several authors have proven VAS reliability and validity using the test–retest reliability. Regarding construct validity, the VAS correlates well with other pain intensity scores such as the FPS-R and the Oucher scale.
The acute pain phase in paediatric fractures has been described as the time between fracture occurrence and immobilization.10 During this phase of acute pain, there are transfer and waiting times, and patients are also manipulated many times from the moment they are admitted to the emergency service until they are immobilized.
In our setting, the use of analgesics in paediatric patients is limited by their availability and the level of care, but, in particular, by practitioner knowledge of dosing, indications and side effects of the medications available.
In children, physiological processes influencing pharmacokinetics are different than in adults; however, pharmacodynamics changes are not significant.
With its analgesic and antipyretic properties, acetaminophen is the most commonly used analgesic in the paediatric population worldwide. The mechanism of action is believed to be its action as a pro-drug that is metabolized in the central nervous system (CNS) to an active molecule that promotes the endogenous cannabinoid activity through a centrally-acting, cyclooxygenase-5 (COX-5)-mediated prostaglandin (PG). Liver toxicity is seen in doses 150mg/kg/day or greater.
Non-steroidal anti-inflammatory agents (NSAIDs) are effective for the management of mild to moderate pain and have antipyretic, anti-inflammatory and analgesic properties. They act through cyclooxygenase inhibition, and therefore, prostaglandin production inhibition; their analgesic effect results from reduced central and peripheral sensitization; their use should be restricted in patients with a risk of non-union, because of delayed bone healing.11
Opioids are used in the treatment of moderate-to-severe pain. Their analgesic action is mediated by agonists with central action on endogenous opioid receptors. The effects on these receptors vary significantly among individuals and may result in multiple undesired effects, the most serious of all being respiratory depression.
Also known as metamizole, dipyrone is used for the management of acute or chronic pain of moderate to severe intensity. However, its use has been discontinued in countries like the United States,12 the argument being the risk of agranulocytosis.13–15 A study conducted in Latin America identified 52 cases of agranulocytosis, 30 of which were included in the case-control study, and found an incidence of 0.38 per million inhabitants/year. The study concluded that the incidence of agranulocytosis is too low to consider it a public health problem, and that, due to the small number of cases, associations cannot be analyzed.16
Dipyrone is also immunogenic and the most frequent associated adverse effect is anaphylaxis, with manifestations ranging from a mild rash to severe anaphylactic shock, the incidence of which is 1 in 5000 patients.17 In Colombia, dipyrone has been licenced by INVIMA and is widely used for pain management.
The paediatric orthopaedic service at Hospital de la Misericordia sees a large number of children with limb fractures and one of the mainstays of comprehensive care in these children is pain management. Although medical orders always include an analgesic regimen, in some cases effectiveness is assessed more than six hours (h) after the regimen has been instituted, without adequate follow-up.
The objective of the study was to assess pain control with the existing pharmacological regimens in patients 3–17 years of age with upper and lower limb fractures using the Faces Pain Scale – Revised (FPS-R) within the first 6hours of treatment, and to assess the effectiveness of the different medications used in the emergency service.
Materials and methodsObservational cohort study conducted between October 2013 and January 2014 in the emergency service of Hospital de la Misericordia in Bogotá – Colombia, in patients 3–17 years of age immobilized due to limb fractures. Excluded from the study were patients with comorbidities involving the presence of chronic pain or with underlying chronic diseases predisposing to limb pain (sickle cell disease, osteogenesis imperfecta, leukaemia), children with conditions affecting pain perception or the ability to express it (developmental disorders, cerebral palsy, autism), patients who took analgesics or anti-inflammatory drugs regularly (once a week or more), patients with red triage requiring resuscitation, and patients with multiple trauma affecting other organs and systems. After obtaining verbal consent from the parents, the researchers administered the FPS-R four times according to the validated instructions:5 upon admission, before initiating any form of therapeutic intervention, 1h after administering the medication prescribed by the physician who responded to the emergency (emergency physicians or paediatric general practitioner), and then three and six hours after administration of the analgesic. Changes in the analgesic regimen made within that time period by the attending physician in accordance with the organizational structure of the service were also documented.
The variables included for each patient were age, sex, affected limb, type of fracture, weight, radiological image of the injury, the results of the FPS-R and the instituted analgesic drug regimen.
All the data were stored in an Excel 2010 worksheet, and the same application was used for data tabulation.
Ethical considerationsAn informed verbal consent from the guardians of all the subjects (under age children) was obtained in accordance with the principles set forth in the Helsinki Declaration and in Resolution 008430 of October 4, 1993 – given that the research was considered of minimal risk according to Article 10 – and in compliance with the relevant items contained in Articles 6 and 16 of the same Resolution. This study was evaluated and approved by the Ethics Committee of the Fundación Hosptial de la Misericordia.
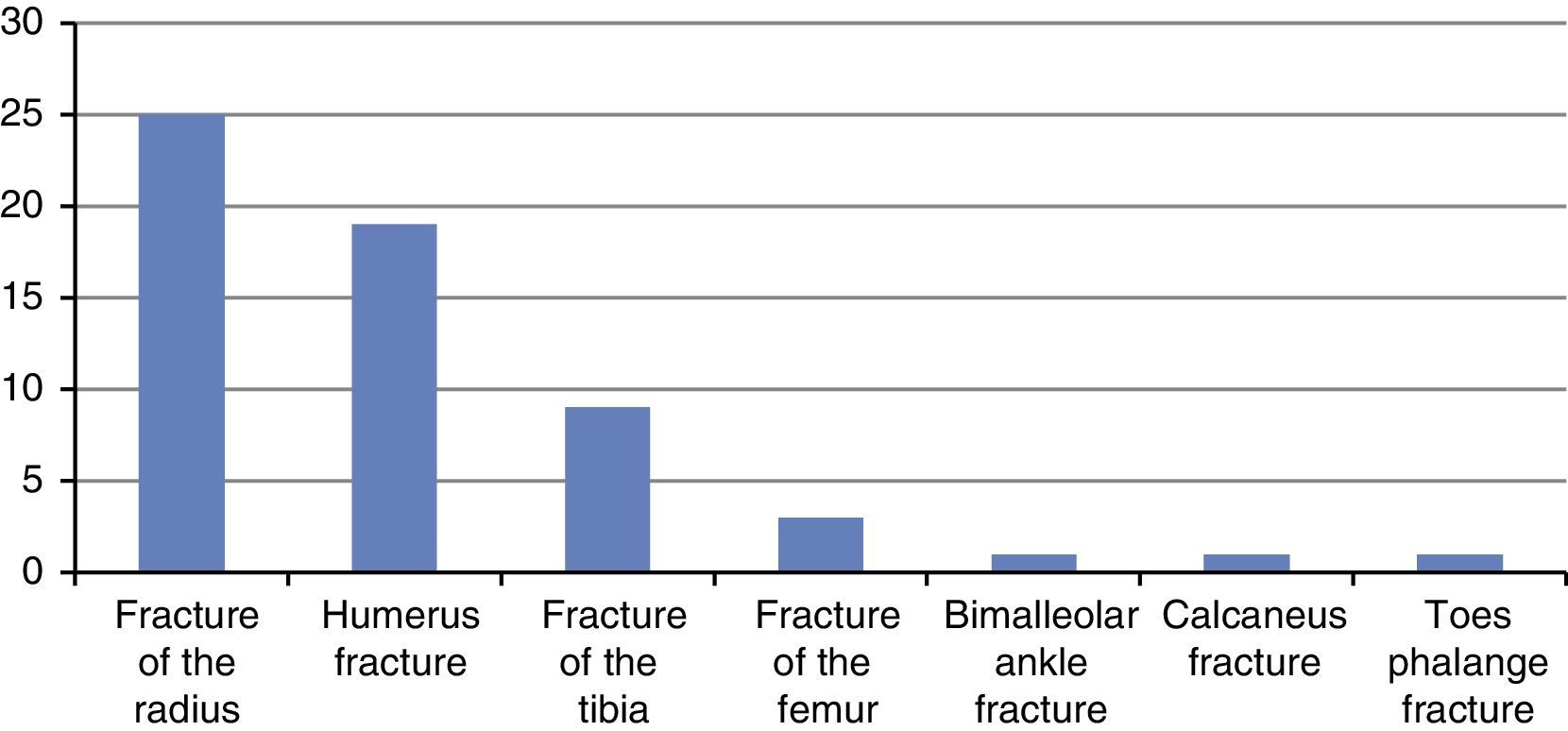
ResultsOverall, 60 patients between the ages of 3–17 were assessed, including 44 males and 16 females, with a mean age of 8.6 and a mode of 8 years, 73.3% of them with upper limb fractures with equal laterality proportion (22 of the right upper limb and 22 of the left upper limb). The most frequent fracture was the Garland III supracondylar fracture of the humerus (23.3%), followed by distal metaphyseal fractures of the radius (21.6%). Fig. 1 summarizes fracture prevalence in the cohort.
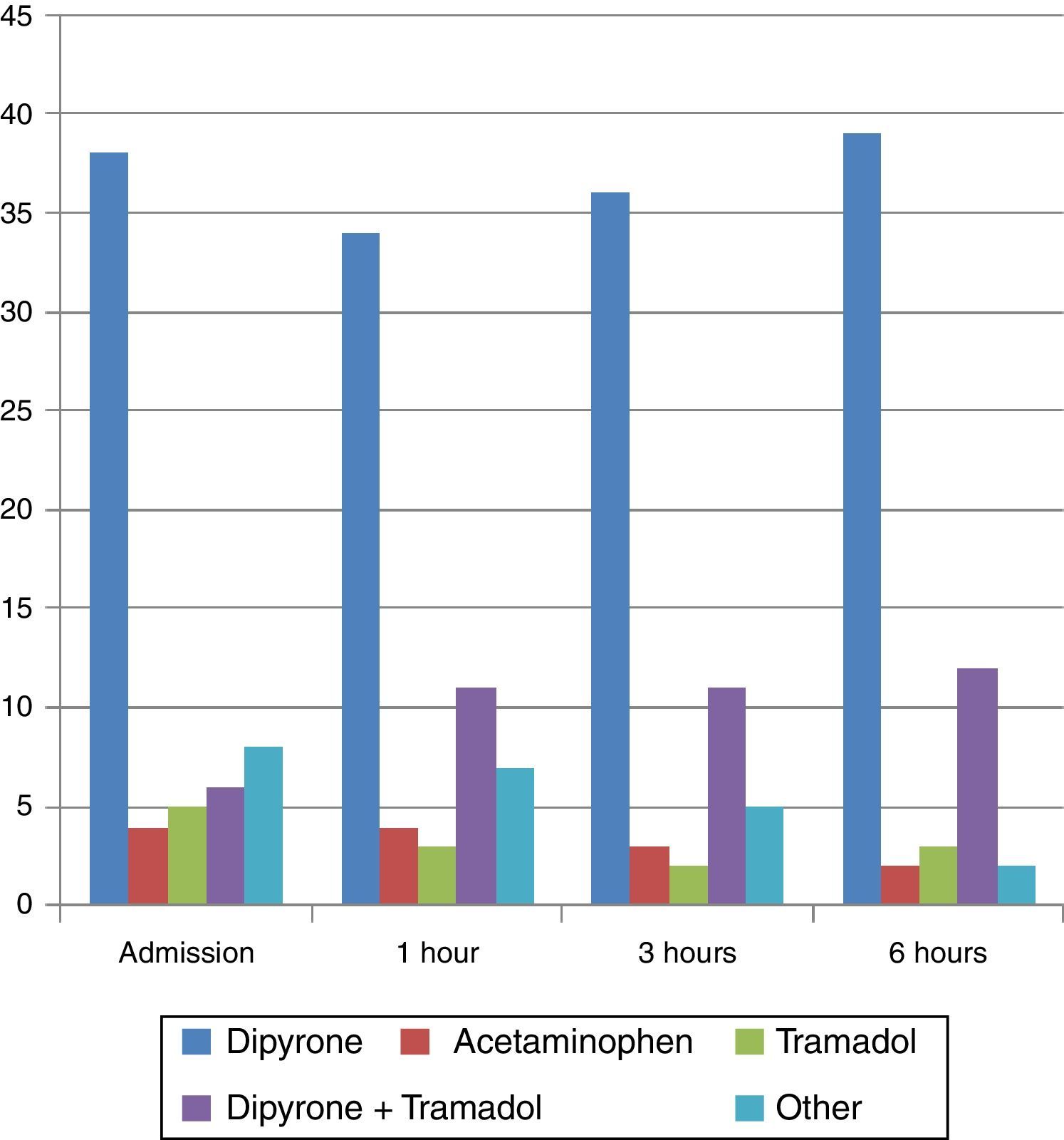
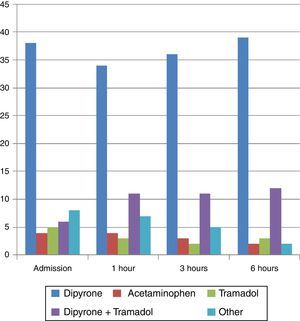
All the patients had already been immobilized. Pharmacological management on admission consisted of 4 main regimens: dipyrone alone in 63.3% of cases, followed by combined therapy with dipyrone and tramadol (10%), tramadol alone (8.3%), and acetaminophen alone (6.6%).
A rescue dose of morphine was used only in one case, and combined with dipyrone in another case. Likewise, diclofenac was used as an adjunct to dipyrone or dipyrone-tramadol therapy in 3 cases, and no form of pharmacological therapy was used in 3 cases (Fig. 2).
For the assessment after 1 hour, changes were made in the pharmacological regimens of 10 patients, consisting mainly of adding a medication to optimize analgesia. At 3h, changes were made in 6 cases, consisting of analgesia optimization but also discontinuation of medications. Between 3 and 6h, most of the changes consisted of medication discontinuation. However, for all assessments, dipyrone alone was the medication used most frequently.
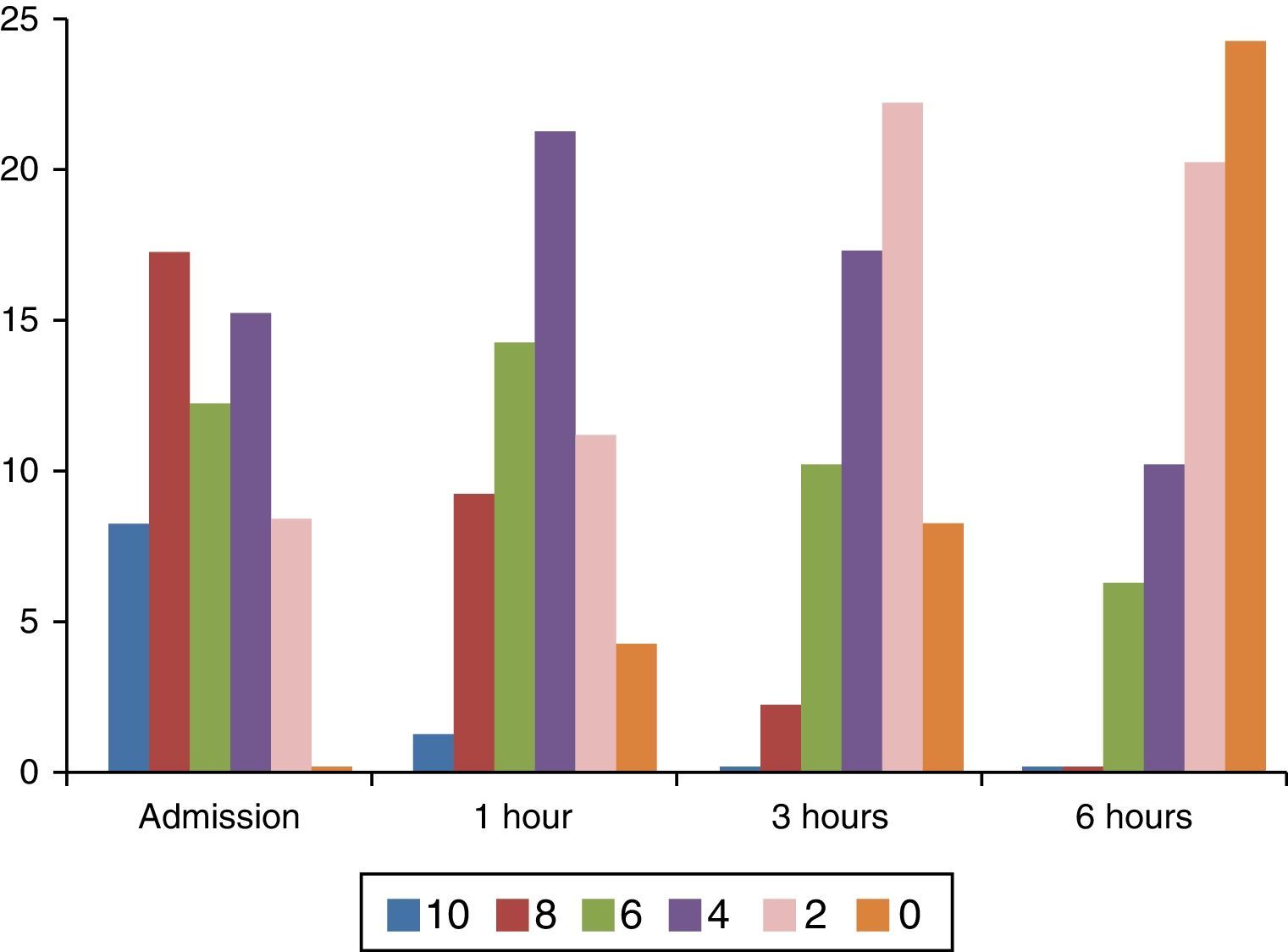
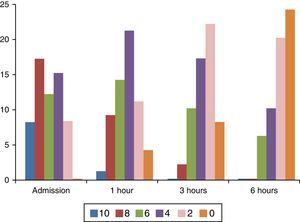
Regarding pain measurement, important intensity variations were found between severe, (8–10) moderate (4–6) or mild pain (2) on the FPS-R, as shown in Fig. 3.
During later assessments, changes in pain intensity were observed and there was evidence of lower pain intensity in 96.6% of cases. In the remaining 3.3% the pain remained stable, and there were no cases of increased pain. Only 40% (24) of the patients reported total absence of pain after 6h.
Findings regarding pain reduction in relation to each pharmacological regimen were the following: with dipyrone, the mean reduction during the first hour was 1.7 points on the FPS-R, and by the end of the 6h of follow-up, the mean reduction was 4 points on the scale; with tramadol, the mean reduction was 1.6 points on the FPS-R during the first hour, and the mean reduction by the end of the 6h of follow-up was 4.6 points. The combined use of dipyrone and tramadol did not result in a significant reduction of pain intensity within the first hour, but by the end of the 6h of follow-up, there was a mean reduction of 2 points on the scale. In the “Other” category, which included associations of dipyrone with tramadol and diclofenac, pain reduction within the first hour was 1.6 points on the FPS-R, and 2.4 points by the end of the follow-up period. Finally, in patients who did not receive any form of pharmacological therapy, there was no pain reduction within the first hour; and in those in whom the therapy was discontinued after the first dose of analgesia, mean pain reduction was 2 points on the scale after 6h of follow-up.
Among patients with moderate or severe pain (6 points or more) on admission, only in 12 was there a reduction of pain intensity down to 4 points or less: 9 patients with the dipyrone regimen, 2 patients with acetaminophen, and 1 patient with tramadol.
DiscussionSeveral situations that limit the results of this study were observed during the follow-up period, including parental psychological impact and rearing standards as influencers of the children's responses when the scale was administered.
Regarding pain, there were no situations in which pain increased and, at the end, only 40% of patients reported absence of pain while the remaining 60% still had some form of pain associated with the fracture, and there was no pain improvement within the first three hours in patients who did not receive pharmacological management. Fracture immobilization is the mainstay of analgesia but it does not suffice for pain management. It is worth noting that some fractures are not comparable due to the wide range of diagnoses and their variability in terms of severity. For example, a metaphyseal fracture of the radius cannot be compared with a femoral fracture because the latter will be associated with greater pain intensity and will require additional therapeutic action in order to provide relief. For this reason, results were tabulated in terms of pain reduction for each case, determining the number of points by which pain intensity dropped as compared to the initial score as a result of the medication used in each individual, and averaging the values. The best results in terms of reduction in the pain scale were achieved with dipyrone or tramadol used as monotherapy, with no important difference between the two. The combined use of dipyrone and tramadol was not found to be better than either medication used alone. However, it is important to consider that the expected reduction in pain intensity is down to 4 points or less on the FPS-R, a result achieved only in 12 patients in this cohort. Consequently, there is a need for protocols for rigorous pain assessment in order to meet the medical intervention targets in these patients.
Worth highlighting is also a very low use of oral therapy for patient management. The use of oral NSAIDs such as ibuprofen and naproxen was notoriously absent, in particular in cases of fractures that required surgical management. This may be explained perhaps by the fear to interrupt the state of fasting required for anaesthesia. Regarding the use of ibuprofen, Drendel et al.18 conducted a randomized trial with 336 patients comparing the use of oral ibuprofen with that of acetaminophen plus codeine for pain management in upper limb fractures, and considered the need for intravenous rescue medication as therapeutic failure. In their results, the authors reported the use of ibuprofen in 20.3% of cases and the use of acetaminophen plus codeine in 30% of cases, with no significant differences between the two in terms of failed treatment, although there were more adverse events with the use of acetaminophen plus codeine. Consequently, they recommend the use of ibuprofen in simple fractures, despite the fact that a large proportion of patients required additional intravenous analgesia mainly with opioids like morphine. In our cohort, this additional analgesia with morphine was used only in one patient with a femoral fracture.
Ortega19 conducted a retrospective study to determine whether the age of the children influenced the type of analgesic prescribed in cases of long bone fractures and found that, in children over 4 years of age, there were no significant differences in terms of the use of opioids versus NSAIDs. However, in children under 4 years of age, opioid prescription was very limited, a fact explained on the basis that injuries in this age range are less severe than in older children. In our cohort, there were no differences in prescription by age range, although there were differences in terms of injury severity.
In conclusion, pain management in children is challenging, starting with intensity assessment all the way to making therapeutic decisions. However, it is of the utmost importance to provide comprehensive care to these patients both in the emergency service as well as postoperatively, which means doing everything possible to not only reduce but also eliminate their pain. According to Mader et al.20 this does not only impact the quality of care but the general condition of the patients. The use of monotherapy is a good option, as suggested by the results of this study. The combined use of NSAIDs and opioids has not been shown to be better. It is important to standardize pain management protocols that consider age, type and fracture site in children with musculoskeletal injuries.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
FinancingThe authors did not receive sponsorship to carry out this article.
Conflicts of interestThe author has no conflicts of interest to declare.
Please cite this article as: Fuentes-Losada LM, Vergara-Amador E, Laverde-Cortina R. Evaluación del manejo de dolor en niños con fractura en extremidades en un servicio de urgencias. Rev Colomb Anestesiol. 2016;44:305–310.