Propofol is one of the most frequently used intravenous anesthetic agents. Due to its lipid-based composition, propofol requires a strict handling protocol to avoid increased risk of extrinsic contamination. Little is known about propofol handling practices in developing countries.
ObjectiveWe conducted a survey among the population of anesthesiologists and anesthesia residents in Colombia, with a view to identify the practices and factors associated with inadequate propofol handling.
MethodsCross-sectional study based on digital and/or telephone surveys addressed to anesthesiologists and residents of anesthesia in Colombia. The data collection tool comprised thirty questions divided into sections considering socio-demographic conditions, practices and knowledge.
ResultsA total of 662 answers were analyzed. The reuse of propofol vials in more than one patient and the reuse of propofol syringes in several patients were described as usual practices by 37.9% (251/662) and 6.2% (41/662) respectively, among the subjects surveyed. Multivariate analysis showed a perception of shortage of Propofol, infrequent use of gloves, and reuse of syringes that were significantly associated with the reuse of propofol vials at institutions with few ORs (<9).
ConclusionsNotwithstanding the economic conditions, the reuse of propofol in Colombia is similar to developed countries. Further research focusing on the impact of external factors such as economic, administrative, and training conditions is required.
El propofol es uno de los agentes anestésicos más usados. Por su composición liposoluble, el propofol requiere un estricto protocolo para su manipulación con el objetivo de evitar el potencial riesgo de contaminación extrínseca. Poco se conoce sobre las practicas de manipulación del propofol en países en vía de desarrollo.
ObjetivoHemos desarrollado un estudio en la población de anestesiólogos y residentes de anestesiología en Colombia con el objetivo de identificar las practicas y factores relacionados con la inadecuada manipulación del propofol.
MetodosSe realizó un estudio de corte transversal basado en encuestas electrónicas y/o telefónicas dirigidas a anestesiólogos y residentes de anestesiología en Colombia. El instrumento de recolección contenía treinta preguntas dividas en secciones que estudiaban condiciones sociodemográficas, prácticas y conocimientos.
ResultadosUn total de 662 respuestas fueron analizadas. La reutilización de viales de propofol en más de un paciente y la reutilización de jeringas con propofol en más de un paciente fueron referidas como prácticas frecuentes en un 37.9% (251/662) y 6.2% (41/662) de los encuestados, respectivamente. El análisis multivariado mostró que un bajo numero de quirófanos (<9), la percepción de escases de propofol, el uso infrecuente de guantes y la reutilización de jeringas son factores significativamente relacionados a la reutilización de viales de propofol.
ConclusionesA pesar de las condiciones económicas, la tasa de reutilización de propofol en Colombia es similar en comparación con la de países desarrollados. Se requiere mayor investigación dirigida a estudiar la influencia de factores externos tales como condiciones económicas, administrativas y formativas.
Anesthesiology is considered a specialty closely associated with patient safety during preparation, surgery, and even several weeks after surgery.1 The daily practice of anesthesiology is characterized by multi-tasking and the settings to which anesthesiologists are exposed each day are diverse.2,3 It is well known that strict compliance with the protocols for handling drugs and the practice of basic recommendations for aseptic procedures are fundamental pillars to ensure satisfactory results.4 During the last few years educational tools have been developed to disseminate the principles of good clinical practice in the OR among healthcare workers, and at the same time to create awareness about the potential risks of failure to comply with these quality standards.5
The recommendations for handling intravenous anesthetic agents have been widely debated and reported through the scientific media and the industry.4 A clear example of this problem is propofol, that due to its lipophilic preparation becomes a favorable environment for the growth of contaminating agents.6 Despite the efforts to educate and learn about the positive impact of the aseptic technique, there are still postoperative infectious complications associated with contaminated propofol.7 Most of the recommendations on propofol handling are based on the guidelines of the Centers for Disease Control and Prevention (C.D.C.). Recent studies have shown that the rate of propofol reuse ranges between 5 and 15%.4,8–10 Notwithstanding the fact that this topic has not been thoroughly studied at the national level, our current situation is quite challenging.11
Based on a number of local publications,7,11 our group has expressed the need to learn about the details of the healthcare professionals practice when involved in perioperative care in Colombia. The objective of this study was to identify the practice and factors associated with improper handling of propofol by anesthesiologists and residents in anesthesiology training in Colombia.
MethodologyPopulationThe study sample included anesthesiology specialists and residents registered in S.C.A.R.E. database (with the exception of Valle del Cauca), valid until January 30, 2016, that responded to the electronic survey. The membership database of the Valle del Cauca affiliate (S.A.R.V.A.C), valid until January 2016 was also used, in order to administer telephone surveys; however, if the respondent could not make him/herself available, an electronic form was e-mailed. The surveyors were selected upon an open invitation of the Clinical Research Center, Fundación Valle del Lili, and were then trained, though they were unaware of the study objectives throughout the data collection phase, in order to avoid manipulating questions or information biases.
Ethical considerationsThe Ethics Committee for Biomedical Research (No. 484-2015), of Fundación Valle del Lili, approved this cross-sectional study. Simultaneously, the project was also approved by the Research Committee of the Colombian Society of Anesthesiology and Resuscitation (S.C.A.R.E.). All the participants in the study had to be verbally approved prior to their inclusion in the trial, but no written informed consent was required, due to the low risk established, and the confidentiality strategies used for handling the data.
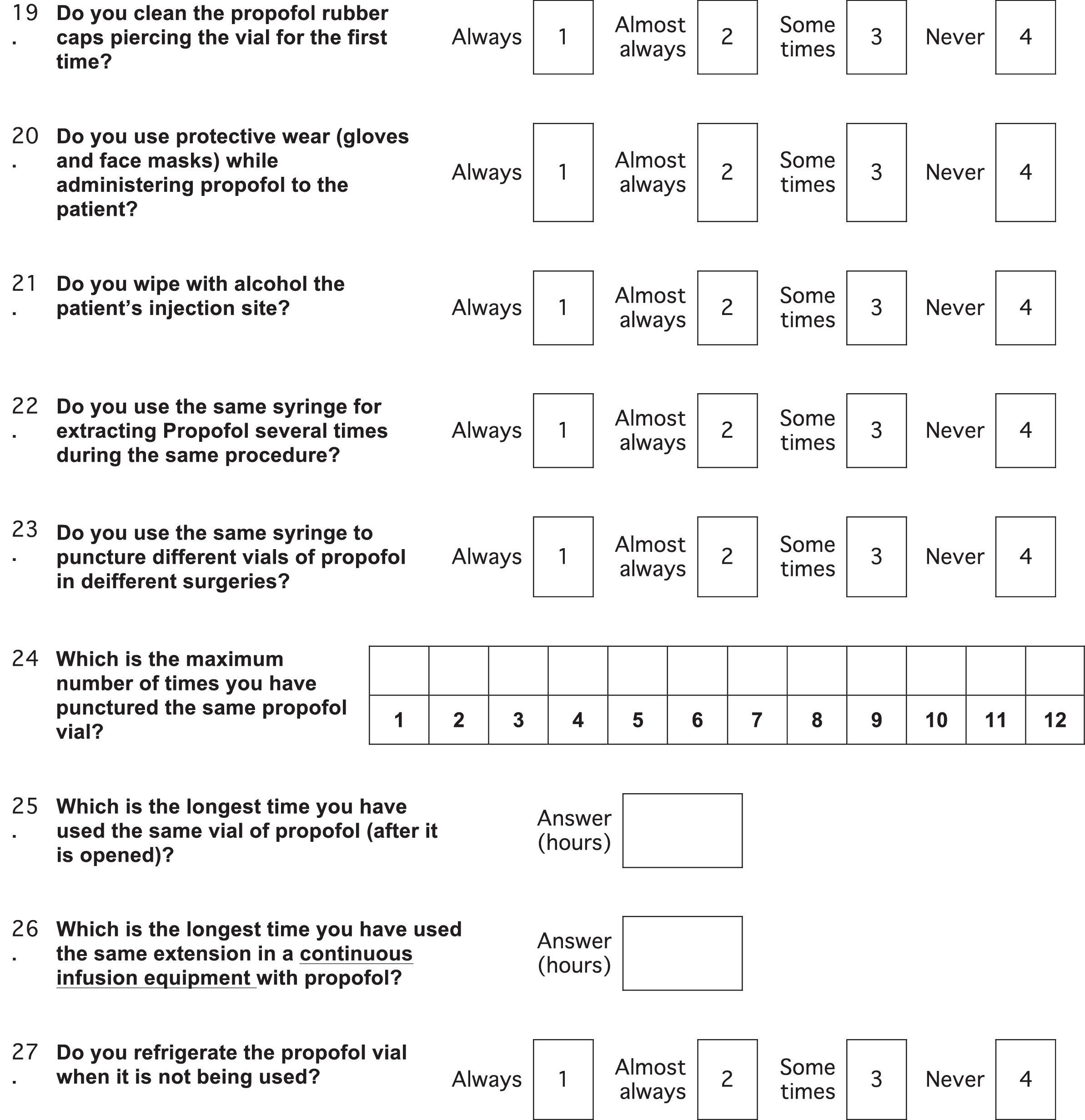
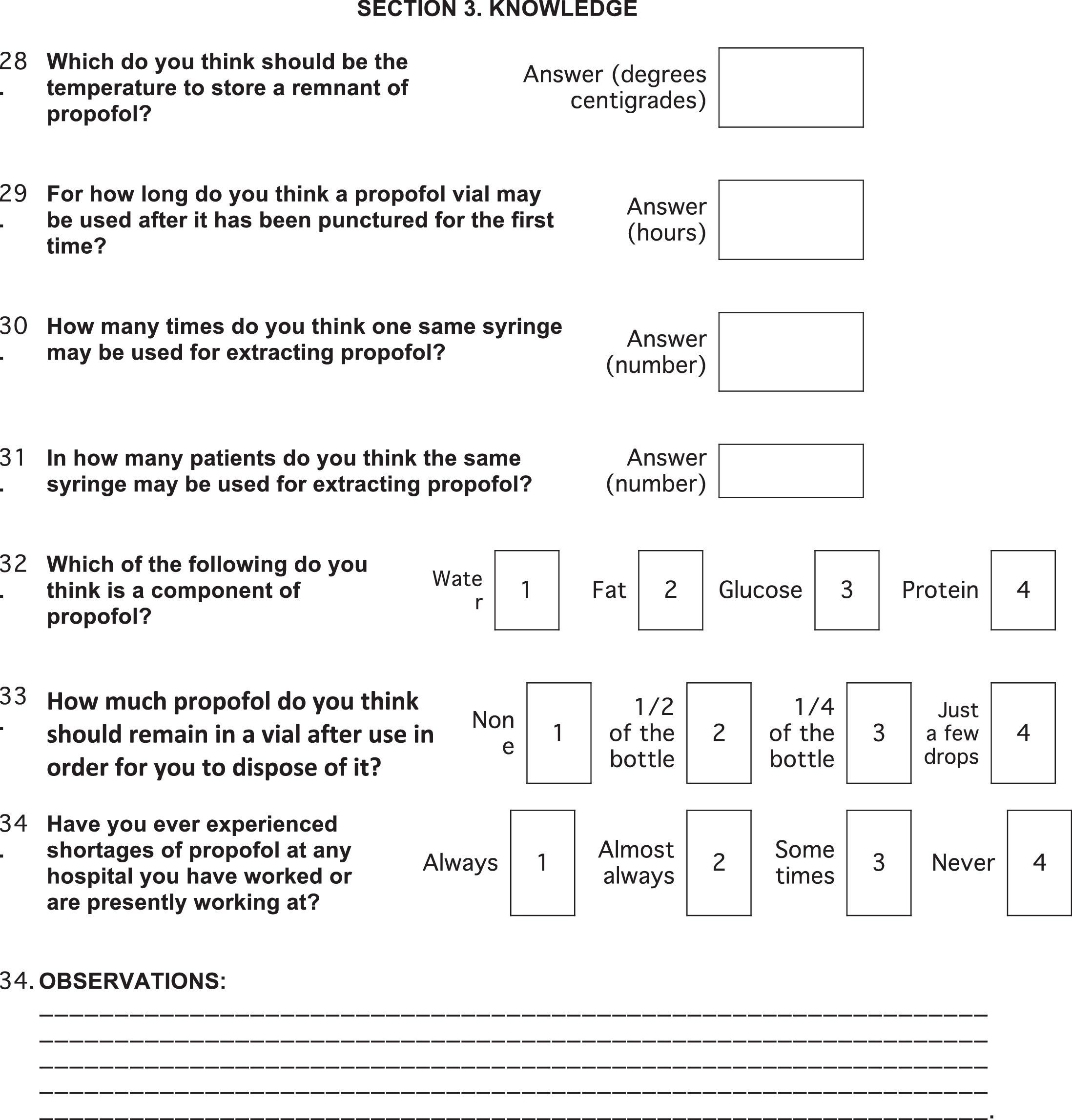
Survey content and variablesThe survey comprised 34 questions (Annex 1). The variables were divided into socio-demographic groups (age, gender, academic status, level and type of medical institution, number of ORs at the institution, City, State), practices (reuse of supplies), and knowledge (time limits for use after opening vials/blisters and practices to discard propofol).
In order to assess the propofol reuse practice, a passive question was asked to avoid interviewer biases (Ex. “For the induction of anesthesia, do you use the same propofol vial for more than one patient?”. The answers were classified into “Always”, “Almost always”, “Some times”, “Never”). The same type of question was asked to learn about the reuse of syringes and infusion pumps. The answers “Always/almost always” were considered frequent practice for the analysis, and the answers “Never/Some times” were considered infrequent practice. Some quantitative variables such as the number of operating rooms, were dichotomized post hoc with selected cut points, in order to define association measures.
Validity of the surveyThe interphase of the virtual survey was designed by S.C.A.R.E. specialists. Before e-mailing the surveys to the responders, the authors analyzed and discussed the lay out and the type of answers on at least three occasions. With regards to the telephone survey a pilot test was done with ten subjects to assess the quality of the questionnaire, the clarity of the questions, the response times, and the accuracy of the surveyor when recording the answers in the electronic database. The database of the virtual survey was to be managed by S.C.A.R.E. staff, while the telephone surveys were to be managed by the Clinical Research Center of Fundación Valle del Lili. The final consolidated results were emailed to the authors for their corresponding analysis. Incomplete surveys with missing information in one or more sections were excluded from the final analysis.
Statistical analysisInitially, an exploratory analysis of the total sample was planned, describing all of the quantitative variables such as average and standard deviation or means and inter-quartile ranges (IQR), according to the type of data distribution. The qualitative variables were expressed in frequency or percentage values, together with their corresponding confidence intervals 95% (CI). For comparative purposes, subjects were classified between those that re-used the propofol vials and those that did not. The qualitative data were compared using the chi square test (χ2) and the quantitative data using t-Student or U-Mann–Whitney, in accordance with normal distribution. The Shapiro–Wilk test was used to evaluate the type of data distribution. A univariate analysis with its respective measurements in terms of percentages and P values was made for all qualitative variables. Any variables with P<0.1 were included post hoc in the multivariate analysis (Poisson regression with robust adjustment for sample variance error) with the respective measures of association (odds ratio [OR], 95% CI). A P<0.05 was considered statistically significant for this model. The data were analyzed using Stata SE13.0 (StataCorp LP, College Station, TX) statistical software.
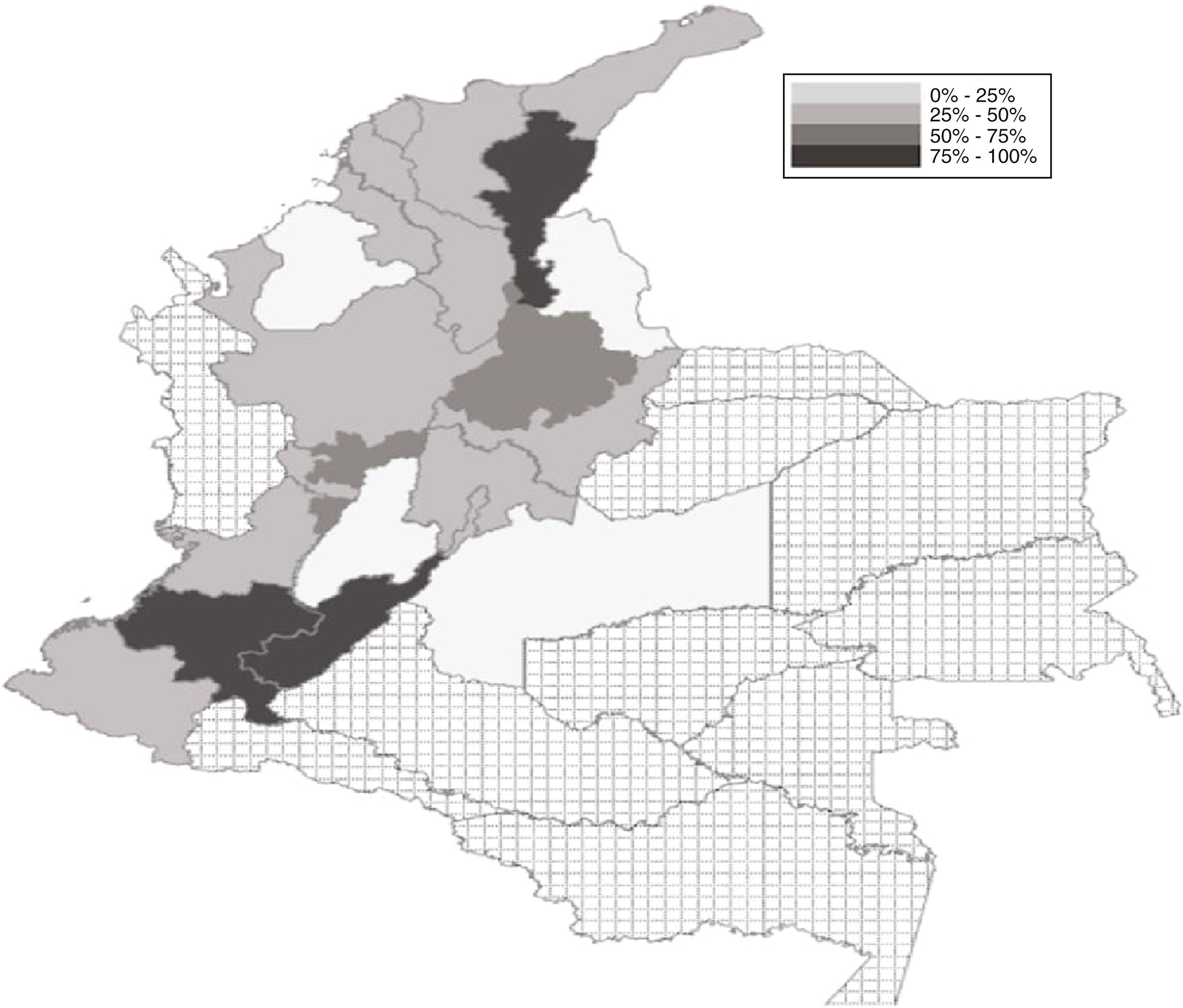
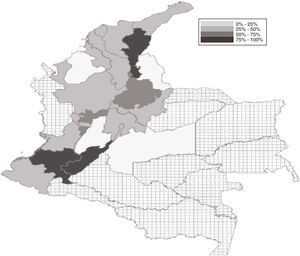
ResultsSample characteristicsOut 3198 anesthesiologists and anesthesia residents, 662 answered the survey (response rate: 20.7%), of which 364 (54.9%) were via Internet and 298 (45.1%) were over the telephone. Most of the sample was made up by general anesthesiologists, followed by anesthesia residents and subspecialists. The mean age of the responders was 44.9 (38.6–48.3) for males and 41.4 (39.5–46.5) for females and the average time as practicing physicians was 15.5 years. Most of the participants were males (472/662, 71.3%), while 70.4% were practicing in private hospitals (mostly level 3). The city with the highest number of responders was Cali, followed by Bogota and Medellin (Fig. 1).
Propofol handling practicesMost of the professionals surveyed stated a level of propofol use of 80% (IQR: 60–90%) over the number of cases intervened per day. With regards to the presentation of the drug, the 20mL vials were more frequently used (73.9%), followed by the 50 (20.2%) and 10mL (4.1%) vials. 37.9% of the responders usually reused propofol vials in more than one patient and 34.6% often reused propofol when handling continuous infusion systems. The rate of reuse of propofol vials in more than one patient was particularly high in certain States such as Cauca, Cesar, and Huila (Fig. 1).
The habit of using one same syringe in more than one patient was described as a frequent practice by 6.2% of the responders. The reasons argued for reusing propofol included savings (72.5%), convenience (10.6%), environmental impact (9%) and time (8%).
When asked about the practices for preparing propofol infusions, only 43.9% of the responders said they used gloves and 59.2% facemasks. Only 34.3% and 47% of the responders did aseptic cleaning of the rubber cap on the propofol vial prior to the initial puncture and of the injection site, respectively.
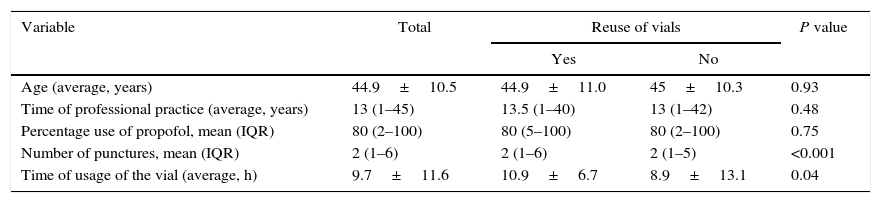
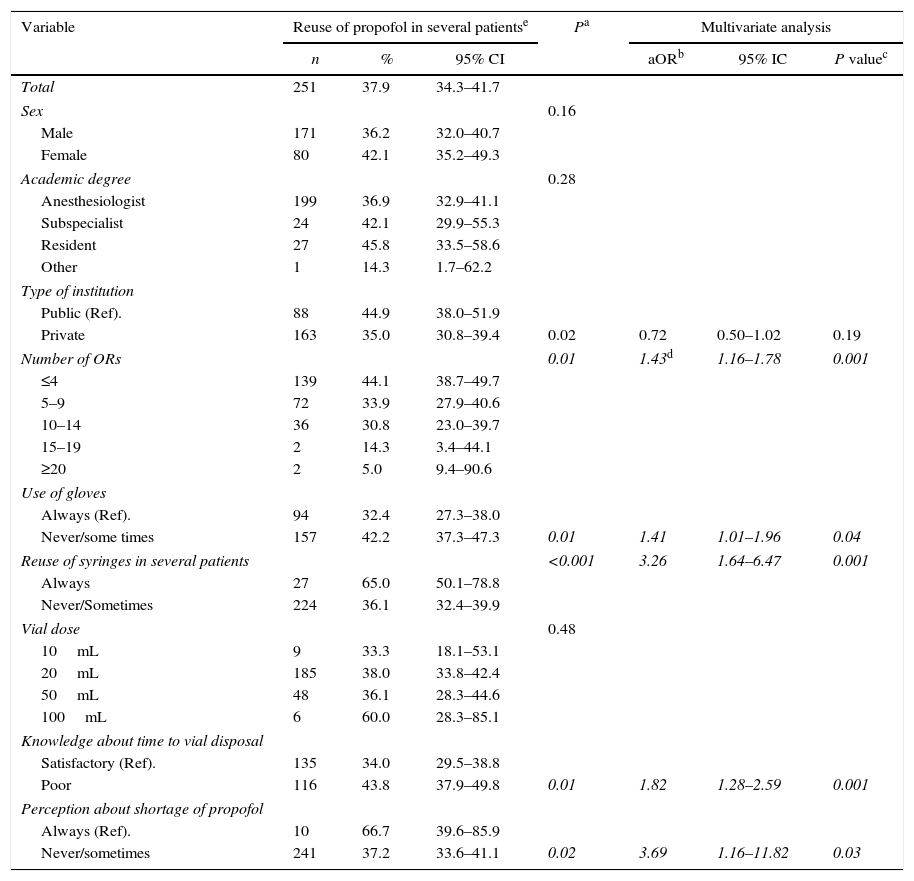
There was a direct relationship between the number of propofol vial punctures and the reuse of the vial in more than one patient, and usage times of the vials was longer in the group that reused the vials (Table 1). The univariate analysis showed that the reuse of propofol is practiced more often in public versus private institutions (44.9% vs. 35%, P=0.02). Similarly, this practice was significantly more prevalent at institutions with fewer ORs (48.2% for <9 ORs vs. 33.8% for ≥9 ORs, P=0.001), and also when there was the perception of shortage of the medication (67.7% vs. 37.2%, P=0.02) low usage of gloves during anesthetic induction (42.2% vs. 32.4%, P=0.01), and concomitant reuse of syringes in more than one patient (65% vs. 36.1%, P<0.001). With the exception of the type of institution (public vs private), all the variables analyzed in the multivariate model showed a statistically significant association (Table 2).
Characteristics of quantitative variables of the anesthesiologists and residents surveyed.
| Variable | Total | Reuse of vials | P value | |
|---|---|---|---|---|
| Yes | No | |||
| Age (average, years) | 44.9±10.5 | 44.9±11.0 | 45±10.3 | 0.93 |
| Time of professional practice (average, years) | 13 (1–45) | 13.5 (1–40) | 13 (1–42) | 0.48 |
| Percentage use of propofol, mean (IQR) | 80 (2–100) | 80 (5–100) | 80 (2–100) | 0.75 |
| Number of punctures, mean (IQR) | 2 (1–6) | 2 (1–6) | 2 (1–5) | <0.001 |
| Time of usage of the vial (average, h) | 9.7±11.6 | 10.9±6.7 | 8.9±13.1 | 0.04 |
Abbreviations: IQR: interquartile range.
Source: Authors.
Factors associated with the reuse of propofol vials in several patients.
| Variable | Reuse of propofol in several patientse | Pa | Multivariate analysis | ||||
|---|---|---|---|---|---|---|---|
| n | % | 95% CI | aORb | 95% IC | P valuec | ||
| Total | 251 | 37.9 | 34.3–41.7 | ||||
| Sex | 0.16 | ||||||
| Male | 171 | 36.2 | 32.0–40.7 | ||||
| Female | 80 | 42.1 | 35.2–49.3 | ||||
| Academic degree | 0.28 | ||||||
| Anesthesiologist | 199 | 36.9 | 32.9–41.1 | ||||
| Subspecialist | 24 | 42.1 | 29.9–55.3 | ||||
| Resident | 27 | 45.8 | 33.5–58.6 | ||||
| Other | 1 | 14.3 | 1.7–62.2 | ||||
| Type of institution | |||||||
| Public (Ref). | 88 | 44.9 | 38.0–51.9 | ||||
| Private | 163 | 35.0 | 30.8–39.4 | 0.02 | 0.72 | 0.50–1.02 | 0.19 |
| Number of ORs | 0.01 | 1.43d | 1.16–1.78 | 0.001 | |||
| ≤4 | 139 | 44.1 | 38.7–49.7 | ||||
| 5–9 | 72 | 33.9 | 27.9–40.6 | ||||
| 10–14 | 36 | 30.8 | 23.0–39.7 | ||||
| 15–19 | 2 | 14.3 | 3.4–44.1 | ||||
| ≥20 | 2 | 5.0 | 9.4–90.6 | ||||
| Use of gloves | |||||||
| Always (Ref). | 94 | 32.4 | 27.3–38.0 | ||||
| Never/some times | 157 | 42.2 | 37.3–47.3 | 0.01 | 1.41 | 1.01–1.96 | 0.04 |
| Reuse of syringes in several patients | <0.001 | 3.26 | 1.64–6.47 | 0.001 | |||
| Always | 27 | 65.0 | 50.1–78.8 | ||||
| Never/Sometimes | 224 | 36.1 | 32.4–39.9 | ||||
| Vial dose | 0.48 | ||||||
| 10mL | 9 | 33.3 | 18.1–53.1 | ||||
| 20mL | 185 | 38.0 | 33.8–42.4 | ||||
| 50mL | 48 | 36.1 | 28.3–44.6 | ||||
| 100mL | 6 | 60.0 | 28.3–85.1 | ||||
| Knowledge about time to vial disposal | |||||||
| Satisfactory (Ref). | 135 | 34.0 | 29.5–38.8 | ||||
| Poor | 116 | 43.8 | 37.9–49.8 | 0.01 | 1.82 | 1.28–2.59 | 0.001 |
| Perception about shortage of propofol | |||||||
| Always (Ref). | 10 | 66.7 | 39.6–85.9 | ||||
| Never/sometimes | 241 | 37.2 | 33.6–41.1 | 0.02 | 3.69 | 1.16–11.82 | 0.03 |
Note: Italic numbers means the variables with statistical significance in multivariate analysis.
aOR=adjusted “odds ratio”; CI=confidence interval.
The mean recommended time for the use of a propofol vial, according to the professionals surveyed was 6 (IQR 4–12) hours. 6% (n=40) of the respondents felt that reusing the same propofol syringe in more than one patient was appropriate. In terms of the storage conditions for propofol, 41.4% of the responders felt it was appropriate to refrigerate any propofol vials not in use. 38% of the responders considered that “no more product left in the vial” was the adequate condition for disposal of the vial.
DiscussionThe study results reveal important characteristics in the practice of anesthesiologists and anesthesia residents with regards to the proper handling of propofol. The percentage of responders that reused propofol syringes in more than one patient was 6.2% and the reuse rate of vials was 37.9%. Our study suggests that a small number of ORs and the perception of shortage of propofol were the main factors associated with the reuse of propofol vials. We also realized that this practice could be associated with other unsafe actions such as the infrequent use of gloves, reuse of syringes, and poor knowledge about the time limits for the safe use of propofol vials after opening the vial.
Most of the clinical research in anesthesiology focuses on comprehensive patient care.1,12 Advances in this field have brought about new challenges for practitioners, being one of the most relevant infection prevention and control.1 There are numerous biosecurity guidelines and protocols for handling devices and medicines in anesthesiology.2,4,5 One of the guidelines most commonly used by healthcare workers and referenced by the American Society of Anesthesiologist (A.S.A.) are the guidelines designed by the Centers for Disease Control and Prevention (CDC).5,13 These guidelines are relevant to alert the healthcare staff about the potential risk of infection whenever there is a breach in the continuity of the natural barriers or in case of manipulation of peripheral or central vascular accesses. These guidelines clearly state that the way in which devices or medications that will be in direct contact with the different tissues are handled, may compromise postoperative outcomes. In the case of propofol, there are handling protocols, both for induction and maintenance infusions. The handling guidelines for this drug have been widely disseminated since its commercialization as intravenous anesthetic, due to the potential risk of contamination associated with its high liposolubility.7
Some studies have established that the rate of contamination of propofol at the time of disposal of the vial ranges between 1% and 20%. A systematic review recently published by the authors found that the main causes of contamination are the extended use of propofol and the reuse of syringes in more than one patient.7 Other cross-sectional studies similar to ours have been done, evaluating the handling practices of propofol in developed countries, where the reported rates of reuse of propofol vials range from 5% to 41.3%.7,11 In our study, the rate of vial reuse was 37.9%, very similar to the reports in other publications (Table 1).
There were no data available on this issue in developing countries prior to this study. Our study developed in Colombia reports a 6.2% rate of reuse of propofol syringes, similar to the rates reported in developed countries (between 1.2% and 59.2%). In New Zealand the rate of reused syringes reported was as low as 0%14; in Brazil 1.2%15; France 2%16; the United Kingdom 6.9%17; and the rate in United States of America (USA) has been dropping from 59.2% in 1995 to 4% in 2013.2,8,18
Consistent with our literature search, our study may be considered as the first to establish the factors associated with propofol reuse, and its relationship with other poor biosecurity practices. The number of ORs and the perception of shortage of propofol were the only variables significantly associated with the reuse of propofol.
Our study exhibits strengths that further the validity of the results obtained. Despite a response rate of only 20%, the size of the sample in our study could be considered as representative, in contrast to similar studies with only 100 to 500 responders. One of the strengths of our study is that surveyors were blinded to the steps in the data collection and processing processes. No information was given to the responders about the objectives of the study, in order to avoid information biases. The applicability of our results is huge, as these results will impact the actions taken to educate the healthcare staff involved with anesthesia, in addition to sharing information about the key unsafe practices of the healthcare staff.
We feel that the major limitations of our study are the inability to evaluate the handling practices through direct observation, and the difficulty to show the validity of the associations due to the cross-sectional design used. Nonetheless, the multivariate statistical analysis identified statistically significant relationships and facilitated the interpretation of the descriptive analysis. Moreover, it important to highlight that we did not receive answers from all the Colombian States (Departments) since only 27 of the 32 Departments responded and this is a limitation to the representativeness of the sample.
ConclusionsThe reuse of propofol vials and syringes in several patients is a relatively frequent practice among Colombian anesthesiologists and anesthesia residents. There is a need for a sustainable culture of drug handling, including educational interventions directed to the population of anesthesiologists and anesthesia residents in Colombia, with higher risk of using unsafe practices (in accordance with our study, these are practitioners in public hospitals with less than 9 ORs, that share the perception of a shortage of propofol, and that also reuse syringes and fail to use gloves during the administration of propofol).
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
FundingThis study was co-financed by the Colombian Society of Anesthesiology and Resuscitation (S.C.A.R.E.) and Clínica Fundación Valle del Lili (Cali, Colombia).
Conflicts of interestNone declared.
We want to express our deepest gratitude to Lina Maria Gonzalez from S.C.A.R.E. and to all the members of the Research Group at Fundación Valle del Lili for their kind collaboration during the development of the study.
Please cite this article as: Zorrilla-Vaca A, León T, Ariza F. Practicas de manipulación del propofol: resultados de un estudio colombiano de corte transversal. Rev Colomb Anestesiol. 2017;45:300–309.