The use of pediatric regional anesthesia has grown to become the standard of care, because of its effective pain control, improved safety profile of the local anesthetic agents, in addition to the introduction of ultrasound.
ObjectiveTo perform a non-systematic review of pediatric regional anesthesia.
Methods and materialsA search was conducted on the available scientific evidence in databases (Pubmed/Medline, ScienceDirect, OVID, SciELO), for a non-systematic review.
ConclusionsThe use of pediatric regional anesthesia has increased due to its notable effect on pain management and furthermore as a result of the incremented use of ultrasound technology.
El uso de anestesia regional en niños ha aumentado hasta convertirse en estándar de manejo, debido al efectivo control del dolor, mejor perfil de seguridad de anestésicos locales y a la implementación del ultrasonido.
ObjetivoRealizar una revisión no sistemática sobre evidencia científica disponible en anestesia regional pediátrica.
Métodos y materialesSe realizó una búsqueda, sobre la evidencia científica disponible, en bases de datos (Pubmed/Medline, ScienceDirect, OVID, SciELO), para realizar una revisión no sistemática.
ConclusionesEl aumento en el uso de la anestesia regional pediátrica, se debe a que proporciona control adecuado del dolor y al uso del US. La realización de bloqueos en niños anestesiados o sedados es más segura que en pacientes despiertos.
Notwithstanding the benefits of pediatric regional anesthesia (PRA), just a few practitioners originally used it. During the last decade, the use of PRA has grown1, due to the introduction of local anesthetics (LA) with improved profiles and tools such as ultrasonography that provides improved safety and has been associated with better nerve blocks2. However, with the exception of ilio-hypogastric and ilio-inguinal nerve blocks (II–IH), the safety advantages of ultrasound (US) over the traditional techniques have not been proven in children because of the limited number of trials3.
PRA provides intra- and postoperative anesthesia and is considered an integral part of the pain management guidelines4, in addition to preventing the harmful effects of improper pain management5.
Ultrasound guidance is not totally risk-free. A number of trials have shown that a practitioner that begins training may make mistakes when visualizing the needle and as a result of inadvertent probe movements. For this reason, the American Society of Regional Anesthesia prepared a document recommending the inclusion of US-guided regional anesthesia training as part of the medical school syllabus6.
The purpose of this article was to review the literature on the key aspects of PRA techniques.
MethodologyA non-systematic literature search was performed using PUBMED/MEDLINE, ScienceDirect and OVID, based on the terms “regional anesthesia”, “pediatric”, “ultrasound”, and “new local anesthetics”. The search and the selection of articles were done in an independent manner, and were restricted to meta-analysis, systematic reviews, Cochrane reviews, clinical essays, and non-systematic reviews. The date of publication was not limited and no Spanish articles were included.
Historical evolutionThe history of PRA began with the discovery of the anesthetic properties of cocaine. Bier introduced spinal anesthesia and two of his patients were children7. Gaston Labat began to teach RA and wrote the book: Regional anaesthesia: Its techniques and clinical applications8.
The number of PRA reports has increased as pediatric anesthesia has evolved. Despite the considerable interest in PRA since 1980, its use was not generalized because general anesthesia was the standard, in addition to the existing concern about causing neurological injury9 to the sedated or anesthetized patient.
In 1998 over 50 pediatric anesthesiologists published an article10 showing that the outcome of a nerve block in an anesthetized child is safer than in a patient that is awake and excited. Other authors wrote an editorial called Regional Anesthesia: children are different, stressing the need to avoid considering pediatric patients as small adults11. Later on, other papers were published describing new techniques, local anesthetics, and adjuvants12,13. Today, RA represents an unquestionable advantage for pain control and plays a relevant role in clinical practice14.
Neuraxial blocksEpidural and caudalEpidural analgesia, including the caudal approach, has been the cornerstone for postoperative pain management in children. It is currently indicated for open chest surgery, major abdominal and spine surgery. The current trend in lower limb surgery is the use of peripheral nerve blocks, including perineural catheters15.
The risk of serious complications is 1:10,000 in epidural anesthesia and 0.2:10,000 in caudal anesthesia15. The anatomic characteristics of children should be considered in order to avoid accidentally puncturing important anatomical structures6.
Neuraxial blocks in children based on anatomical landmarks are safe and currently there is no evidence of the need for the routine use of ultrasound16,17.
The loss of resistance in the smaller patients should be done with air because it facilitates the identification of any unintended puncture of the dura mater6. The advancement of caudal catheters in neonates is not recommended because of the high rates of contamination15. In older patients, the recommended approach is from the low lumbar area, ideally inserting the catheter as close as possible to the surgical site. Visualizing the tip of the catheter using ultrasound, radiology aids, and electrical stimulation are all modern techniques used to confirm the position of the catheter6,15.
Spinal anesthesiaThis approach was quite popular early in the twentieth century and had a comeback three decades ago, due to the successful results shown in preterm babies undergoing herniorrhaphy, since these babies were at high risk of postoperative apnea. Spinal anesthesia is safe for infants, school children and adolescents18 undergoing lower limb surgery and any procedure below the umbilicus18,19.
The contraindications are: puncture site infection, rise in intracranial pressure, degenerative axon disease and severe hypovolemia18,20.
The key limitation is the length of time – between 70 and 90min because of the increased CSF volume, heart rate, and blood flow, both through the bone marrow as through epidural space. In order to do a spinal puncture, sedation or prior administration of a local anesthetic is required to control movement18.
The puncture is made at L4–L5 or L5–S1, in lateral decubitus or with the patient sitting down. The injection shall be administered in over 20s and the Trendelemburg position should be avoided due to the risk of total spinal anesthesia. The local anesthetic agents of choice are levobupivacaine and ropivacaine, both at a 0.5mg/kg dose18.
Peripheral nerve blockAll of the peripheral nerve blocks performed in adults may also be administered to children16.
General considerationsIt is absolutely crucial to define whether the block will be done under sedation or general anesthesia21. Fasting time should be considered, keeping in mind that trauma children should be considered as having a full stomach22. If a neurological injury is suspected, it should be documented with a physical examination prior to administering the block. The extent of neurological injury may be assessed early during the postoperative period, using low LA concentrations.
The likelihood of compartment syndrome is not a contraindication for regional anesthesia since the block does not mask its diagnosis because of the severity of the pain and also because there are diagnostic aids to confirm the condition, such as infrared spectroscopy23.
The presence of infection does not represent an absolute contraindication either, and the block may be administered at a site away from the surgical area.
Technical considerationsA comfortable position is critical, with the ultrasound screen facing the operator24. The anatomic structures in children are superficial and the recommendation is to use a high frequency lineal probe (>13MHz). Echogenic blunt-tip 22–24G needles, with a separate injection line are the most suitable21.
Upper limb blocksThe following are the most common ultrasound-guided brachial plexus approaches.
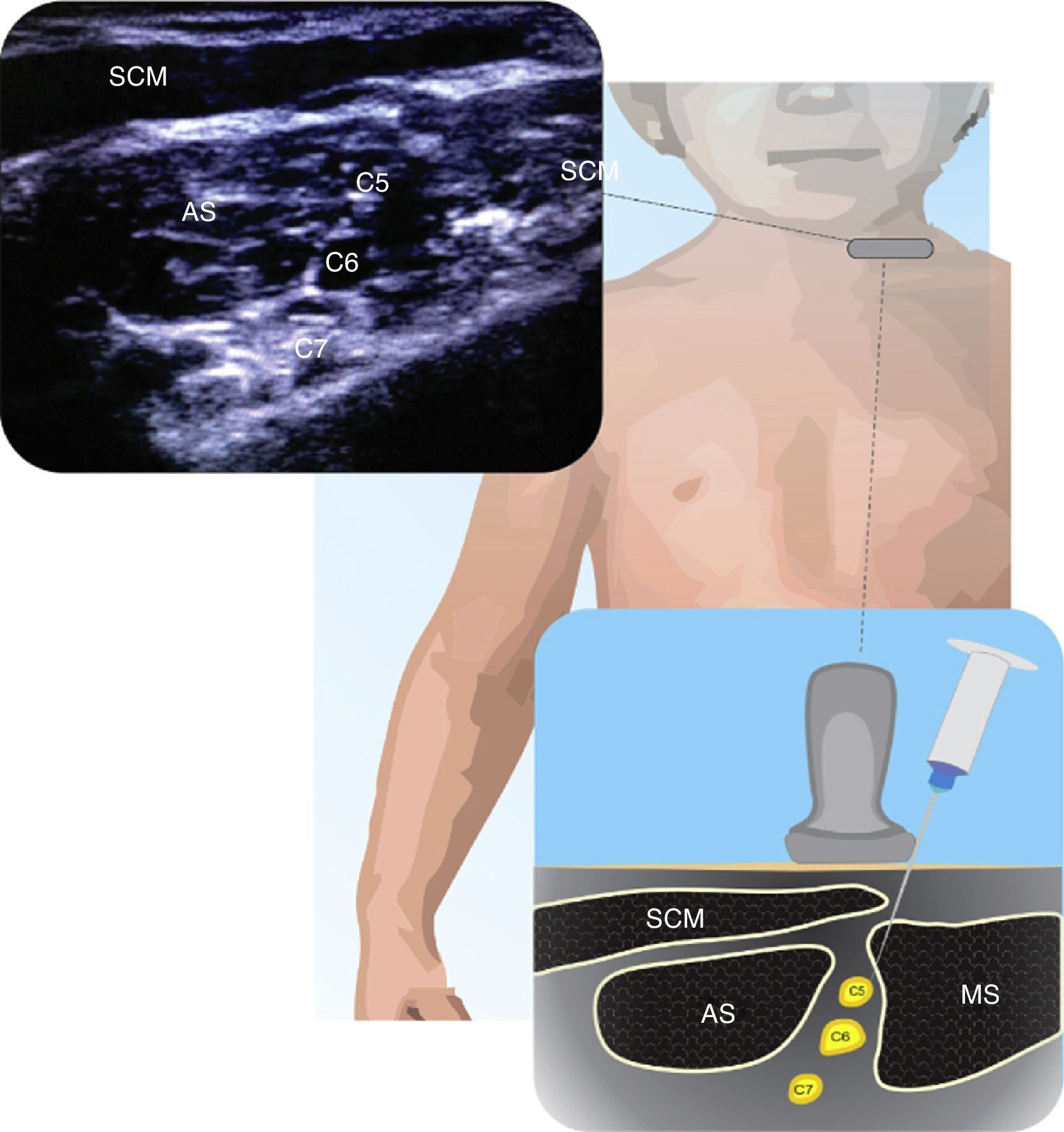
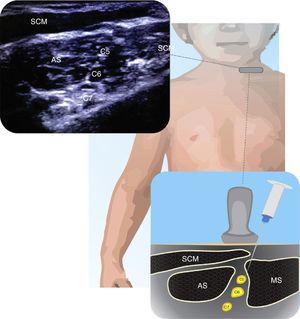
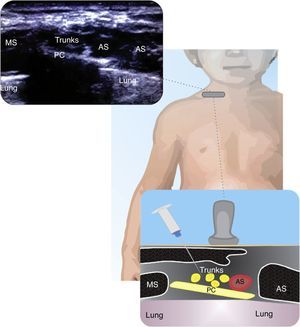
InterscaleneThere are few publications on the interscalene block approach in children25. This is a useful approach for shoulder procedures and subcapital fractures of the humerus. Fig. 1 illustrates the anatomy of the C5–C7 nerve roots within the interscalene groove. The block may be done both inside and outside the plane, but the superficial location of these structures requires careful needle manipulation. The volume and concentration of the LA depend on the patient and the procedure21.
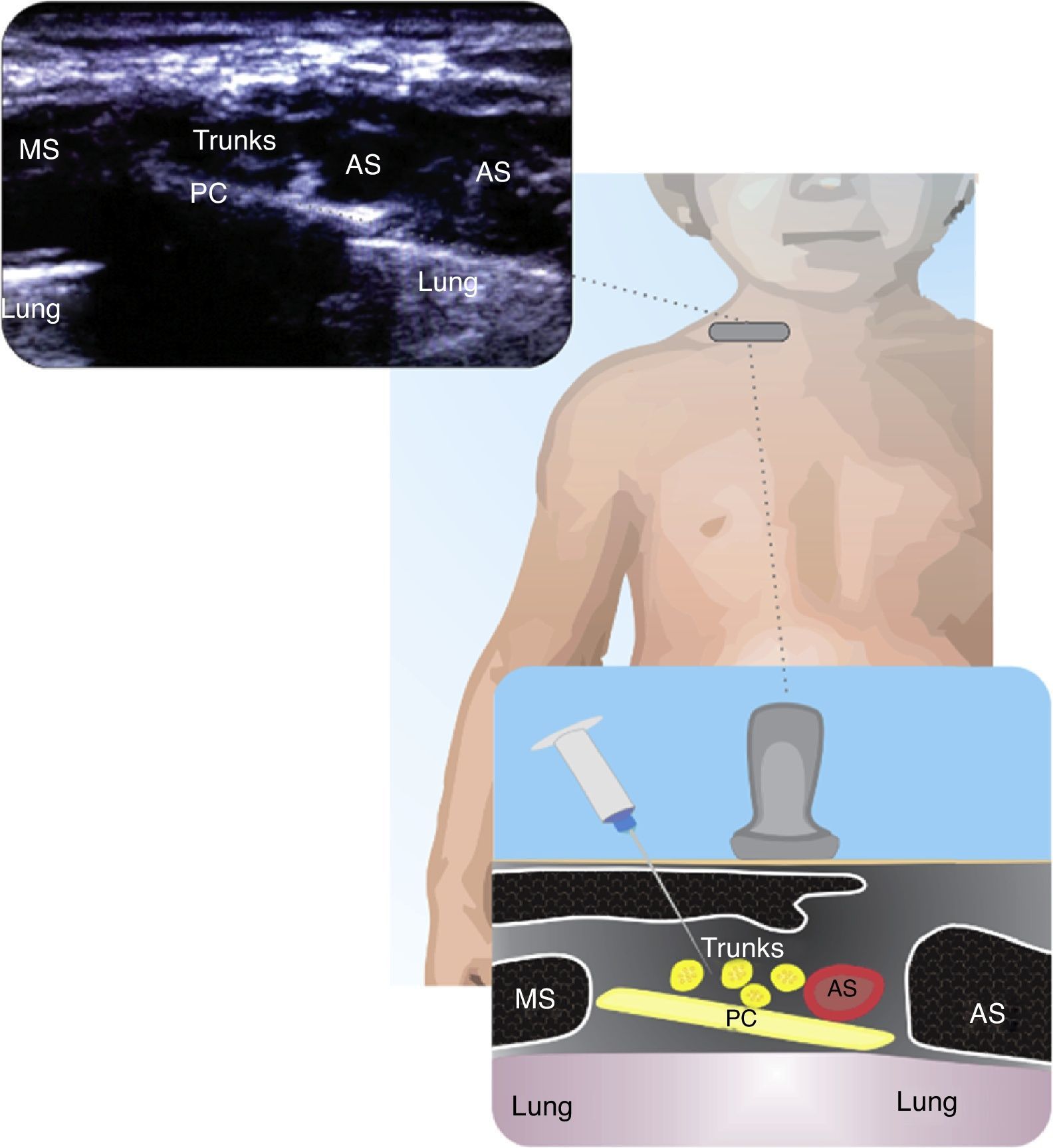
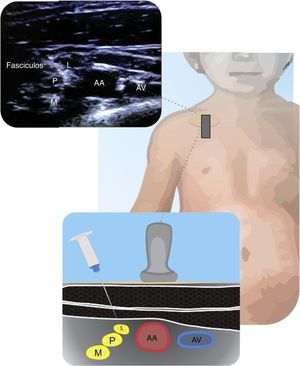
SupraclavicularThis has been a controversial block because of the proximity of the subclavian vein and the pleura. The use of ultrasound has increased this approach and the recommendation is to proceed from the lateral to the medial plane. The supraclavicular approach is indicated for procedures below the mid humeral level. As compared against the infraclavicular approach, the supraclavicular has a lower latency and higher efficacy21. Fig. 2 shows the relationship of the brachial plexus with the subclavian artery, the pleura and the first rib.
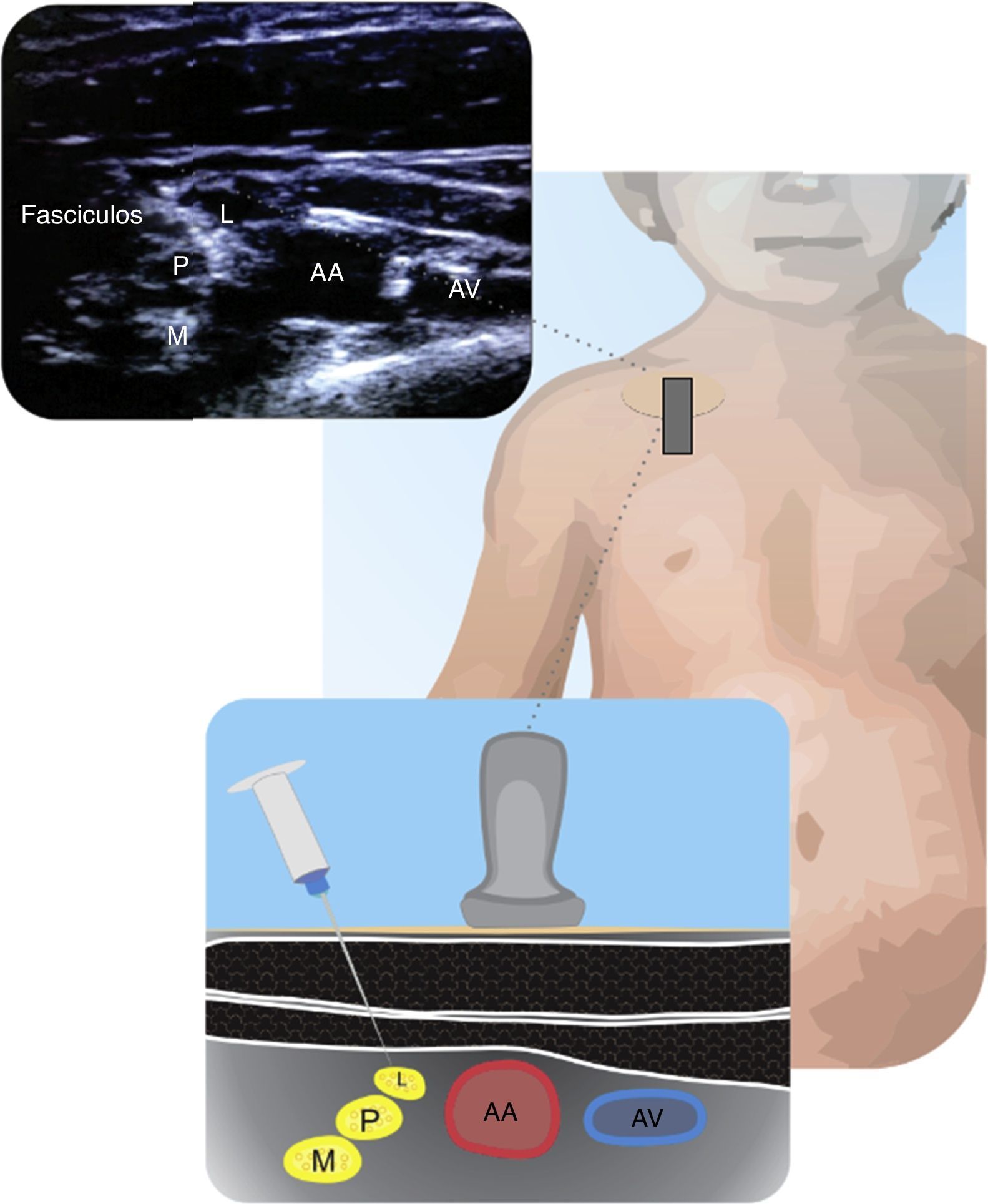
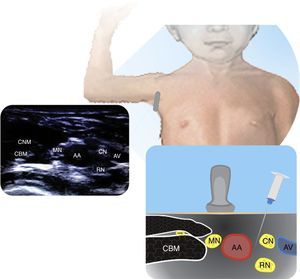
InfraclavicularThis is an alternative to the previously described approach and it is recommended when the ultrasound visualization of the infraclavicular is better than the supraclavicular approach. Both outside and inside the plane techniques yield adequate results26. Fig. 3 shows the neurovascular bundles and their relationship to the axillary artery.
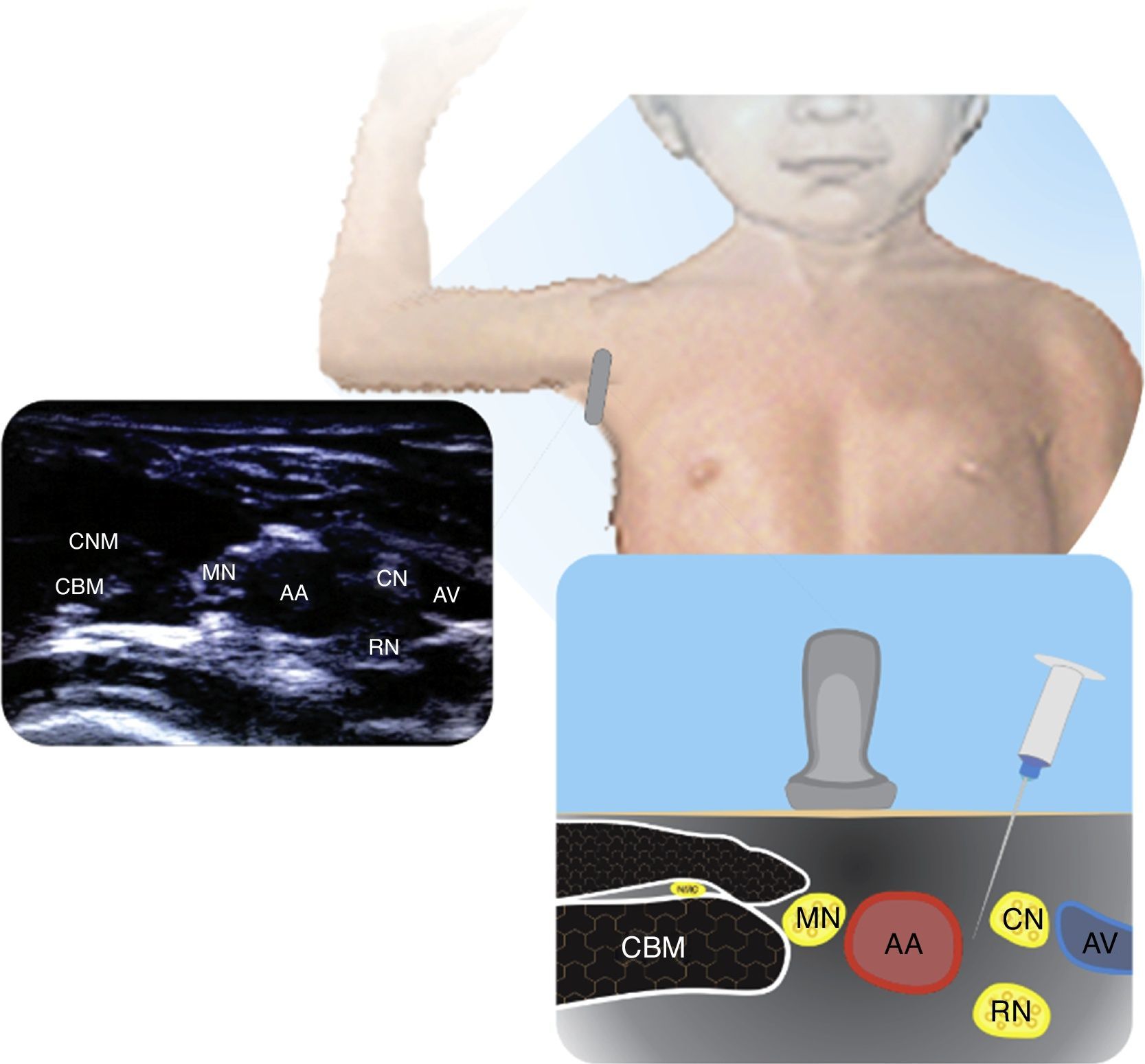
AxillaryAlthough this is a popular approach in adults, periclavicular approaches are preferred in children because these avoid the abduction of an injured upper extremity and also because in many cases the visualization of very superficial structures is difficult. The axillary approach is indicated for forearm and hand surgical procedures and the recommendation is to use inside the plane techniques21. Fig. 4 illustrates the position of the axillary artery relative to the nerves.
Lower limb blocksMost lower limb procedures may benefit from regional techniques, although they may frequently require at least two nerve blocks24. The following are the most frequent approaches using ultrasound guidance.
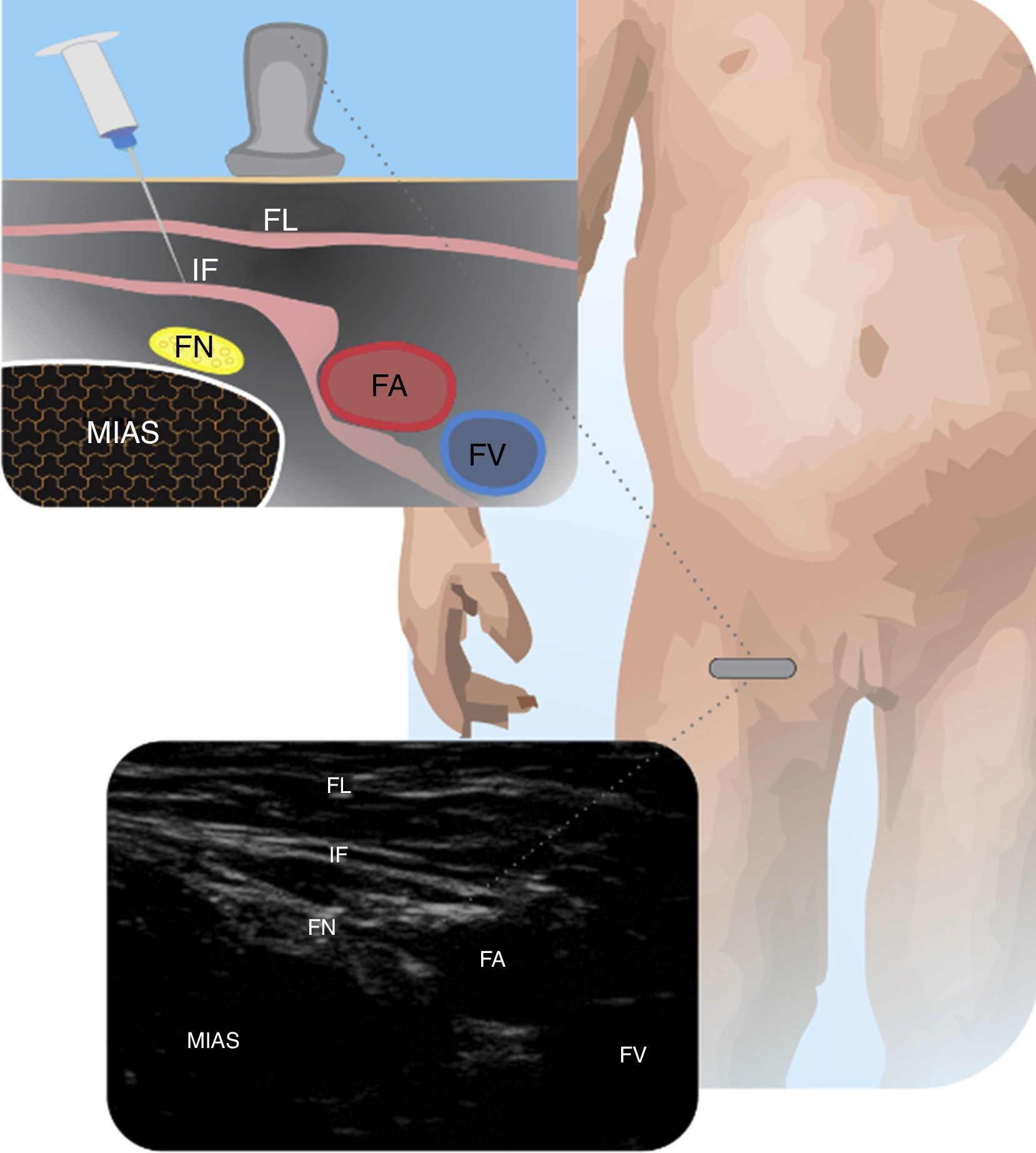
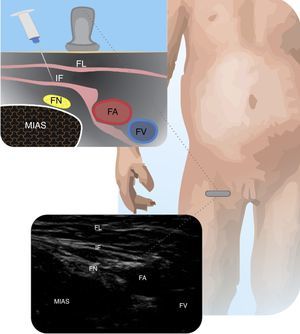
Femoral nerveThis approach is useful in femur fractures, in arthroscopy and for the reconstruction of knee ligaments, inter alia27. It is done by placing the probe on the femoral fold and localizing the femoral artery (FA). The recommendation is to insert the needle inside the plane and move from lateral to posteromedial24. Parents should be advised that the child should not stand up until the complete resolution of the block. Fig. 5 depicts the femoral nerve and its anatomical relationships.
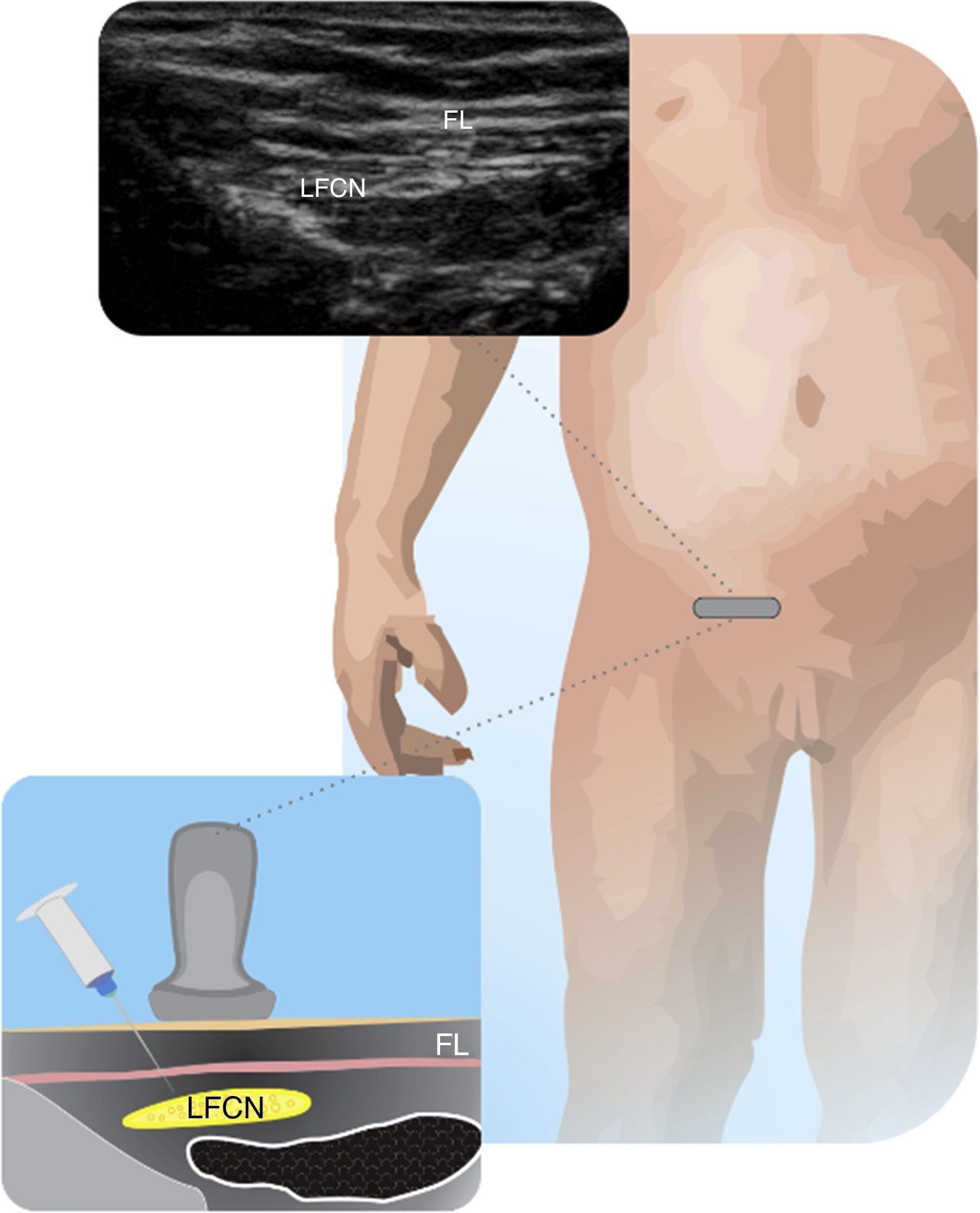
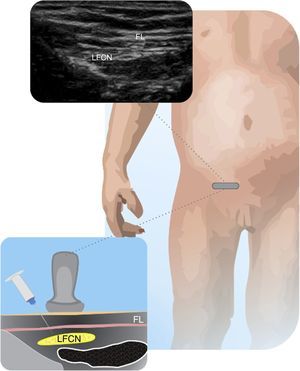
Lateral femoral cutaneous nerveThis approach is helpful for grafting or taking biopsies of the innervated zone, for preventing tourniquet pain and to complement knee surgery24. The femoral nerve and vessels should be located and the iliac fascia must be traced towards the anterior superior iliac spine, until a round hyperechogenic structure us identified. Either out-of-plane or in-plane approaches may be used24. Fig. 6 shows the LFCN and its anatomical relationships.
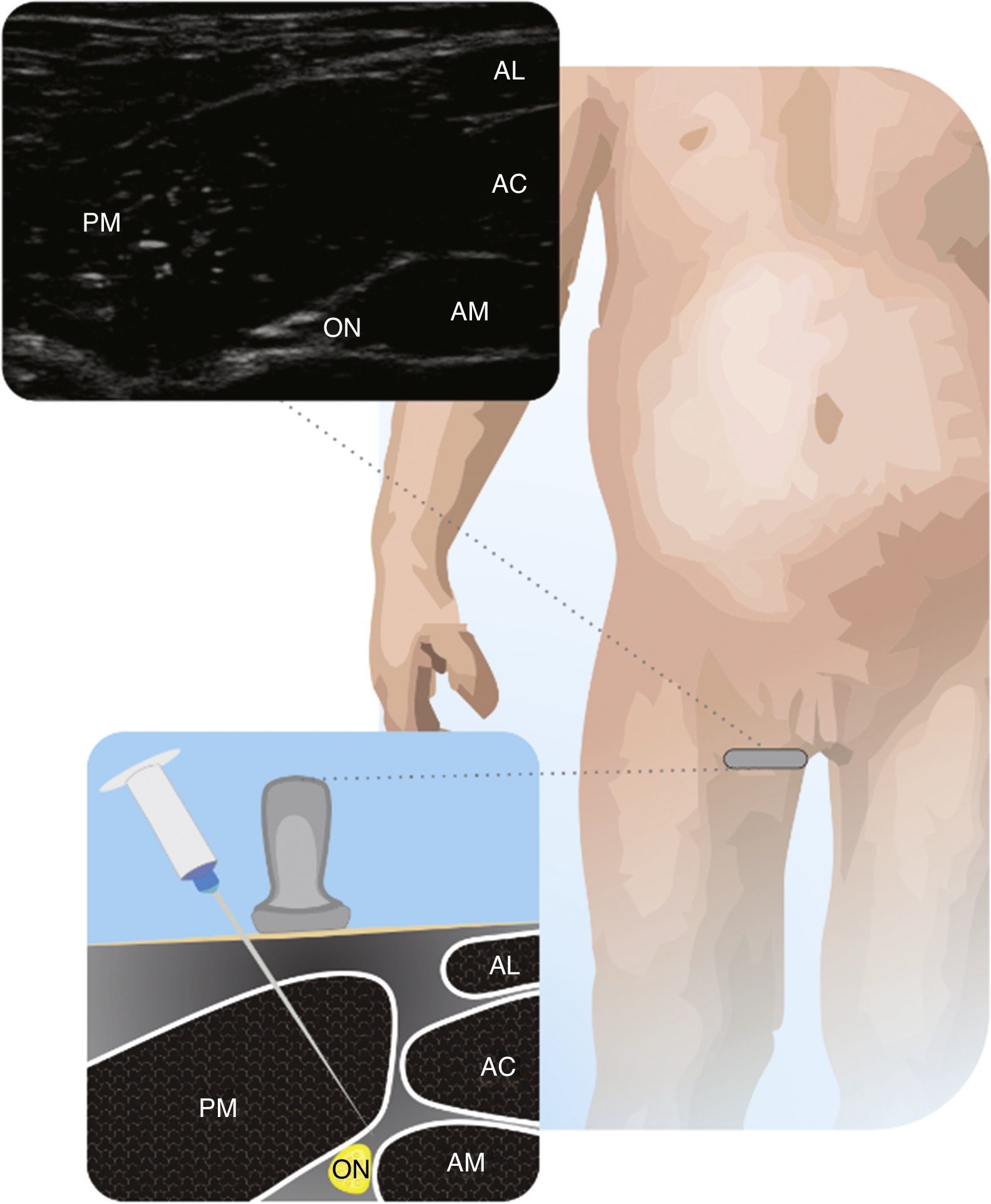
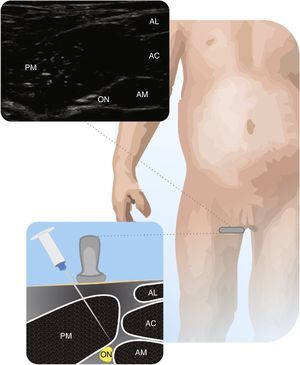
Obturator nerveRecommended to complement femoral block analgesia in knee surgery. The number of literature reports on pediatric ON block is limited28. To perform the ON block, the FA is identified in the inguinal fold, and the probe advances medially toward the pubic symphysis until the three adductor muscles are identified. The two branches of the ON are superficial and deep to the short adductor, and the approach may be either in-plane or out-of-plane24. Fig. 7 illustrates the Obturator Nerve relative to the adductor muscles.
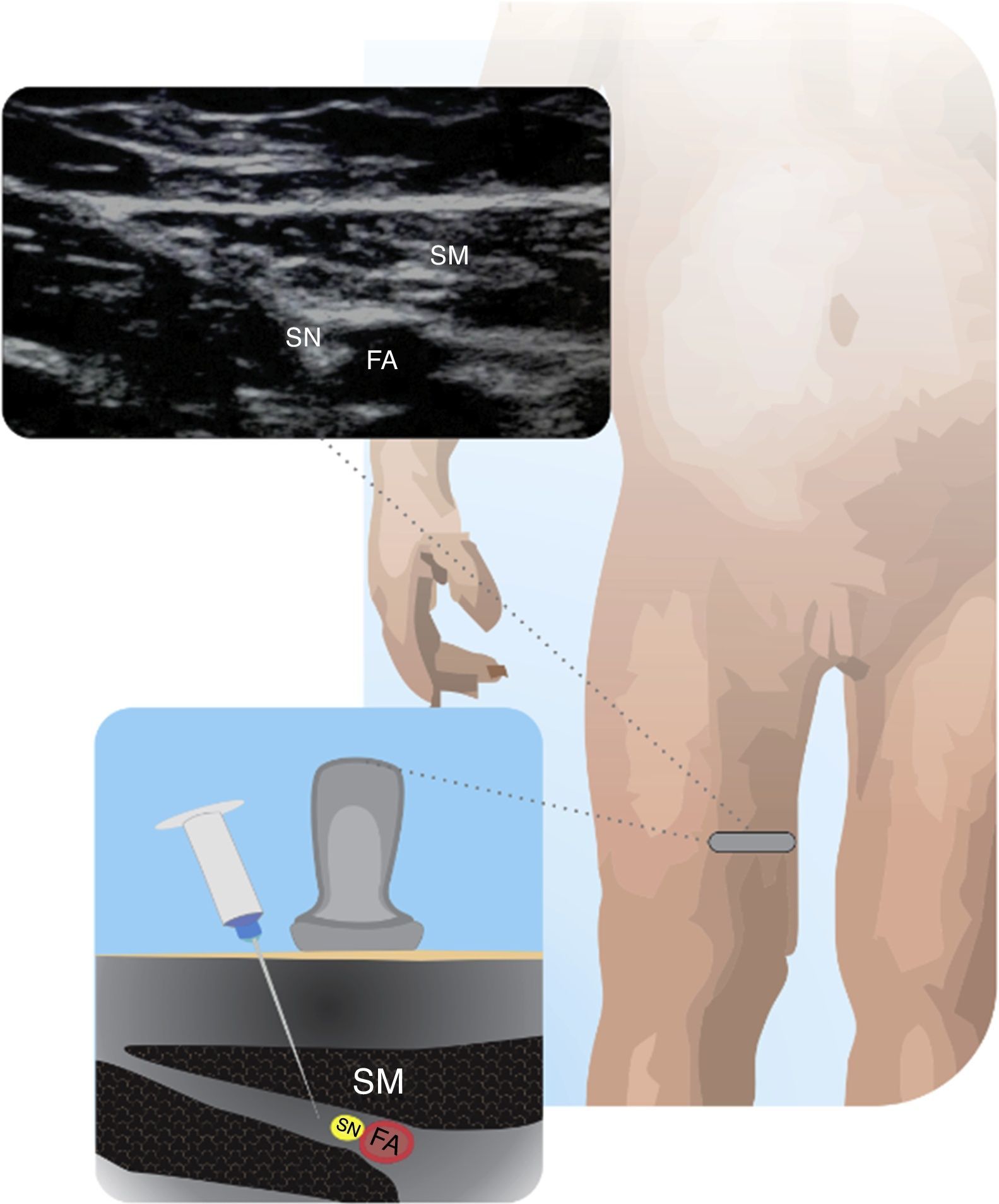
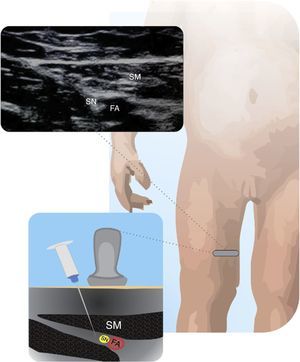
Saphenous nerveIt can be used to complement the sciatic nerve block for foot and ankle surgeries. Selective SN block avoids weakening the femoral quadriceps. For the subsartorial approach, the child is placed with a slight external rotation of the hip and knee flexion. The FA is localized medially to the muscle. Advance caudally until the separation of the artery and the nerve. The needle enters in an anteroposterior direction, between the vastus medialis and the sartorium29,30. Fig. 8 shows the SN in relationship to the FA and the sartorium muscle in the distal third of the muscle.
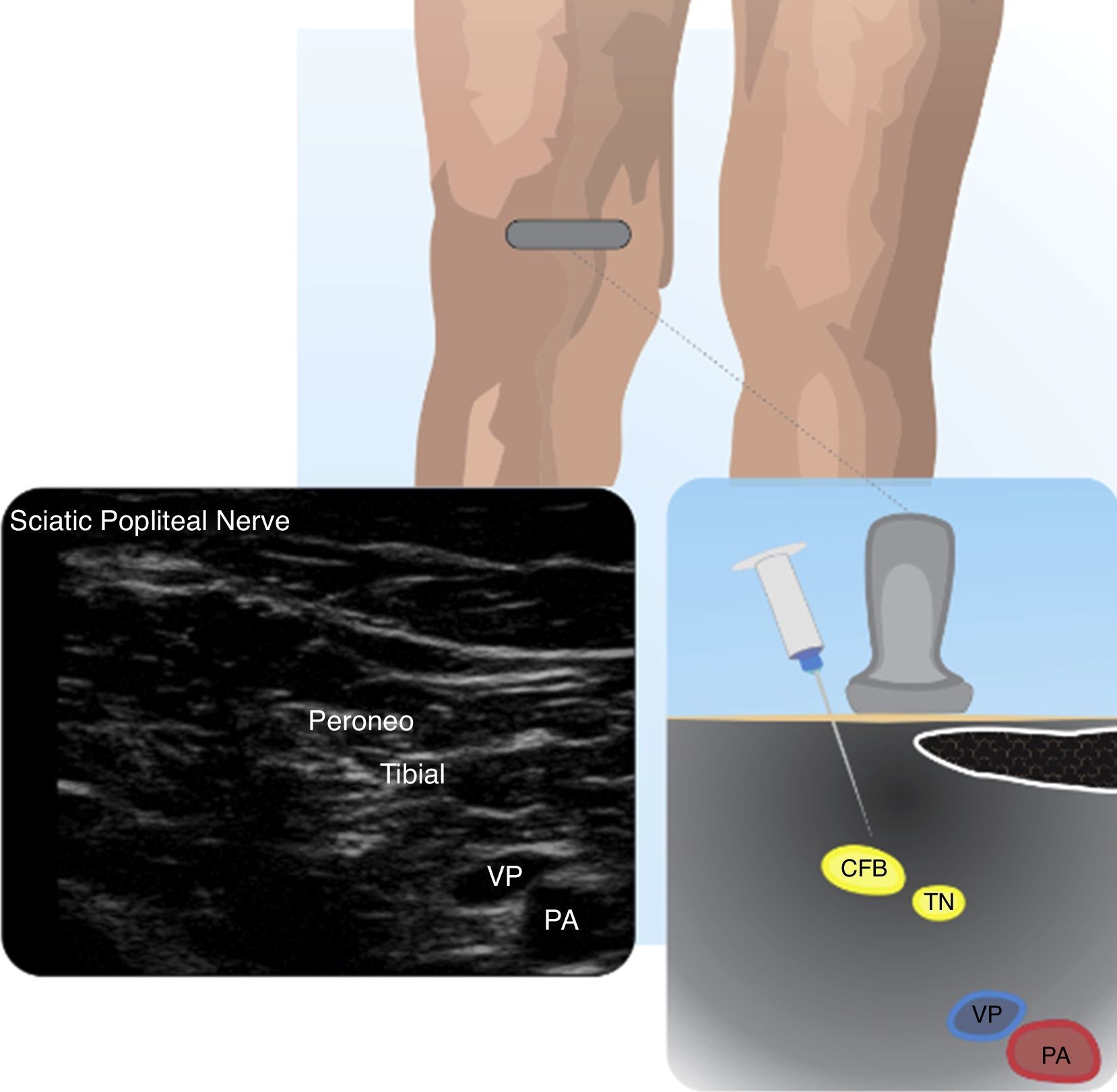
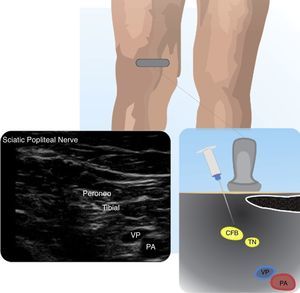
Popliteal sciatic nerve blockThis approach is useful for surgical procedures of the tibia, the fibula, the posterior aspect of the knee, ankle and foot24. The spread of the anesthetic agent around the nerve is an important parameter for the rapid block onset31. Both in-plane and out-of-plane approaches may be used24. Fig. 9 illustrates the PSN with its two components and the position versus the popliteal vessels.
Abdominal wall blocksAlthough the pediatric neuraxial blocks have been used as analgesic techniques with excellent results, these blocks have undesirable side effects. Peripheral blocks may avoid these side effects and provide similar analgesia32. The use of ultrasound to guide these blocks has led to higher efficacy than the techniques based on anatomical landmarks32.
These include:
Transverse abdominis plane blockThis block was described by Rafi33 as a blind technique, and although it was used for many years, US has further expanded its use.
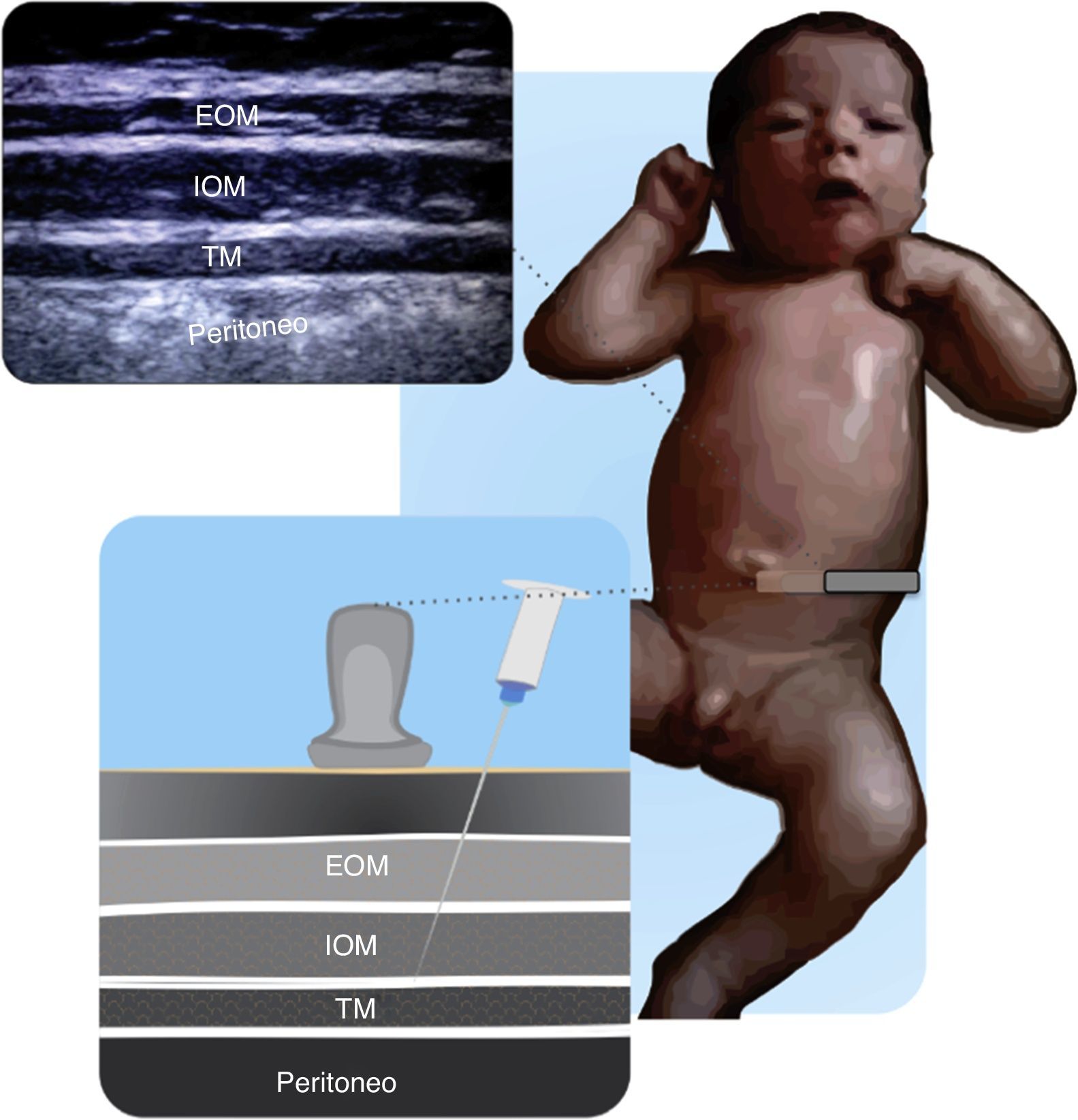
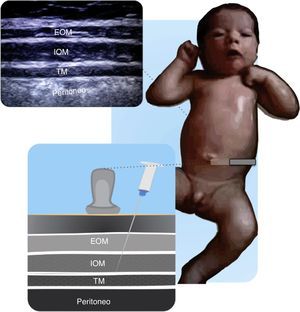
The abdominal wall is innervated by the anterior branches of T6 to L1, running between the internal oblique and transverse muscles of the abdomen34,35. Figs. 10.1 and 10.2 illustrate the technique for placing the transducer and visualizing the muscle groups.
The indications for this particular block are abdominal wall surgeries, urology, and any patient conditions that are a contraindication for neuraxial blocks. The TAPB has a longer effect and improved quality of analgesia than infiltration of the surgical wound in children between 2 and 8 years old36. Being an analgesic block, the use of long-lasting local anesthetic agents at low concentrations is recommended.
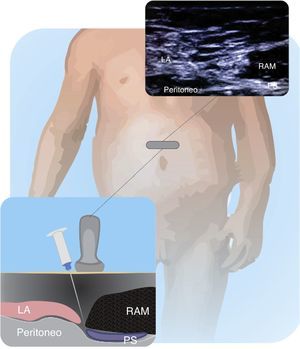
Ilioinguinal and iliohypogastric block (II–IH)Used for procedures in the inguinal region and for urological surgeries, this approach was shown to be equivalent to the caudal block32, and some reports claim longer analgesia and less frequent use of rescue analgesics37.
This block was performed for many years using anatomical landmarks, but some trials report the correct placement of the LA in only 14% of the cases38, in addition to other complications such as intestinal puncture39.
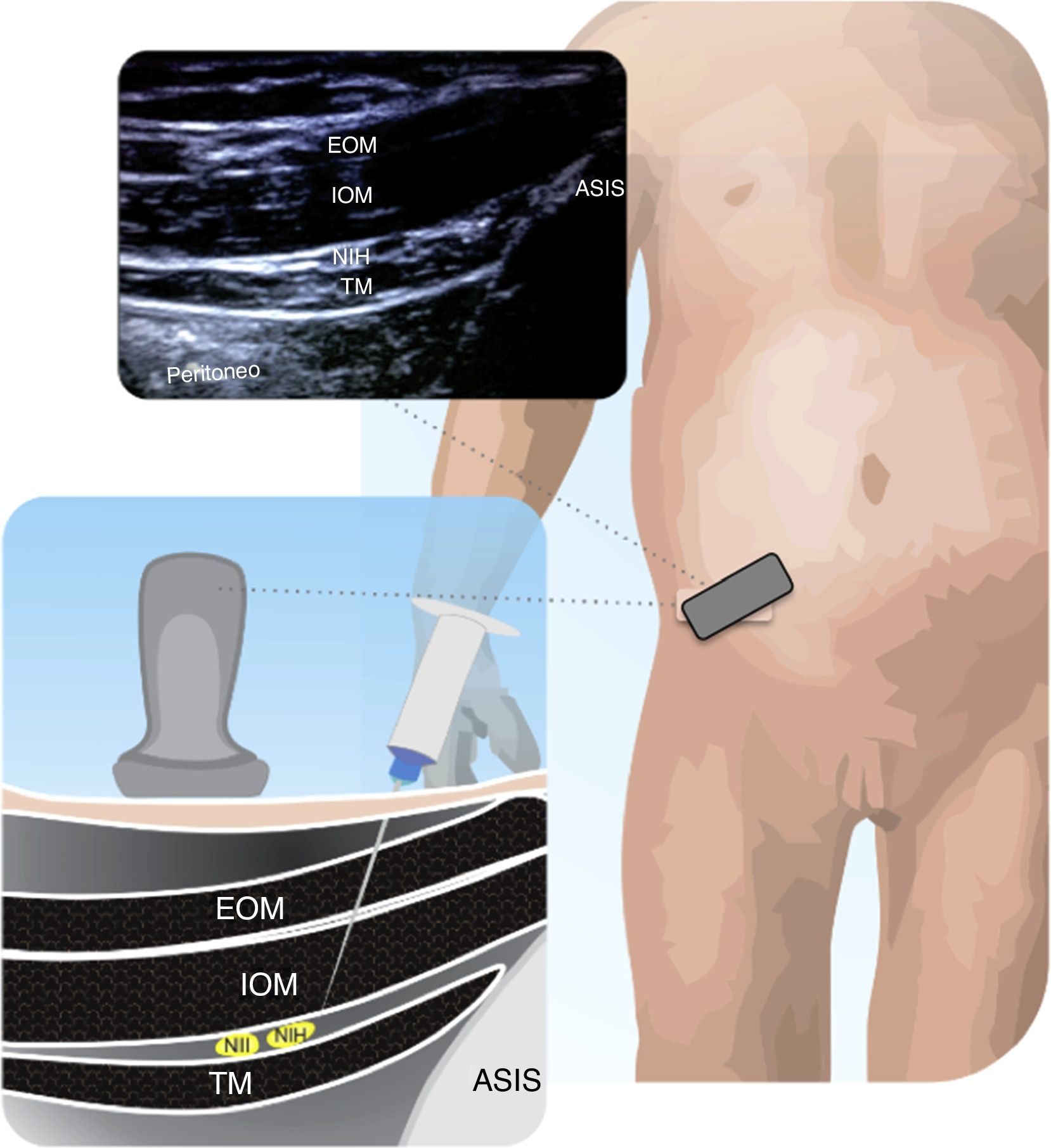
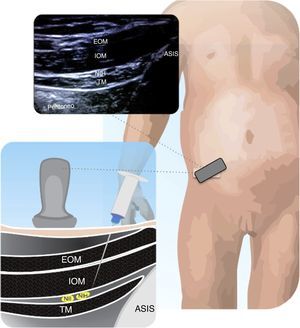
Figs. 11.1 and 11.2 illustrate the technique for placing the probe, enabling the visualization of the iliac crest, the Ilioinguinal and Iliohypogastric nerves, the muscle groups and the peritoneum. The objective of the block is to reach the fascia separating the internal from the transverse oblique40.
Anatomical structures identified during an ilio inguinal and ilio hypogastric (II–IH) nerve block. Transverse muscle (TM), Internal oblique (IO) and external oblique (EO); anterosuperior iliac spine (ASIS). The local anesthetic agent deposits between the TM and the IOM.
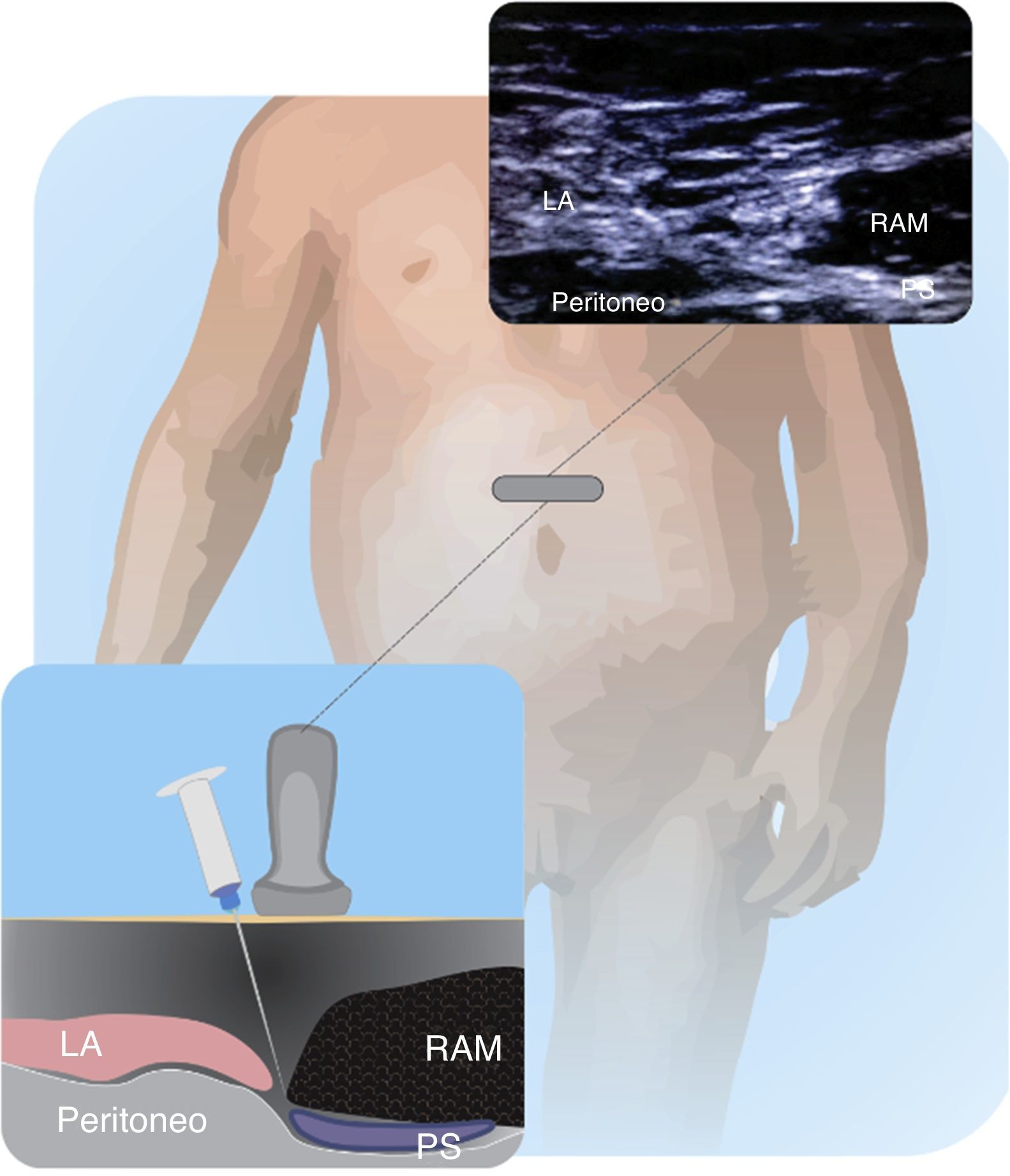
The use of the rectus sheath block in children was originally described by Ferguson et al41. and Courreges et al42. for umbilical hernia repair, pyloric-myotomies and abdominal mid-line incisions. The nerve roots run between the posterior sheath formed by the fascia of the internal and transverse oblique muscles. US has expanded the use of this block because it is easy and effective. Figs. 12.1 and 12.2 depict the technique for placing the transducer and the block target structures.
NE was introduced in the 1960s as an alternative to the paresthesia technique, objectively localizing the nerve and allowing for the injection of the agent as close as possible avoiding any injuries43. Following the introduction of ultrasound, the technique has been compared against other existing tools in an attempt to emphasize its advantages in terms of safety and the prevention of complications; however, since the occurrence of adverse events is rare in Regional Anesthesia, no significant differences have been identified44,45.
One of the current advantages of NE is the combined use with ultrasonography to prevent intraneural injection. Neurostimulation with less then 0.2mA is indicative of intraneural localization. This explains why using both techniques is useful and may prevent complications46.
NE may be used to check the position of the needle and the catheter into the epidural space in 80–100% of the cases, particularly if the procedure is performed with the patient anesthetized or sedated47.
New local anestheticsLevobupivacaine and ropivacaine have an improved safety profile as compared against racemic bupivacaine and should be used as a routine for central and peripheral blocks1,15,48. Both agents are pure enantiomers S(−) with an improved profile and adequate sensory block, in addition to lower risk of cardiac fiber block. Local Anesthetics bind to plasma proteins, particularly to the acid alpha-1 glycoprotein that has a low concentration at birth and increases during the first year of life. Cytochrome CYP1A2 that metabolizes lidocaine and ropivacaine is immature until age 4–749. Hence, neonates and infants are prone to LA toxicity because of the increased free fraction, reduced clearance and increased susceptibility to cardiac toxicity.
The recommended doses vary depending on the block; however, the average dose is 2mg/kg for ropivacaine and 2.5mg/kg for levobupivacaine1,50. Dosing for continuous infusion in epidural and perineural blocks ranges from 0.2 to 0.6mg/kg/h in both cases50.
ConclusionThe renewed interest on Pediatric Regional Anesthesia is due to its adequate pain control and the use of ultrasound that enables the visualization of the anatomical structures, the needle and the spread of the local anesthetic agent, all of which translates into an improved safety profile and less complications. Administering blocks to anesthetized or sedated children is safer than in patients who are awake. Ultrasound guidance is not absolutely risk-free and therefore, it is recommended to include training in ultrasound guided regional anesthesia as part of the standard curricula, to develop skills for everyday clinical practice.
Conflict of interestNone.
FundingThe authors have no conflicts of interest to declare.
Please cite this article as: Ríos Medina AM, Caicedo Salazar J, Vásquez Sadder MI, Aguirre Ospina OD, González MP. Anestesia regional en pediatría – Revisión no sistemática de la literatura. Rev Colomb Anestesiol. 2015;43:204–213.