Mild Cognitive Impairment (MCI) is common in Parkinson's Disease (PD). Few studies have compared the Health-Related Quality of Life (HRQoL) in patients with and without MCI due to PD (PD-MCI), and its correlation to patients’ subjective cognitive and communicative difficulties has not been explored.
ObjectiveWe aimed to compare HRQoL in PD-MCI and PD without MCI (PD-nMCI), and explore its possible relationship to subjective cognitive and communicative complaints.
MethodsWe included 29 PD-nMCI and 11 PD-MCI patients. The HRQoL was assessed with the Parkinson's Disease Questionnaire-39 (PDQ-39): its Cognition dimension was used as a measure of subjective cognitive complaints, its Communication dimension for subjective communicative complaints, and the summary index (PDQ-39 SI) as an indicator of HRQoL. Non-parametric partial correlations between the Cognition and Communication dimensions, and the adjusted PDQ-39 SI were conducted.
ResultsPD-MCI patients had greater subjective cognitive and communicative complaints and worse HRQoL than PD-nMCI patients. In the PD-MCI group, both subjective cognitive and communicative complaints exhibited significant direct correlations with the adjusted HRQoL scores.
ConclusionsHRQoL seems to be affected in PD-MCI, and it might be influenced by greater subjective cognitive and communicative complaints. Including patient-reported outcome measures of HRQoL, and providing cognitive and speech rehabilitation, as well as psychotherapeutic strategies to face these deficits can enhance the patient-centred approach in PD.
El deterioro cognitivo leve (DCL) es frecuente en la enfermedad de Parkinson (EP). Pocos estudios han comparado la calidad de vida relacionada con la salud (CVRS) en pacientes con DCL debido a EP (EP-DCL) sin explorar la relación entre la CVRS y las quejas subjetivas cognitivas y comunicativas de los pacientes.
ObjetivoComparar la CVRS en EP-DCL y EP sin DCL (EP-nDCL) explorando sus posibles relaciones con las quejas subjetivas cognitivas y comunicativas.
MétodosSe incluyó a 29 EP-DCL y 11 EP-nDCL. La CVRS se evaluó con el cuestionario PDQ-39: su dimensión Cognición se usó como medida de las quejas subjetivas cognitivas; su dimensión Comunicación, como medida de las quejas subjetivas comunicativas y su puntuación resumen (PDQ-39 SI), como indicador de CVRS. Se realizaron correlaciones parciales no paramétricas entre el PDQ-39 SI ajustado y las dimensiones Cognición y Comunicación.
ResultadosLos pacientes EP-DCL presentaron una peor CVRS y mayores quejas subjetivas cognitivas y comunicativas. En el grupo EP-DCL, tanto las quejas subjetivas cognitivas como las comunicativas mostraron correlaciones directas significativas con la puntuación de CVRS ajustada.
ConclusionesLa CVRS de los pacientes con EP-DCL parece estar afectada e influida por las quejas subjetivas en cognición y comunicación. Incluir los resultados de CVRS reportados por los pacientes, proveer rehabilitación cognitiva y del lenguaje y estrategias de psicoterapia para afrontar dichos déficit podría mejorar el abordaje centrado en el paciente en la EP.
Parkinson's disease (PD) is one of the most prevalent neurodegenerative diseases and is primarily related to motor symptoms.1 However, cognitive decline in PD patients has been highlighted among other non-motor symptoms due to its high prevalence, and its negative effects on wellbeing and prognosis.2,3 Mild cognitive impairment (MCI) due to PD (PD-MCI) is present in approximately 36% of the PD cases, even at the early stages of the disease, and it has been identified as a risk factor for progression to PD dementia (PDD).1,3 In PD patients with normal cognition, the presence of subjective cognitive complaints seems to be an indicator of the subsequent onset of MCI and functional decline.4 On the other hand, up to 89% of PD patients present speech difficulties such as dysarthria, hypophonia, and loss of articulation.11 Also, language disorders at the pragmatic level have been reported in PD.12 Therefore, speech and language disorders could generate subjective communication complaints related to language impairment at the phonemic and pragmatic level.
As evidence-based medicine perspectives have emphasized the importance of patient-reported outcome measures in chronic diseases, the health-related quality of life (HRQoL) has become a crucial target in neurodegenerative diseases.5 The HRQoL is a multidimensional construct including physical and mental health, social, emotional, and other domains.6 Thus, this patient-centered approach considers the patient's beliefs, ideas, and subjective perception of health, as well as the implications of the disease symptoms over satisfaction and individual well-being.7 However, the perception of mental health (especially of subjective cognitive complaints) could be affected by several factors in PD patients, such as mood symptoms,8 lack of knowledge about the cognitive symptoms related to PD,9 and lack of self-awareness for cognitive deficits (i.e. anosognosia).10 Given the possible confounder effect of anosognosia in advanced stages of PD (i.e. PDD),10 evaluating the impact of subjective cognitive and communicative complaints on HRQoL in earlier stages of PD (i.e. MCI) is valuable for a better understanding of the determinants of HRQoL.
Few preliminary studies have compared the HRQoL in PD patients with and without MCI,11 thus, our first objective is to compare the HRQoL in PD patients with and without MCI. In addition, we are not aware of studies exploring the relationship between self-perceived cognition-communication difficulties and HRQoL. Therefore, our second objective is to explore the potential relationships between the subjective cognitive and communicative complaints, and HRQoL in PD patients with and without MCI. We hypothesize that PD patients with MCI will have a worse HRQoL, and they will report more subjective cognitive and communicative complaints that will be correlated with a worse HRQoL. Our exploratory results may contribute to the patient-centered approach enhancing the comprehension of the effects of MCI over self-perceived mental health and HRQoL in PD patients.
Materials and methodsSample selectionThis is a secondary analysis of the project “Procesamiento del lenguaje de acción en pacientes con enfermedad ganglio-basal desde una perspectiva neuropsicológica y neurofisiológica” (“Action language processing in patients with basal-ganglia disease from a neuropsychological and neurophysiological perspective”) carried out at the Universidad de Antioquia (University of Antioquia) in 2015, recruiting a convenience sample of PD patients from the outpatient service of the Grupo de Neurociencias de Antioquia (Neurosciences group of Antioquia).12 The study had the approval of the Ethical Research Committee of the Universidad de Antioquia (Certificate No. 15-10-569), and all participants signed an informed consent form before enrolment in the study. The data was handled and kept following national health and data privacy protocols.
We included 29 PD patients without MCI (PD-nMCI) and 11 PD patients with MCI (PD-MCI). Participants with parkinsonian syndromes other than PD, other major neurological or psychiatric disorders, and dementia (based on impairment in cognition and function)13 were excluded. Detailed criteria regarding PD and MCI diagnosis are stated below.
Clinical assessmentA team of two neurologists and one trained physician (general practitioner) determined the PD diagnosis following the Movement Disorder Society clinical diagnostic criteria.14 The Hoehn & Yahr scale15 and the Unified Parkinson's Disease Rating Scale part III (UPDRS-III)16 were used for evaluating the severity of the disease and motor symptoms, respectively. Levodopa Equivalent Daily Dose (LEDD) was determined from the individual medication of patients, all patients had received stable antiparkinsonian treatment during at least 4 weeks before evaluations. All assessments were completed in phase ‘On’ of levodopa treatment.
The PD-nMCI patients had a Montreal Cognitive Assessment score (MoCA) of 23 or above (according to validation in the Colombian population17,18). The PD-MCI patients were diagnosed following the level one task force criteria of the Movement Disorders Society19 (i.e. observed or self-reported gradual cognitive decline in the clinical or neuropsychological examination, MoCA<23, and no significant cognition-related functional decline). In those who reported subjective cognitive complaints, the evaluator examined carefully if the difficulties are related to functional decline (i.e. impairs the ability of the patient to perform instrumental or basic activities of daily living). The latter was assessed using the Barthel Index20 and Lawton & Brody scale.21
All the neuropsychological tests (i.e. Neuropsychiatric Inventory, MoCA, Lawton & Brody, Barthel, and HRQoL scale) were performed by a team of 3 trained psychologists who also confirmed PD-MCI diagnosis and absence of exclusion criteria (i.e. dementia).
Since anosognosia could influence subjective cognitive or communicative complaints, due to the lack of awareness deficits,10 we evaluated anosognosia especially in the PD-MCI group through the clinical examination but none of the patients presented this symptom.
Health-related quality of lifeHRQoL was assessed with a specific scale for PD, the Parkinson's Disease Questionnaire-39 (PDQ-39)22 due to its reliability, psychometric properties, validation across different countries, and translations in many languages.8. The questionnaire consists of 39 items grouped in eight dimensions (i.e. mobility, activities of daily living, emotional wellbeing, stigma, social support, cognition, communication, and bodily discomfort) and an averaged summary index of the 8 dimensions of PDQ-39 (PDQ-39 SI) that provides a measure of the global HRQoL. Each of the 39 items was scored using a 5-point Likert scale, ranging from “never” to “always/cannot do at all”. Therefore, a higher score in PDQ-39 indicates a poor HRQoL.22 To avoid mistakes while filling up the PDQ-39, the evaluator marked the patients’ answers in the form, and clarified any item if necessary.
Based on previously reported methods,23 we analyzed the Cognition dimension as a measure of subjective cognitive complaints, and the Communication dimension was used as a measure of subjective communicative complaints. High scores in the Cognition or Communication dimension indicates high subjective cognitive or communication difficulties reported by the patient. The Cognition dimension includes 4 items (items 30-33) of the PDQ-39, such as impaired concentration, memory complaints, and cognitive fluctuations (e.g., Had problems with your concentration when reading, watching TV, etc.?; Felt your memory was bad?).22 The Communication dimension includes 3 items (items 34-36) of the PDQ-39 that point out speech and communication problems (e.g., Had difficulty with your speech?; Felt unable to communicate with people properly?; Felt ignored by people?).
Finally, the PDQ-39 SI was taken as our outcome variable, indicating the global HRQoL. To face the circularity problems when analyzing the influence of a dimension over the summary index of the PDQ-39, we adjusted the PDQ-39 SI excluding the items of the Cognition or Communication dimension as appropriate (see “Statistical analysis”).
Statistical analysisDemographic and clinical group differences were assessed using Mann-Whitney's U for continuous variables, and the Chi-square test for categorical variables. Group differences in clinical and demographic variables (i.e. age, years of education, gender, and motor symptoms) were explored to evaluate the homogeneity between groups, and the effect size was calculated using the Wilcoxon r statistic (r=Z/√N) for continuous variables24, and the phi coefficient —φ=√(X2/N)— for categorical variables.25 Given the possible confounder effect of motor symptoms due to heterogeneity between PD-nMCI and PD-MCI groups, we controlled the subsequent analyses for the score of the UPDRS-III.
For our first objective, group differences of the non-adjusted PDQ-39 SI were evaluated and its effect size was calculated using the Wilcoxon r statistic.24 An additional analysis of the group differences in each of the eight dimensions of PDQ-39 was also performed, and these resulting p-values were corrected for multiple testing using the false discovery rate (FDR) method setting a significance threshold of .05.26
For our second objective, non-parametric bivariate correlations between the Cognition dimension score and an adjusted PDQ-39 SI (excluding the items of the Cognition dimension) were performed following previous methods.27 Similarly, to explore the correlations between the score of the Communication dimension and PDQ-39 SI, we computed an adjusted summary index (PDQ-39 SI) excluding the items of the Communication dimension to avoid circularity issues. Each correlation model was performed separately in both groups, and the resulting P-values of these correlations were corrected for multiple testing using the FDR method.
In addition, given the differences in motor symptoms (UPDRS-III), the results of the latter correlation models were checked in each group using non-parametric partial correlations controlling for the effect of UPDRS-III. These resulting P-values were also corrected for multiple testing (Table 3).
Motor-adjusted partial correlations between the global HRQoL, and the Cognition and Communication dimensions in PD-nMCI and PD-MCI groups.
| Dimension | Group | Correlation with adjusted PDQ-39 Summary Indexa | ||
|---|---|---|---|---|
| Partial (controlling for) UPDRS-III | ||||
| Coefficient | P-value (FDR) | S-value | ||
| Cognition | PD-MCI | .728 | .017 (.034) | 5.88 |
| PD-nMCI | .14 | .477 (.477) | 1.07 | |
| Communication | PD-MCI | .76 | .011 (.043) | 6.51 |
| PD-nMCI | .37 | .052 (.070) | 4.27 | |
FDR: false discovery ratio; PD-MCI: Parkinson's disease with mild cognitive impairment; PD-nMCI: Parkinson's disease without mild cognitive impairment; UPDRS-III: Unified Parkinson's Disease Rating Scale part III (motor subscale).
For the correlations with the Cognition dimension, the PDQ-39 Summary Index was calculated excluding the “Cognition” items. For the correlations with the Communication dimension, the PDQ-39 Summary Index was calculated excluding the “Communication” items.
Spearman partial correlations were adjusted for UPDRS-III to control the effect of motor symptoms in the model estimations.
Previous reports suggested a possible confounder effect of depression or anxiety in the self-perception of cognition difficulties.8,28 Thus, we used the Neuropsychiatric Inventory (NPI)29 to measure depression and anxiety symptoms based on synoptic scores (i.e. frequency × severity) of the corresponding items. In each group, we conducted non-parametric bivariate correlations between the PDQ-39 variables (i.e. Cognition and Communication dimensions, as well as each of the adjusted summary indexes of the PDQ-39), and the NPI scores of depression and anxiety, in order to examine these potential confounders (Table 1 of the supplementary material). Additional non-parametric partial correlations controlling for the effect of depression, anxiety, as well as the combined effect of depression, anxiety, and motor symptoms, were explored (Table 2 of the supplementary material).
Non-parametric bivariate correlations between the Cognition and Communication dimensions, and the non-adjusted PDQ-39 SI, were also conducted to explore the dimension-total correlations as part of the psychometric characteristics of the instrument (Table 3 of the supplementary material).
Given the possible effect of our sample size in the P-values, and to provide a better scale for measuring the amount of information the test supplies against the hypothesis, we rescaled all the P-values into Shannon information values (S-values) following previously described methods.30 Statistical analyses were performed using SPSS (version 25)31 and JASP (version 0.13.1.0).32
Results40 patients were included. Table 1 summarizes the characteristics of PD-MCI and PD-nMCI patients, showing homogeneity between groups except for the MoCA test (used for classification of the cognitive status of the groups), and motor symptoms. The latter can be seen in the higher UPDRS-III score of the PD-MCI group. We did not find significant differences for age, neuropsychiatric symptoms (NPI Total), nor depression (P=.939; r=–.012) or anxiety scores (P=.397; r=–.134).
Demographic and clinical characteristics of the sample.
| PD-nMCI(n=29) | PD-MCI(n=11) | P-valuea | Effect sizeb | |
|---|---|---|---|---|
| Age (years) | 63 [54-66] | 65 [60-69] | .225 | –.192 |
| Gender (F/M) | 11/18 | 3/8 | .528c | .115d |
| Education (years) | 13 [8-16] | 13 [5-16] | .693 | –.063 |
| Years since diagnosis of PD | 5 [3-8] | 7 [3-8] | .394 | –.135 |
| LEDD | 600 [350-800] | 750 [300-1048] | .379 | –.139 |
| Hoehn & Yahr | 2 [2-2] | 2 [2-2] | .854 | –.029 |
| UPDRS-III score | 27 [22-31] | 35 [28-41] | .030 | –.343 |
| MoCA total score | 26 [26-28] | 22 [19-23] | <.001 | –.771 |
| NPI total score | 5 [1-9] | 8 [0-19] | .428 | –.126 |
F/M: female/male; LEDD: levodopa equivalent daily dose; MoCA: Montreal Cognitive Assessment; NPI: neuropsychiatric inventory; PD-MCI: Parkinson's disease with mild cognitive impairment; PD-nMCI: Parkinson's disease without mild cognitive impairment; UPDRS-III: Unified Parkinson's Disease Rating Scale part III (motor subscale).
The median score of the PDQ-39 SI in the PD-nMCI group was 13.6, and in the PD-MCI group was 29.3. The PDQ-39 SI scores were higher in PD-MCI patients (P=.030), exhibiting a moderate effect size of the difference (r=–.342) (figure 1).
Self-perception of HRQoL in PD-nMCI and PD-MCI. PD-MCI: Parkinson's disease with mild cognitive impairment; PD-nMCI: Parkinson's disease without mild cognitive impairment; PDQ-39 SI: Parkinson's Disease Questionnaire-39 Summary Index; r: effect size (Wilcoxon); P: p-value; S: Shannon information-value.
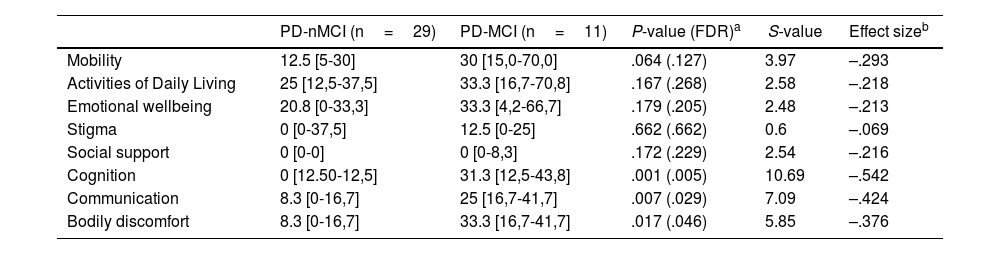
Patients diagnosed with PD-MCI reported higher scores in all the dimensions of PDQ-39 (Table 2). Differences were found in three dimensions after correction for multiple testing (i.e. Cognition, Communication, and Bodily discomfort). Estimations of the effect size showed large differences in the Cognition dimension. A moderate effect size of the difference was found in the Communication, and Bodily discomfort dimensions.
Dimensions of the PDQ-39 in PD-nMCI and PD-MCI groups.
| PD-nMCI (n=29) | PD-MCI (n=11) | P-value (FDR)a | S-value | Effect sizeb | |
|---|---|---|---|---|---|
| Mobility | 12.5 [5-30] | 30 [15,0-70,0] | .064 (.127) | 3.97 | –.293 |
| Activities of Daily Living | 25 [12,5-37,5] | 33.3 [16,7-70,8] | .167 (.268) | 2.58 | –.218 |
| Emotional wellbeing | 20.8 [0-33,3] | 33.3 [4,2-66,7] | .179 (.205) | 2.48 | –.213 |
| Stigma | 0 [0-37,5] | 12.5 [0-25] | .662 (.662) | 0.6 | –.069 |
| Social support | 0 [0-0] | 0 [0-8,3] | .172 (.229) | 2.54 | –.216 |
| Cognition | 0 [12.50-12,5] | 31.3 [12,5-43,8] | .001 (.005) | 10.69 | –.542 |
| Communication | 8.3 [0-16,7] | 25 [16,7-41,7] | .007 (.029) | 7.09 | –.424 |
| Bodily discomfort | 8.3 [0-16,7] | 33.3 [16,7-41,7] | .017 (.046) | 5.85 | –.376 |
FDR: false discovery ratio; PD-MCI: Parkinson's disease with mild cognitive impairment; PD-nMCI: Parkinson's disease without mild cognitive impairment.
The median [interquartile range] cognition-adjusted PDQ-39 SI in the PD-nMCI group was 15.6 [6.43-28.99], and in the PD-MCI group was 30.48 [10,12-43,63], as reported in figure 2J of the supplementary material.
Figure 2A depicts the bivariate non-parametric correlation between the Cognition dimension and the Cognition-adjusted PDQ-39 SI (i.e. excluding the items of the Cognition dimension). After correction for multiple testing, significant strong correlations were seen in the PD-MCI group but not in the PD-nMCI group.
Correlation between the global HRQoL and the Cognition and Communication dimensions. 95%CI: 95% confidence interval; FDR: false discovery ratio; PD- nMCI: Parkinson's disease without mild cognitive impairment; PD-MCI: Parkinson's disease with mild cognitive impairment; PDQ-39: Parkinson's Disease Questionnaire-39.
Similar findings were observed when controlling the correlations for the group differences in motor symptoms (UPDRS-III). The Cognition dimension and the global HRQoL (i.e. cognition-adjusted PDQ-39 SI) were significantly correlated only in the PD-MCI group, as reported in Table 3.
Self-perceived communication and its relation to HRQoLThe median [interquartile range] Communication-adjusted PDQ-39 SI in the PD-nMCI group was 12.86 [6.43-27.80], and in the PD-MCI group was 29.88 [12.80-43.33], as showed in figure 2K of the supplementary material.
Figure 2B shows the bivariate correlation between the Communication dimension and the Communication-adjusted PDQ-39 SI (i.e. excluding the items of the Communication dimension). Significant correlations were seen in both groups after correcting for multiple testing. However, the PD-MCI group exhibited strong correlations while the PD-nMCI had moderate correlations.
Table 3 shows the results of these correlations when controlling for motor symptoms (UPDRS-III). The Communication dimension and the global HRQoL (i.e. communication-adjusted PDQ-39 SI) were significantly correlated only in the PD-MCI group.
Effect of depression and anxiety symptoms over self-perceived cognition or communication difficulties and global HRQoLAs we did not find group differences in depression or anxiety scores, we did not adjust the main correlation models for these confounders. In spite of it, we explored in each group the correlation of these symptoms, and the PDQ-39 dimensions, adjusted and unadjusted PDQ-39 summary indexes. Given the exploratory nature of these correlations, we did not perform correction for multiple testing.
As showed in Table 1 of the supplementary material, none of the groups exhibited significant correlations between the depression or anxiety scores and the analyzed PDQ-39 scores (i.e. Cognition, Communication, PDQ-39 SI, cognition-adjusted PDQ-39 SI, and communication-adjusted PDQ-39 SI). Depression only showed significant strong correlations in the PD-MCI group with the Emotional well-being dimension (ρ=.62; P=.002). In the PD-nMCI group, depression was significantly correlated with the stigma (ρ=.479; P=.009) and the social support dimensions (ρ=.545; P=.002). Anxiety scores were not correlated with any of the PDQ-39 variables in none of the groups (Table 1 of the supplementary material).
Finally, we explored non-parametric partial correlations between the Cognition and Communication dimensions, and the adjusted PDQ-39 SI, these correlations were controlled for depression, anxiety and the combination of motor and mood symptoms (Table 2 of the supplementary material). In the PD-MCI group, our findings regarding the relationship between the Cognition and Communication dimension and the adjusted PDQ-39 SI remain consistent after controlling the correlation models for all the possible confounders. Also, the PD-nMCI group exhibited significant correlations between the Communication-adjusted PDQ-39 SI and the Communication dimension after (Table 2 of the supplementary material).
DiscussionThis work explored the relationship between the HRQoL and the subjective cognitive and communicative complaints in PD patients with and without MCI. Our results showed that subjective cognitive and communicative complaints are significantly correlated with the HRQoL in PD-MCI patients, but not in the PD-nMCI group. Therefore, assessing the HRQoL and the subjective cognitive and communicative complaints might be useful to quantify important patient-reported outcomes in PD patients,33,34 especially those with MCI.
Cognitive decline has been recognized as an important factor that impacts negatively the HRQoL in PD patients.3,35–37 We found a poorer HRQoL in the PD-MCI group with the largest effect size of the difference in the Cognition dimension of HRQoL. One preliminary work has tried to determine what can be considered “minimally important differences in HRQoL” using the PDQ-39, showing that those differences vary across the PDQ-39 dimensions.38 Thus, in our study, the observed differences between PD-MCI and PD-nMCI in the PDQ-39 SI, the Cognition, and the Communication dimensions seem to be considered important for the patients.38 Also, in line with our findings, preliminary reports have shown worse HRQoL in PD patients with an abnormal cognitive screening test,36 those with PD-amnestic MCI,37 as well as those with more severe MCI.39 By contrast, other studies found non-significant differences in the Cognition dimension or the global HRQoL when comparing PD patients with and without MCI.11,28 However, their conclusions only can be generalized to populations with a high level of education (>15 years).11 In our case, the median years of education was below 15 years in both groups. Therefore, even if cross-sectional evidence suggests that low education level does not impact the HRQoL in PD patients,36 further research including larger and heterogeneous samples will elucidate the effect of education over subjective cognitive complaints and HRQoL in PD-MCI patients.
Beyond, multiple factors can affect the scores of the Cognition dimension: Lack of awareness of the cognitive deficits (i.e. anosognosia) that emerges in severe cognitive decline (i.e. PD dementia).10 Other mental health factors such as anxiety, depression, and optimism seem to explain the worsening in the Cognition subscale and the HRQoL better than the neuropsychological performance.8,28 Comparable results were described in PD patients with normal cognition: the higher the depressive symptoms, the higher the subjective cognitive complaints.35 However, our results did not show significant correlations between depression or anxiety and the HRQoL nor the Cognition or Communication dimensions. Also, our findings regarding the relationship between HRQoL and subjective cognitive and communicative complaints were consistent in the PD-MCI group after controlling for depression, anxiety, and motor symptoms, but the relationship of HRQoL and Communication dimension in the PD-nMCI group seems to be significant. We are aware that these results should be interpreted with caution since the NPI is not a specific instrument for mood disorders, and future studies with specific protocols will contribute to unveil the role of mood disorders as determinants of HRQoL in PD.
It has been proposed that the Cognition dimension (specifically the attention and memory items) could be valuable measures of how the patients subjectively rate their cognitive performance and should be examined as an endpoint and predictor of cognitive decline in larger studies.40 In addition to the Cognition dimension, we have evaluated the Communication dimension (i.e. phonetic and pragmatic level of language). In line with our findings, preliminary reports have suggested increased subjective communication difficulties in PD-MCI patients.11,28 Also, the prognostic impact of the subjective cognitive complaints has been recently evaluated in PD patients with normal cognition, showing that subjective cognitive complaints have been associated with greater decline in cognition-related functional abilities, suggesting that subjective cognitive complaints may be a sensitive indicator of the cognitive decline in PD.4 Therefore, considerìng the subjective cognitive and communicative complaints as well as HRQoL in PD patients with and without MCI, may lead the clinicians to a more comprehensive patient-centered approach with relevant prognostic implications.
The PDQ-39 is an instrument that has been widely applied in clinical research to assess the HRQoL and the multiple dimensions of this construct, demonstrating acceptability, internal consistency, and a holistic approach to both motor and non-motor symptoms of PD.41 Nevertheless, the validity of the Cognition dimension as a subjective measure of cognitive abilities has been questioned since some of the items are not directly related to cognition (such as presence of hallucinations).40,42 Further research analyzing the meaningful items of the Communication and Cognition dimensions as well as the PDQ-39 SI could represent an important patient-reported outcome measure.
Several limitations to this study should be considered when interpreting our results. The small convenience sample size can affect the external validity of our results and the statistical power, it also precluded us from the use of other statistical methods better suited for larger samples. In order to get the external validity of our results, the authors encourage future research to reproduce our exploratory findings. Also, the lack of follow-up reduces the possibility of establishing causal effects. Besides, given the retrospective nature of our study, we did not use a specific measure for subjective cognitive and communicative complaints, nor specific tests for anosognosia or mood symptoms. Additionally, the subtle nonsignificant age difference in our groups should be considered as a potential bias. However, few publications have evaluated the HRQoL in the Colombian population,43,44 and this is the first work examining the impact of subjective cognitive and communicative complaints on HRQoL in PD-MCI patients.
ConclusionsHRQoL seems to be affected in PD-MCI patients, and it might be influenced by greater subjective cognitive and communicative complaints. Thus, including patient-reported measures of HRQoL, and providing cognitive and speech rehabilitation, as well as psychotherapeutic strategies, to face the cognitive and communicative deficits of the patients, could enhance the patient-centered approach in PD.
FundingThis work was supported by the Research Development Committee (CODI) - Universidad de Antioquia (University of Antioquia), Colombia, under the research project: “Procesamiento del lenguaje de acción en pacientes con enfermedad ganglio-basal desde una perspectiva neuropsicológica y neurofisiológica” (grant reference PRG-2014-768). In addition, this paper represents independent research supported by the Norwegian government, through hospital owner Helse Vest (Western Norway Regional Health Authority), and was partly funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Conflicts of interestsThe authors have no conflicts of interests to declare. The sponsor has no role in the collection, analysis, interpretation of the data, and writing of the final manuscript.
We want to thank all the participants and their families, researchers that collaborated, the Research Development Committee (CODI) and Young Researchers program at Universidad de Antioquia, as well as the staff and facilities provided by Grupo de Neurociencias de Antioquia (GNA), Grupo Neuropsicología y Conducta (GRUNECO), Semillero SINAPSIS (research incubator program) and the School of Medicine at the Universidad de Antioquia in Colombia, as well to the Centre for age-related medicine (SESAM) in Norway.