Reactive arthritis describes the relationship between the host and the environment. This leads to urogenital or gastrointestinal infections. It clinically presents with inflammatory lumbosacral pain, asymmetric oligoarthritis and enthesitis of the Achilles tendon and plantar fascia. Among the extra-articular manifestations are acute anterior uveitis, skin lesions, genital lesions, and oral ulcers, with the rarest being cardiovascular. A case is presented of a patient with a urogenital infection and cardiovascular manifestations, interpreted and managed as acute coronary syndrome. After further studies an acute myopericarditis was considered as a primary manifestation of reactive arthritis.
La artritis reactiva describe la interrelación entre el hospedero y el medio ambiente. Aparece después de infecciones urogenitales o digestivas. Clínicamente presenta dolor lumbosacro inflamatorio, oligoartritis asimétrica y entesitis del tendón de Aquiles y la fascia plantar. Entre las manifestaciones extraarticulares, se encuentran la uveítis anterior aguda, lesiones en piel, lesiones genitales y úlceras orales. Las más infrecuentes son las cardiovasculares. Describimos el caso de un paciente con infección urogenital y manifestaciones cardiovasculares interpretadas y manejadas como síndrome coronario agudo, pero que a la luz de estudios posteriores se consideró finalmente una miopericarditis aguda como manifestación primaria de una artritis reactiva.
Reactive arthritis is one of the types of inflammatory arthritis that clearly describes the interrelationship between the host and environment factors. It appears after urogenital or digestive infectious conditions in predisposed individuals.1 Different infectious agents have been implied, depending on the origin of the infection; however, it is considered that there is an extensive underreporting of the agents, due to the lack of documentation of the disease, as well as to the difficulty in the microbiological or serological diagnosis, given the temporary nature of the clinical picture and the asymptomatic course of some cases, such as those triggered by Chlamydia.2 Clinically, the symptoms of reactive arthritis develop between one and four weeks after the triggering infection and are characterized by inflammatory lumbosacral pain, asymmetric oligoarthritis, enthesitis and extra-articular symptoms, that in order of frequency are: acute anterior uveitis (50–75%), skin lesions, genital lesions (20–40%) and oral ulcers.1,2 Cardiovascular manifestations are uncommon, although some series have reported them in 10% of patients.2 Below we present the case of a patient with orchiepididymitis who develops a secondary acute myopericarditis.
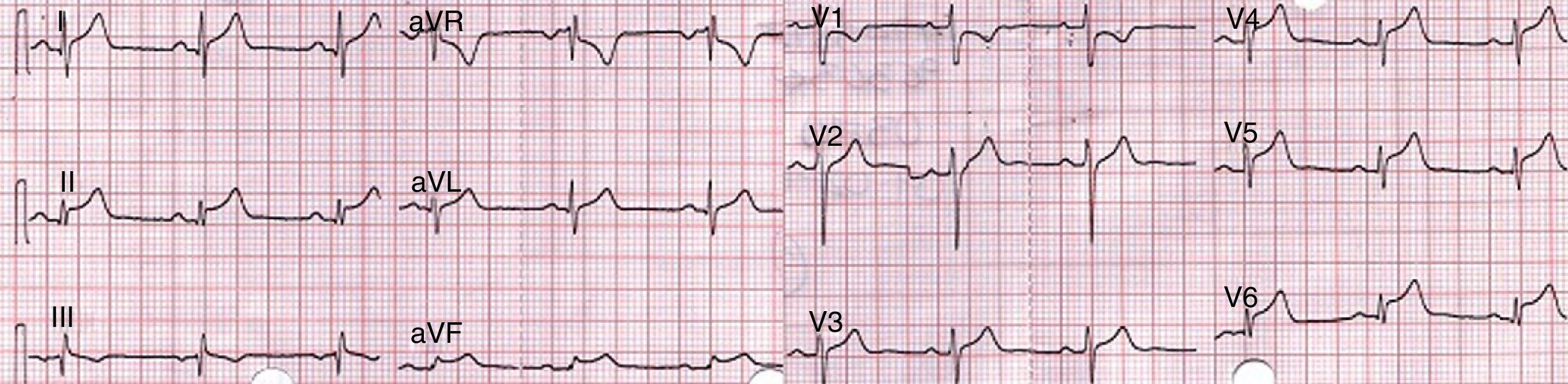
Case presentationA 44-year-old patient, with a clinical picture of a week of pain and edema in left testicular region, associated with constitutional symptoms. He consulted at our center, where acute orchiepididymitis was diagnosed and antibiotic management with ceftriaxone and doxycycline was started. After 48h of observation, the patient reported sudden onset of precordial pain, with dyspnea and evident dysautonomia. It was taken an EKG which shows concave elevation of the ST segment in inferior and lateral leads with elevation of PR in aVR (Fig. 1).
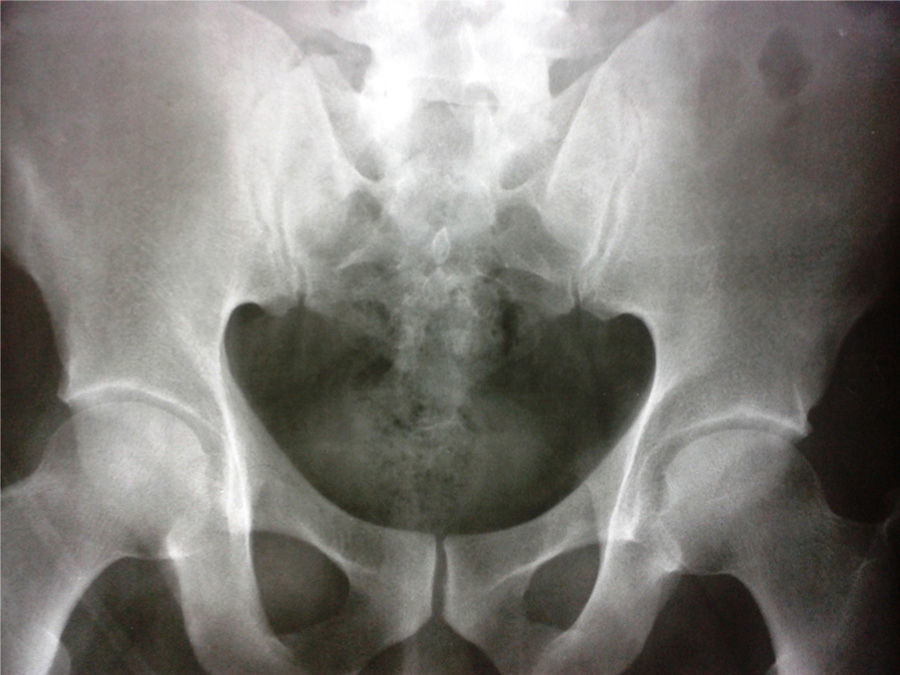
The first observers considered an acute myocardial infarction with ST elevation, reason by which they decided to carry out thrombolysis with alteplase, with improvement of the precordial pain and reduction in the ST segment in the control post-thrombolysis EKG. The paraclinical tests showed: initial troponin of 2.88ng/mL (positive>0.5); second control troponin of 6.53ng/mL and third troponin of 14.7ng/mL. The TT echocardiogram showed a concentric hypertrophic cardiomyopathy and hyperdynamic myocardium. LVEF: 70%. Mild inferior hypokinesis. PSP: 33mmHg. Normal atria. Mildly dilated ascending aorta. The patient was taken to coronary angiography, which reported coronary arteries without lesions. During his evolution the patient referred lumbosacral pain, and for this reason it was requested a pelvic radiograph, which showed findings suggestive of grade 2 sacroiliitis (Fig. 2).
The additional paraclinical tests showed, urinalysis: negative nitrites, leukocyte esterase: 70leu/ul, sediment with leukocytes: 8–10 per field, red blood cells: 6–8 per field, eumorphic, bacteria: +. Urine culture: negative. CRP: 16.2mg/dl, ESR: 52mm3/h. Given the negative result of the urine culture associated with leukocyturia and the onset of cardiovascular symptoms, despite the initial empirical management, it was decided to request a semen culture looking for a possible microorganism not yet covered. The semen culture reported more than 100,000 colony forming units of Staphylococcus haemolyticus (S. haemolyticus) methicillin-resistant, which required management with vancomycin with adequate evolution. It was considered, then, that the patient had a reactive arthritis of urogenital origin, with cardiac involvement (myopericarditis), given the electrocardiographic changes suggestive of pericarditis associated with elevation of biomarkers of myocardial injury. Anti-inflammatory management with Naproxen was started with rapid resolution of precordial and lumbosacral pain. The patient was discharged after 2 weeks of hospital care.
DiscussionReactive arthritis, considered within the group of seronegative spondyloarthropathies, occurs most frequently in individuals between 20 and 40 years old, and tends to be more common in males when its origin is urogenital and of equal distribution in the cases of gastrointestinal origin. In Colombia there are no data on the prevalence or incidence of this disease, however, some data of developed countries report an incidence of 1–30 cases per 100,000people/year.2 The agents mainly involved in the etiopathogenesis of the disease vary according to the origin of the infection. In the gastrointestinal tract are found: Yersinia, Salmonella, Shigella, Campylobacter jejuni. In the urogenital tract: Chlamydia trachomatis (C. trachomatis), Neisseria gonorrhea, Mycoplasma genitalium, Ureaplasma urealyticum (U. urealyticum).1,2 Other different infectious agents have been reported as triggers of the disease, but their prevalence is really low, so that to date there are only few case reports in the literature, none of them including infections with S. haemolyticus.
The cardiovascular commitment of the rheumatologic diseases has been widely described, mainly the correlation with early coronary artery disease and rheumatoid arthritis (OR 1.59, 95% CI: 1.46–1.73) or systemic lupus erythematosus (OR 52.43; 95% CI: 21.6–98.5), with regard to the general population of the same age and gender.3 Regarding the seronegative spondyloarthropathies, ankylosing spondylitis was the best documented in echocardiographic and autopsy studies, reporting proximal aortitis with secondary valve damage (prevalence of 18%), AV conduction disturbances (7–15%), myocarditis and heart failure with reduced EF (18% in the study of Brewerton, very similar to what was reported by Pizepiera-Bedzak in patients with psoriatic arthritis), symptomatic pericarditis (1%) and asymptomatic pericardial effusion, not explained by other causes (4–42%).4–6 All the above manifestations are associated with the presence of HLA-B27.7
In reactive arthritis, the data are scarce, confined to case reports since the 1940s and only one series of 344 patients described by Paronen in 1948, of which 69.8% met the classification criteria for Reiter's syndrome and 6.7% (23 patients) had cardiovascular involvement. By that time the diagnosis of myocarditis, pericarditis and valve damage was based on clinical and electrocardiographic findings.8 With regard to the first ones, pericardial rub usually associated to pain due to acute pericarditis, gallop rhythm and apical systolic murmur stood out. The main electrical alterations reported were: PR interval prolongation, atrioventricular block, prolongation of QRS, and ST or T wave alterations, as the most frequent. Other 6 cases were reported between 1960 and 1993, with data available only in 3 of them, among which are described events similar to those reported by Paronen, electrocardiographic alterations and abnormalities on the cardiovascular physical examination; however, by 1992 Gran et al., provided echocardiographic measurements finding dilation of the heart chambers and decreased LVEF in his patient with myocarditis and reactive arthritis secondary to Chlamydia pneumoniae.8–13 It should be taken into account that in patients with persistent active disease, a syndrome similar to that of the ankylosing spondylitis finally develops, leading to aortic valve insufficiency and conduction disorders in a period that may vary between 4 and 31 years, after the primary infection.7,8
In the case of our patient, several interesting aspects were found, such as the myocardial and pericardial commitment, evidenced by the elevation of biomarkers of injury and the electrocardiographic changes suggestive of pericarditis, associated with the angiographic confirmation of health coronary arteries, in the setting of an acute orchiepididymitis. From a microbiological point of view, at admission, an inflammatory process of the urinary tract could be established by the leukocyte and erythrocyte count and the positive leukocyte esterase but with a negative urine culture, reason why we proceeded to take a semen culture in order to determine a microorganism different from gram-negative bacteria, C. trachomatis, U. urealyticum and Mycoplasma hominis, already covered empirically with the initial treatment. The semen culture reported S. haemolyticus, with a colony count greater than 100,000CFU. While some studies such as the one published by Strebel et al., establish that the main commensal microorganisms in the urethra are alpha-hemolytic Streptococcus and coagulase-negative Staphylococcus, they were also able to determine that the presence of these microorganisms should be assessed on the basis of the colony count in the semen culture and the personal history of the patient, since up to 33% of patients with a significant colony count (higher than 105), have already had a previous episode of epididymitis or prostatitis in a lesser degree. Another striking finding in this study is that more than 50% of the isolates in cultures or by PCR were obtained only in semen samples.14 For these reasons, although the recommendations in the guidelines for management establish that the urine culture is the cornerstone of the diagnosis, it should not be forgotten the usefulness also offered by the semen culture, with the above mentioned caveats,15 Pathophysiologically is of the utmost importance to know the virulence of this microorganism, since like all the coagulase-negative Staphylococcus, it has the ability to form biofilms on prosthetic devices, as well as macrocolonies that limit the bactericidal effect of antibiotics. On the other hand, S. haemolyticus has the ability to produce hemolysin, cytolisyn and enterotoxin, which gives rise to tissue involvement, increased virulence and leads to the retrograde ascending colonization of the urinary tract causing local (epididymitis, cystitis) or systemic (bacteremia) infectious processes, mainly in immunosuppressed patients.16 With the above mentioned is finally considered that it is a case of reactive arthritis secondary to an infection with methicillin-resistant S. haemolyticus isolated from the semen culture, whose primary manifestation is an acute myopericarditis.
It remains to be clarified what mechanism led to the improvement of the electrocardiographic changes after thrombolysis, which was interpreted as successful reperfusion. In this regard there is not enough bibliographical support that would lead to a pathophysiological explanation, given the small number of cases reported in this specific clinical context. However, Millaire et al., in 1995, published a series of 9 cases of myopericarditis erroneously diagnosed as acute myocardial infarction with ST elevation, who received thrombolytic therapy. In this patients it was reported a low rate of complications (only one out of the 9 patients had a non-critical pericardial effusion) and an improvement of the electrocardiographic changes after thrombolysis.17 It is unlikely that such regression of the electrocardiographic changes would be due to a side effect of the thrombolytic agent (since no anti-inflammatory properties have been described), and very possibly it was due to the concomitant use of statins (administered because of the initial diagnostic consideration), given their anti-inflammatory and immunomodulatory effects, that have already been clearly documented both in myocarditis of viral origin and in experimental autoimmune myocarditis in murine models, from the echocardiographic and histopathological points of view.18,19 On the other hand, it could simply be the natural evolutionary course of the electrocardiographic changes in this type of processes.
Another aspect to discuss and that can lead to multiple opinions, is given by the ideal management in this group of pathologies, since there is not strong evidence in this regard.
In the first instance we must be clear about the concepts and types of myopericardial syndromes that many times are used indistinctly in the literature to refer to the same disease. The spectrum of this syndrome can range from a pure pericarditis, to varying degrees of myocardial involvement (myopericarditis when there was primary pericardial involvement or perimyocarditis when there was a primary myocardial involvement), to a pure myocarditis,20 which will address the proper treatment for the patient. Today is considered that the first line of management in this type of cardiovascular manifestations associated with collagen diseases are still the non-steroidal anti-inflammatory drugs (NSAIDs) and colchicine as nonspecific anti-inflammatory therapy, mainly in the context of pure acute pericarditis and in the myopericarditis/perimyocarditis with preserved left ventricular ejection fraction (LVEF).21 Imazio et al., in 2013, published a prospective cohort study of patients with pericarditis and myocardial involvement (n=486 patients) with a mean follow-up of 36 months, in which they described that in 10% of the studied population there was an association with a collagen disease. There was a clear trend to the management with NSAIDs or aspirin in the patients with pericarditis or myopericarditis (94% and 88%, respectively), but in the patients with perimyocarditis the use of NSAIDs was only 17% and their treatment was based primarily on angiotensin-converting enzyme inhibitors and beta blockers. As an additional data, they described that during the follow-up no increased mortality was reported in those patients with high troponin levels and that the highest recurrence rate was observed in patients with pure pericarditis, which led to the deduction that the natural history of the disease usually has a good prognosis with the treatments described.20
When there is primary myocardial involvement, evidenced by the presence of segmental disorders and especially a decrease in LVEF, the degree of recommendation of NSAIDs decreases, given the existent and extrapolated evidence, in which they are involved with an increased mortality in viral myocarditis in murine models.22 It is considered that the ideal management in these cases is the systemic immunosuppressive therapy, where the glucocorticoid therapy has a role3,4,23,24 and probably the tumor necrosis-alpha blockers.3
In the context of a myocarditis as an extra-articular manifestation of a rheumatic disease, there are no studies with methodological designs of high statistical power for the use of corticosteroids, given the low prevalence of the disease, so that their recommendation results from case reports. However, it should not be confused the existing and growing evidence of this group of drugs in studies such as the Myocarditis Treatment Trial, ESETCID and TIMIC, in which were included patients with viral myocarditis, viral cardiomyopathy (demonstrated and classified by PCR for cardiotropic viruses in endomyocardial biopsy and lymphocytes count) or negative-virus myocarditis, histologically classified as acute lymphocyte myocarditis or autoreactive myocarditis,23 whose main cause is still the viral infection and not the inflammatory commitment by collagenopathies. Despite the foregoing is very striking the finding of the ESETSID study in which it was found a spontaneous resolution of the myocarditis in 45% of patients in the placebo group, as well as of the LVEF and the functional class.25 Finally, it should be mentioned that the use of corticosteroids in the context of pericarditis or perimyocarditis/myopericarditis, without compromise of the LVEF, has a deleterious effect, especially when used at high doses, with respect to NSAIDs or colchicine.26 More recently it has been introduced within the therapeutic armamentarium the use of the biological blockers of the tumor necrosis factor, which have clearly changed the therapeutic vision in spondyloarthropathies and can generate a remarkable clinical efficacy in extra-articular manifestations. However, some authors call attention to the adverse effects and precautions (for example: rhythm disorders, hypertension and thrombosis) with the use of these drugs, mainly in patients with early heart failure and probably not in all those with advanced heart failure.3,27
Finally it was considered that the importance of the description of this case lies in remembering and always keeping in mind the cardiovascular manifestations of rheumatologic diseases, different from the coronary disease, in order to take an adequate clinical approach in this group of patients.
Ethical responsibilitiesProtection of people and animals subjectsThe authors declare that no experiments were performed on human beings or animals for this research.
Confidentiality of dataThe authors state that patient data do not appear in this article.
Right to privacy and informed consentThe authors state that patient data do not appear in this article.
FundingNone.
Conflict of interestThe authors declare that they have no conflict of interest.
Please cite this article as: Coral Enríquez J, Nates Burbano J. Miopericarditis aguda como manifestación primaria de artritis reactiva urogenital por Staphylococcus haemolyticus resistente a meticilina. Rev Colomb Reumatol. 2016;23:121–125.