Tumor necrosis factor inhibitors have become one of the most important treatments of rheumatoid arthritis. Adalimumab is a monoclonal antibody used for the treatment of this condition. A case is described of adalimumab induced cutaneous lupus.
La terapia antifactor de necrosis tumoral se ha convertido en los últimos años en uno de los pilares fundamentales para el tratamiento de la artritis reumatoide. El adalimumab es un anticuerpo monoclonal humanizado empleado en el tratamiento de la artritis reumatoide. Se describe un caso de lupus cutáneo inducido por adalimumab.
Anti-tumor necrosis factor (TNF) therapy is widely used in the treatment of rheumatoid arthritis (RA), in those patients who do not respond to conventional therapy with disease-modifying antirheumatic drugs (DMARDs). Adalimumab is a human monoclonal antibody, in most cases with good tolerance and few adverse effects. In the present case is reported a 55-year-old female patient who after having received intermittent therapy with adalimumab for 7 years, is diagnosed with cutaneous lupus induced by this drug.
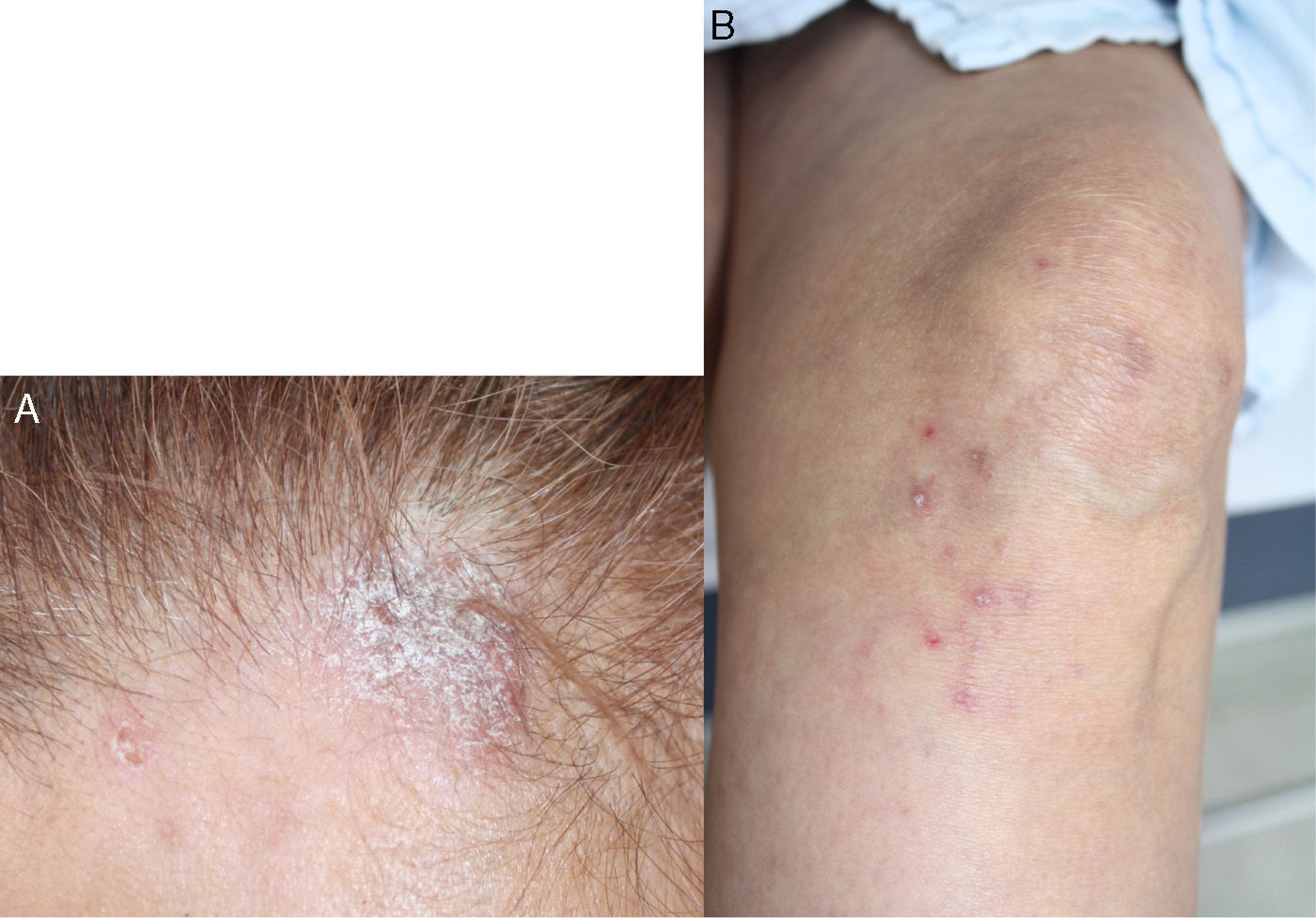
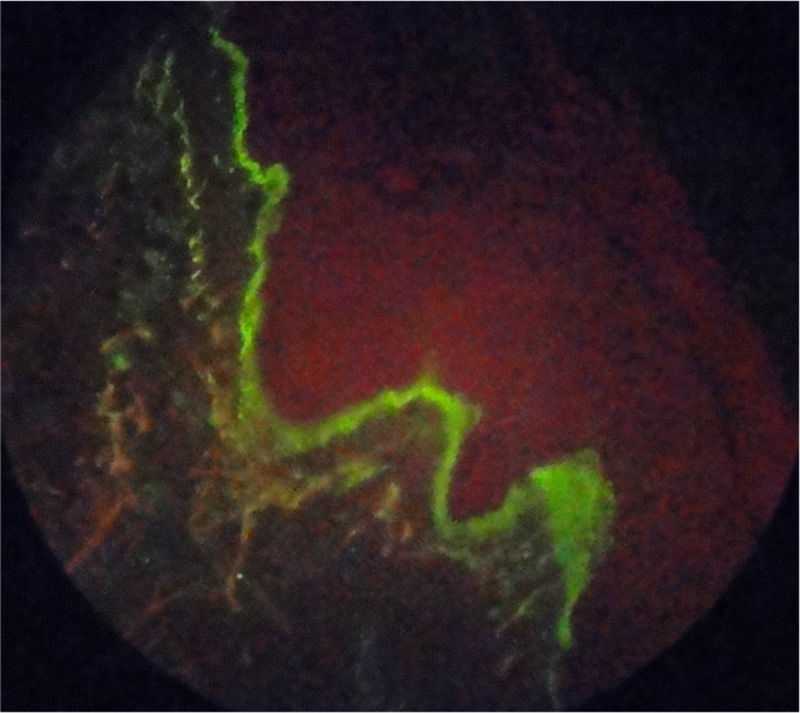
Case reportA 55-year-old patient with a history of RA diagnosed at the age of 39 years who received biological therapy with adalimumab for 4 consecutive years (2005–2009); however, this therapy had been suspended 4 months before the consultation due to administrative difficulties with her healthcare administrator agency, and since then she continued under management with methotrexate 12.5mg weekly and sulfasalazine 500mg every 12h, drugs that she had received since 1994. She reported excellent response and adequate tolerance to the established biological therapy; however, since the moment of its suspension the activity of her underlying pathology began, consisting of arthritis of the wrists and proximal interphalangeal joints, with evident deterioration of her functional class and limitation for the performance of activities of daily living. In the paraclinical tests is evidenced an elevation of acute phase reactants, RCP 4.5mg/dl (reference value: 0 to 1mg/dl) and ESR of 65mm/h (reference value lower than 20mm/h) and therefore is decided to restart biological therapy with adalimumab 40mg subcutaneously every 15 days, overcoming at this time the administrative inconveniences that prevented the application of this biological agent for 4 months. In the month of June of 2011 she consulted due to the appearance of skin lesions consisting in xeroderma on the hands and legs with emergence of psoriasiform lesions type erythematous scaly plaques in photoexposed areas, in the first and second fingers of the left hand and the anterior region of the neck, which extended over the course of one month to the hands, legs, back, pinna and scalp, with associated pruritus (Fig. 1). It was taken a skin biopsy which reports interface dermatitis with thinning of the epidermis and vacuolar degeneration of the basal layer, with positive immunofluorescence for IgM in a strong membrane pattern (Fig. 2). Laboratory tests were requested finding normal blood count, negative anti-DNA, normal complement, anti-Ro 24 units (weakly positive, reference value: less than 20U), negative anti-La, anti-RNP and anti-Sm, positive antinuclear antibodies (ANAS) 1/640 speckled pattern and anti-histones antibodies 7.4U/l (reference value: less than 1U/l), confirming the diagnosis of subacute cutaneous lupus. The patient does not have previous studies of autoimmunity to contrast these results with previous exams.
The patient presents progression and intensification of her cutaneous symptoms, which were also associated with livedo reticularis. A diagnosis of adalimumab-induced cutaneous lupus is made and the application of the drug is suspended (October of 2011). In the subsequent follow-up is observed a complete disappearance of the cutaneous lesions 4 weeks after having discontinued the biological medication.
DiscussionAnti-tumor necrosis factor therapy is used for the management of rheumatoid arthritis. Among its main adverse effects are the increased risk of opportunistic infections, mainly tuberculosis1 and the appearance of autoimmune phenomena, including the formation of ANAS and anti-double stranded DNA antibodies (anti-dsDNA) associated with drug-induced lupus syndrome.2–6 The reports of cases of adalimumab-induced lupus are scarce,6–9 and among those found there are few that describe only cutaneous manifestations as in the described patient, prevailing the cases secondary to other biological therapies, mainly infliximab and etanercept and the involvement by systemic lupus.7,8
Among the cases found in the literature are described two patients with a diagnosis of RA managed with etanercept and one with infliximab; however, they appeared with systemic involvement associated with cutaneous manifestations.7 Later is published a new case of a 53-year-old female patient with diagnosis of infliximab-induced discoid-like lupus erythematosus with a underlying diagnosis of RA confirmed by anatomopathological study and immunofluorescence.9
In the French record published in 2005 are presented 10 cases of patients with isolated cutaneous manifestations or toxicoderma associated with the use of biological therapy in the context of autoimmunity, 4 of them with etanercept and 6 with infliximab; however, the number of patients who presented systemic manifestations was higher (12 in total), 9 associated with infliximab and 3 with etanercept.4
We found an additional case series of 14 patients who were followed-up during 8 years, 10 of them with diagnosis of Crohn's disease and 4 with RA, 13 of whom were treated with infliximab and one with adalimumab, who presented lupus-like syndrome with systemic manifestations, with resolution after the suspension of the biological therapy.5
With regard to cases reported with adalimumab, the reports are scarce, confined to patients with diagnosis of RA and Crohn's disease (the majority of cases); however, those that exhibit cutaneous involvement as the only manifestation like our patient are few. The systemic manifestations of the adalimumab-induced lupus are variable, regarding the degrees of severity and the target organ involvement. Among the dermatological findings described in the literature, local reactions associated with the anatomical site of administration, such as the presence of papulopustular exanthema in the site of application and in the palms and soles have been reported, with complete resolution after the suspension of the drug.10
In 2011 was described a case of a 53-year-old female patient with a diagnosis of Crohn's disease in treatment with adalimumab complicated with lupus erythematosus with involvement of the central nervous system, associated with pleural effusion, pericardial effusion and ascites, requiring management with high doses of glucocorticoids; however, unlike our patient, the immunological profile was normal before, during and after therapy with this medication.2
There have been reported cases similar to ours in terms of immunological seroconversion evidenced by laboratory tests such as ANAS, anti-DNA and lupus anticoagulant, in a patient with Crohn's disease, whose systemic manifestations consisted of oral ulcers, arthritis, fever, rash on the back of the hands and fatigue; however, as it was previously described, our patient did not have systemic manifestations.5
In the literature reviewed, we found a case of adalimumab-induced cutaneous lupus erythematosus with similar clinical characteristics in a 61-year-old woman with diagnosis of RA, who after months of having received biological therapy with adalimumab at doses of 40mg subcutaneously every 2 weeks presents positivity of ANAS of homogeneous pattern with titers that were increasing in the following two years (1/160 to 1/640) with subsequent appearance of erythematous scaly plaques on the face and the trunk, not associated with any other symptom, with a biopsy report compatible with cutaneous lupus (immunofluorescence was not performed, unlike in the case of our patient), for this reason adalimumab was discontinued and after two months of its suspension the dermatological lesions disappeared.11 Determination of anti-histones antibodies was not reported. A second case was reported in Brazil12 in a 42-year-old woman with psoriatic arthritis who presents adalimumab-induced lupus with leukocytoclastic vasculitis, after 22 months of treatment with this biological therapy. The clinical picture was characterized by myalgias, arthralgias in elbows and wrists, low-grade fever (38°C) and dermatological lesions (purpuric papules on the fingertips of the hands with periungual telangiectasias). From the paraclinical point of view, she presented positivity for ANAS (1/640 homogeneous pattern) and anti-histone antibodies (the exact value is not recorded).
The immunopathological mechanism of the adalimumab-induced lupus is still unknown, it is believed that it is a cell-mediated immunity process which involves two phases, the first where the activation of TH1 lymphocytes increases the production of proinflammatory cytokines, especially interferon gamma and IL2, which stimulate the production of autoantibodies, and the second phase which corresponds to the production of other cytokines by the TH2 lymphocytes, that are responsible for perpetuating the blood levels of these antibodies. It is proposed that this therapy blocks preferentially the production of interferon gamma (INF gamma) and with this inhibition the INF gamma-dependent up-regulation of Fas is blocked, with the consequent lack of inhibition of cytokines such as IL4, IL6, IL10, suggesting that the therapy with adalimumab promotes the appearance of humoral autoimmunity through the selective inhibition of the response of cytotoxic T lymphocytes, which under normal conditions should inhibit the autoreactive B cells.13,14 Other authors suggest that this drug exerts an effect similar to ultraviolet rays, inducing cell apoptosis and thereby, accumulation of autoantibody forming nucleosomal material, or that the increase in the frequency of infectious complications in these patients predisposes to polyclonal activation of B lymphocytes and overexpression of Toll-like receptors 9, with the consequent formation of antibodies11,15; however, it is believed that the participation of genetic predisposition plays a key role in the development of these manifestations, among the described the TNF alpha 308 A polymorphism.15
The suspension of the therapy is the main therapeutic measure and most patients present resolution of their clinical manifestations after this, in a period of approximately 6–12 weeks.
ConclusionWe present a case of adalimumab-induced cutaneous lupus, with compatible histopathological changes and presence of ANAS, positive anti-Ro and anti-histone antibodies, without systemic manifestations, with complete resolution of the clinical picture after the suspension of the biological drug. The rheumatologist or the physician specialist who prescribes biological therapies such as anti-TNF agents and among them, adalimumab, must be attentive to paradoxical situations such as the one described in the present clinical case, consisting in the appearance of autoimmune phenomena, secondary to the treatment of autoimmune diseases.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflict of interestThe authors declare they do not have any conflict of interest.
Please cite this article as: Tobón T M, Gutiérrez JM, Cuellar I, Fernández-Ávila DG, Díaz MC. Lupus cutáneo inducido por adalimumab. Rev Colomb Reumatol. 2016;23:271–274.