Antimalarials are first-line therapies in the management of systemic lupus erythematosus (SLE). Their immunomodulatory effects have been proven and are generally safe. Hydroxychloroquine (HCQ) has the best safety profile of these therapies. However, antimalarial retinopathy can lead to irreversible loss of vision. It is associated more frequently with the use of chloroquine (CQ) than with HCQ but, in general, the incidence of retinal toxicity is low. It is necessary to perform periodic ophthalmological tests from the beginning of treatment to detect patients with incipient retinopathy early, since at this stage withdrawing the drug leads to resolution of the condition. The main risk factors for retinopathy include duration of treatment, dose, chronic kidney disease, and pre-existing retinal or macular disease. The risk of toxicity is very low for doses of HCQ below 5mg/kg of body weight, so the daily dose should not exceed this threshold. New detection modalities exist for the early detection of asymptomatic HCQ and CQ-induced retinopathy. The ophthalmological study demonstrates permanent retinal pigmentary deposits in the study of the eye fundus. The early detection tests are visual field (VF) together with spectral domain optical tomography (SD-OCT). The multifocal electroretinogram (mfERG) can provide objective corroboration for visual field, and fundus autofluorescence (FAF) can show the topography of lesions. The goal of all these examinations is to detect retinopathy before it is visible in the fundus. Given the low initial risk of HCQ or CQ retinopathy with an appropriate dose and absence of significant risk factors, annual screening may be postponed until 5 years of drug exposure have elapsed. Other than drug cessation, no diet or medical treatment has been shown to be effective in preventing, treating, or reducing the risk of antimalarial-induced retinopathy.
Los antimaláricos son fármacos beneficiosos en el tratamiento del lupus eritematoso sistémico (LES). Su efecto inmunomodulador ha sido demostrado, y son, en general, muy seguros; entre ellos, la hidroxicloroquina (HCQ) ha demostrado el mejor perfil de seguridad. La retinopatía antipalúdica es la toxicidad más grave, ya que puede provocar una pérdida irreversible de la visión. Se asocia, con mayor frecuencia, al uso de cloroquina (CQ) que de HCQ, pero, en general, la incidencia de toxicidad retiniana es baja. Es necesario realizar unas pruebas oftalmológicas periódicas, desde el inicio del tratamiento, para detectar precozmente una retinopatía incipiente, ya que, en esta etapa, la retirada del fármaco conlleva la resolución de la afección. Los principales factores de riesgo de la retinopatía incluyen la duración del tratamiento, la dosis, la enfermedad renal crónica y la enfermedad macular o retiniana preexistente. El riesgo de toxicidad es muy bajo para dosis de HCQ menores de 5mg/kg de peso corporal real, por lo que la dosis diaria no debe exceder este umbral. Existen nuevas modalidades de detección precoz de la retinopatía inducida por antipalúdicos. El estudio oftalmológico consiste en la demostración de depósitos pigmentarios retinianos permanentes en el estudio del fondo de ojo. Las pruebas de detección precoz son los campos visuales (VF), junto con la tomografía óptica de dominio espectral (SD-OCT). El electrorretinograma multifocal (ERGmf) puede proporcionar una corroboración objetiva de los campos visuales, y la autofluorescencia del fondo de ojo (FAF) la topografía de las lesiones. El objetivo de estos exámenes es detectar la retinopatía antes de que sea visible en el fondo de ojo. Dado el bajo riesgo inicial de retinopatía por HCQ o CQ con una dosis adecuada y en ausencia de factores de riesgo importantes, puede posponerse la revisión anual hasta que hayan transcurrido cinco años de exposición al fármaco. Ninguna dieta o tratamiento médico ha demostrado ser eficaz para prevenir, tratar o reducir el riesgo de retinopatía inducida por HCQ y CQ, salvo la suspensión de la medicación.
- •
Hydroxychloroquine remains a safe and beneficial drug in treatment of systemic lupus erythematosus as long as there is no contraindication.
- •
Retinal toxicity is unpredictable and could occur even with low doses of antimalarials.
- •
New detection modalities exist for the early detection of asymptomatic hydroxychloroquine and chloroquine-induced retinopathy.
- •
When the definitive signs of retinopathy have been detected, the decision to discontinue the medication should be made between the patient and the prescribing physician to ensure that the medical risks of such a decision are controlled.
Three antimalarial drugs are available for the treatment of systemic lupus erythematosus (SLE): hydroxychloroquine (HCQ), chloroquine (CQ), and quinacrine (Qn). HCQ is the most widely used in routine clinical practice due to its better safety profile.
The main indications for HCQ in patients with SLE are skin and joint manifestations and serositis.1 It has also been shown to be effective in preventing flare-ups and improving survival in SLE patients.2 However, given the drug's safety and taking into account the high number of beneficial effects it provides, all clinical practice guidelines recommend the use of HCQ at the time of diagnosing SLE,3,4 regardless of the type of manifestations and the severity of the disease. Only those patients with hypersensitivity or intolerance to the drug, in whom the presence of retinal toxicity or other significant adverse effect is suspected or confirmed, should be excluded.
HCQ has been shown to reduce SLE activity.5 It could also prevent severe flare-ups5 and even be beneficial in patients with lupus nephritis, although this has yet to be confirmed by other clinical trials. It also improves the lipid profile6 (total cholesterol, LDL cholesterol, and triglyceride levels decrease, and HDL cholesterol levels increase), especially if patients continue treatment with glucocorticoids. Antimalarial drugs have a hypoglycemic effect7 and improve some aspects of carbohydrate metabolism, such as insulin resistance. They have antithrombotic effects, especially in patients at high risk of thrombosis,8 as the carriers of antiphospholipid antibodies. They could also have a beneficial effect on bone mass, preventing osteoporosis.9,10 HCQ has been associated with a decrease in chronic organ damage and an increase in survival. In addition, other potentially beneficial effects have included protection against infections,10,11 and a decrease in the incidence of cancer.12
In regard to this, it is worth highlighting a study recently published by our group,13 in which antimalarial treatment was shown to have a protective effect against infection in a group of 339 SLE patients that had been admitted to the hospital. The positive outcome that had been previously associated with antimalarials as a protective factor against serious infections in outpatient settings14 appears to also extend to hospitalization settings.
Lastly, an additional effect attributable to hydroxychloroquine would be its ability to behave as a steroid sparer.9
Mechanism of action of antimalarialsVarious mechanisms by which HCQ could exert its immunomodulatory effect have been suggested.10 The most likely one would be its ability to hinder the antigen processing of antigen-presenting cells by increasing intralisosomal pH, altering the degradation of antigens and making it difficult to bind the resulting peptides to the class II major histocompatibility complex (MHC).15 Antigen presenting cells could not stimulate CD4+ Helper T cells. This inhibition occurs selectively on low affinity antigens, such as autoantigens; however, it would not have an excessive effect on high affinity antigens, such as bacterial ones.
In addition, HCQ could decrease the production of some pro-inflammatory cytokines such as IFNγ, IL-12 and TNFα10 and interfere with the activation of T-lymphocytes and the production of autoantibodies. HCQ could also alter the innate immune response by blocking the activation of toll-like receptors (TLR).
HCQ pharmacologyThe absorption of HCQ from the digestive tract is effective but variable and is not affected by food intake. HCQ is widely distributed throughout the tissues and tends to accumulate in organs such as the liver, spleen, lungs, kidneys, and in melanin-rich tissues such as the skin and retina.9
It has a very long half-life (40–50 days) and can remain in tissues for months, and even years, after suspension. Thus, after drug withdrawal, HCQ can continue to have an effect for weeks as it continues to be released into the circulation from impregnated tissues.16 In cases of toxicity, the withdrawal of the drug would not necessarily cause an immediate improvement of the adverse effects.
It has been suggested that in the maintenance phase, daily administration would not be necessary; it would probably be sufficient to take it three or four times a week in order to prevent the accumulated dose over time and the development of antimalarial retinopathy, although some studies associate drug efficacy with blood HCQ levels.9,16,17
HCQ is metabolized in the liver, which produces active metabolites, and it is mainly eliminated by the kidneys. Therefore, the dose of HCQ must be adjusted in patients with a decreased glomerular filtration rate.9,18
HCQ can be used during pregnancy, since the risk of congenital malformation in exposed fetuses is similar to that of unexposed.19 Furthermore, the risk of experiencing a flare after stopping HCQ is higher, so its withdrawal could even be counterproductive in women who were taking the drug at the time of becoming pregnant.19 Its use is also allowed during breastfeeding.
Adverse effects of antimalarialsAntimalarial drugs are generally very safe, and of them, HCQ has the best safety profile.10 The most common adverse effects are those related to the gastrointestinal tract9; these include nausea, vomiting, abdominal pain, dyspepsia, and occasionally diarrhea. These symptoms are usually transient and improve or disappear with time. In certain cases, it is necessary to reduce the dose, and in exceptional cases, to stop the treatment altogether. Tolerance can improve when the drug is administered with meals.
Cutaneous adverse effects are relatively frequent20 and include a wide range of manifestations, such as hyperpigmentation of the skin and gums; grayish discoloration of the hair root, eyebrows, and beard (especially in very long treatments); itching; alopecia; urticaria; morbilliform or maculopapular eruptions; and exfoliative dermatitis.9
Nonspecific symptoms such as asthenia, arthromyalgia, and flu-like symptoms have been described in a small percentage of patients.10 Antimalarial cardiotoxicity in patients with SLE is very rare.21
Antimalarial retinopathy is the most serious toxicity, as it can lead to irreversible loss of vision.22 It is associated more frequently with the use of CQ than with HCQ. In general, the incidence of retinal toxicity ranges from 0% to 4%.23 However, a recent study reported an incidence of HCQ toxicity close to 7.5% in 2361 patients with treatment durations longer than 5 years.24 This study suggested that HCQ (and probably CQ) toxicity was not that rare.
Many authors suggest that CQ is more toxic than HCQ, but the older literature does not provide details on dose by weight and duration of treatments.25
Pathophysiology of retinal toxicity due to antimalarialsThe pathophysiology of CQ and HCQ toxicity is not well understood.26 High doses used in vitro have a rapid effect on retinal cell metabolism, but it is not clear how these rapid metabolic effects are related to the slow and chronic damage that characterizes the clinical toxicity status.
When HCQ binds to melanin in the retinal pigment epithelium (RPE), it can concentrate the drug and contribute to or prolong its toxicity. However, this binding to melanin could also serve as a removal mechanism for the toxic agents that cause intracellular damage.
Both the inner and outer retina can be affected by exposure to CQ in animal studies, but some work suggests that the inner retina is not significantly damaged by HCQ in humans.27,28
In clinical practice, the main damage is to photoreceptors (reversible), and as the outer nuclear layer degenerates, secondary RPE alteration develops (irreversible).29 There are no anatomical features of the retina and RPE that specifically correlate with the parafoveal or extramacular damage patterns typical of CQ and HCQ toxicity. The macular location of the disease suggests that the absorption of light or possibly the metabolism of the cones may play a role, but this is only mere speculation.
CQ, and less commonly HCQ, can cause spiral intraepithelial deposits in the cornea (cornea verticillata or vortex keratopathy).30 These corneal changes are not correlated with retinal toxicity or associated with visual loss, and unlike retinopathy, they are usually reversible.
Symptoms of antimalarial retinopathyInitially, antimalarial retinopathy is asymptomatic. Over the years, nonspecific symptoms such as difficulty reading, blurred vision, or photophobia may appear before blindness sets in.10
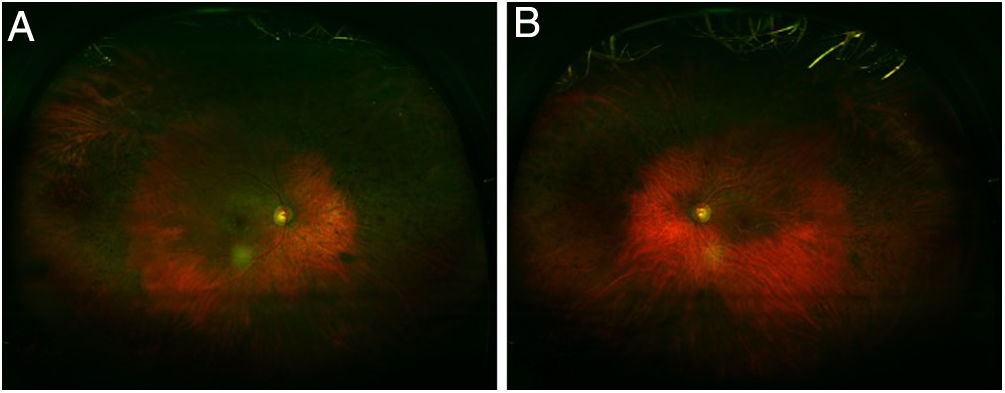
Regardless of any pattern of involvement, visual acuity is usually excellent until the retinal damage is severe. Most patients who develop HCQ toxicity do not have any visual symptoms. Some patients may notice paracentral scotomas while reading. If exposure to the drug continues, the area of disturbance expands, the RPE is involved, and the maculopathy can invade the foveal center with eventual loss of visual acuity (Fig. 1).24,29 Cystoid macular edema can sometimes appear31 and advanced cases show generalized RPE involvement and retinal atrophy with loss of visual acuity, peripheral vision, and nyctalopia.
Hence the need for periodic ophthalmological tests from the beginning of treatment to detect early patients with incipient retinopathy, since at this stage the withdrawal of the drug leads to resolution of the condition.
HCQ and CQ retinopathy can progress even after medication is stopped, although progression and risk to vision are correlated to the severity of the retinopathy at the time it is detected.31,32 It seems doubtful that this late progression of damage after stopping the drug is related to the deposit of the drug in the tissues, although elimination may take several months after it has been withdrawn. The late progression may be related to the gradual deterioration of cells that were metabolically injured during the period of exposure to the drug.10
The clinical picture of HCQ and CQ toxicity has been classically characterized as a bilateral bull's-eye maculopathy, an appearance caused by a ring of parafoveal depigmentation of the RPE that excludes a central island of the fovea (Fig. 2).26 However, this classic pattern should no longer be observed, since it is recommended that early detection tests detect HCQ toxicity long before RPE damage is visible by imaging or fundus examination.
Although the majority of patients of European descent present the initial damage to the photoreceptors in the classical parafoveal distribution, the majority of patients of Asian descent present the initial damage with a pericentral, more peripheral distribution, near the arches.33,34 African Americans and Hispanics33 present a predominant pattern of parafoveal damage like European subjects, although they also have a greater tendency for the evolution of extramacular involvement. The sample of patients of other races has been too small to draw conclusions. Also, a mixed pattern (retinal changes in both the parafoveal and pericentral areas together with a relatively normal central area of the retina) has been described.
DosageBased on existing studies, which suggest that the risk of toxicity is very low for doses below 5mg/kg of actual body weight, the daily dose should not exceed this threshold. Importantly, the efficacy of HCQ in SLE has been established in studies with a prescribed dose of 6.5mg/kg/day,9 so it remains to be confirmed whether a lower dose would have comparable clinical effects. Patients in long-term remission may have the dose reduced, although no studies have formally addressed this strategy.35
The old recommendations used ideal body weight for the calculation of the dose based on the idea that these drugs were retained in fat; however, available laboratory studies show that these drugs are stored primarily in melanin-rich tissues, the liver, and the kidneys, while concentrations are low in muscle, fat, and other organs.36,37 Calculating the dose based on the ideal weight leads to overdose in lean people, while the recommended formula which takes into account the actual weight balances the risk in a wide range of body types.24
There are no comparable demographic data on CQ dose and toxicity. The mechanisms of action are presumed to be similar for both CQ and HCQ, and in the older clinical literature on antimalarials the toxicity was approximately equivalent to 3.0mg of CQ versus 6.5mg of HCQ.25,38 With this estimate, the equivalent of 5.0mg kg of HCQ would be 2.3mg/kg of CQ. Many studies suggest that CQ is somewhat more toxic than HCQ, but there is no good data on its pharmacological equivalence. The increased toxicity of CQ in routine clinical practice may be an artifact of the prescription itself, due to the size of the available CQ tablet (250mg). Almost any patient taking a CQ tablet will receive more than 2.3mg/kg. As HCQ is available in 200mg tablets and CQ is available in 250mg tablets, it may seem difficult to prescribe intermediate doses. However, the blood levels of these drugs are stable for many weeks, so the dosage can be averaged over time. Intermediate doses can be easily obtained by splitting the tablets or simply removing a tablet on certain days of the week.
In theory, blood levels should be useful in calculating the dose or evaluating poor clearance of these drugs. However, the literature on measuring the level of HCQ in blood has shown it to be an unreliable indicator of medical effectiveness or toxicity.10,39 HCQ is metabolized by cytochrome P450 enzymes, which can be affected by a wide variety of drugs, and some of the variability in blood levels may be related to these metabolic pathways.40,41
Risk factors for macular toxicity due to antimalarialsConcern for retinal toxicity from long-term treatment with HCQ led to the use of more sensitive detection techniques for retinal abnormalities, with an estimated prevalence of retinal abnormalities exceeding 10% after 20 years of continuous use.24
The main risk factors for retinopathy include duration of treatment (OR 4.71 for every 5 years of use), dose (OR 3.34 for every 100mg daily dose), chronic kidney disease (adjusted OR 8.56) and pre-existing retinal or macular disease.26
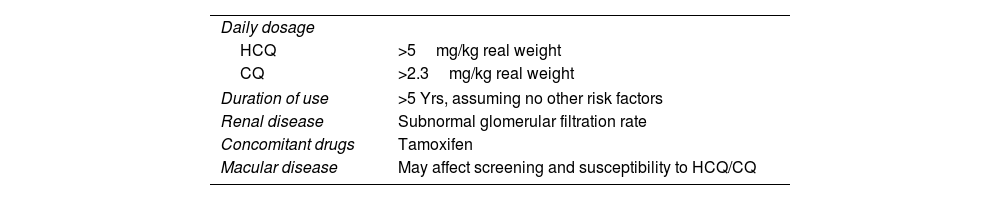
Major risk factors26The most important risk factors are listed in Table 1.26
- 1.
Daily dose and duration of use. The most important risk factor for the development of HCQ toxicity is excessive daily dose by weight.24 Doses above 5.0mg/kg dramatically increase both population risk and annual incremental risk, and extreme doses can be very dangerous. Two reports of patients receiving HCQ 800 to 1000mg daily (up to 20mg/kg) for nonrheumatic diseases show an incidence of retinopathy of 25% to 40% and signs of damage in the next 1 to 2 years.42,43
Major risk factors for toxic retinopathy.26
| Daily dosage | |
| HCQ | >5mg/kg real weight |
| CQ | >2.3mg/kg real weight |
| Duration of use | >5 Yrs, assuming no other risk factors |
| Renal disease | Subnormal glomerular filtration rate |
| Concomitant drugs | Tamoxifen |
| Macular disease | May affect screening and susceptibility to HCQ/CQ |
HCQ: hydroxychloroquine; CQ: chloroquine.
The risk of toxicity depends on the daily dose and the duration of use. At the recommended doses, the risk of toxicity up to 5 years is less than 1% and less than 2% up to 10 years, but it increases to almost 20% after 20 years. On the other hand, even after 20 years, a patient who has not developed toxicity has only a 4% risk of presenting it in the following year.24
- 2.
Kidney disease. HCQ and CQ are largely eliminated by the kidney, so kidney disease increases the circulating level of drugs and the risk of toxicity.24,44 Renal disease is not uncommon in SLE, so careful monitoring is important. Patients with kidney disease may have unpredictably high blood drug levels, and dosage adjustment may often be necessary.
- 3.
Use of tamoxifen. An unexpected finding from a recent large study on the use of HCQ was that the simultaneous use of tamoxifen (a drug used for the long-term treatment of breast cancer) increased the risk of toxicity by approximately 5-fold.24 The reasons are unclear, although tamoxifen is a retinal toxin by itself, and there may be synergy in adverse effects. Newer estrogen analogs, such as anastrozole, have not shown an association with HCQ toxicity to date, but the number of patients studied has been limited. Patients taking tamoxifen need careful dosing and monitoring.
- 4.
Retinal and macular disease. Patients with underlying retinal disease may have an increased risk of toxicity, although there is no specific data to confirm this. It seems reasonable not to add a potentially retinal toxic agent in cases where there is significant retinal dystrophy or degeneration. The other problem with maculopathy is that it can cause test abnormalities that interfere with the interpretation of retinopathy screenings. Therefore, a significant loss of the central photoreceptor would be a contraindication for antimalarial therapy, whereas isolated drusen, which leaves good visual fields and an intact photoreceptor structure, would not be.26
- 1.
Age. Older patients may appear to be at higher risk, as aging tissue may be less resistant to the toxic effects of the drug. However, the demographic study by Melles et al. found that there was no significant association between age and risk of toxicity.24
- 2.
Liver disease. Although the liver is involved in the metabolism of these agents, there is no clear association between liver disease and toxicity.24
- 3.
Genetic factors. It is assumed that some patients have a genetic predisposition to HCQ toxicity (e.g., abnormalities in the ABCA4 gene),45 but another study suggests that some ABCA4 polymorphisms may actually be protective.46 Polymorphisms in the cytochrome P450 gene could influence the blood concentration of the drug.40,41 Genetic factors may be the basis for the difference in the presentation of the disease between European and Asian patients.
HCQ and CQ retinopathy are not reversible, and cell damage can progress even after medications are stopped. When retinopathy is not detected until bull's eye maculopathy develops, the disease can progress for years, often with thinning of the fovea and eventual loss of visual acuity. However, when retinopathy is diagnosed early, before RPE damage occurs, only mild and limited progression is seen after medication is discontinued, and the fovea is not threatened.32 Therefore, early detection cannot prevent damage, but if done correctly, it allows diagnosis of toxicity before vision is significantly affected.
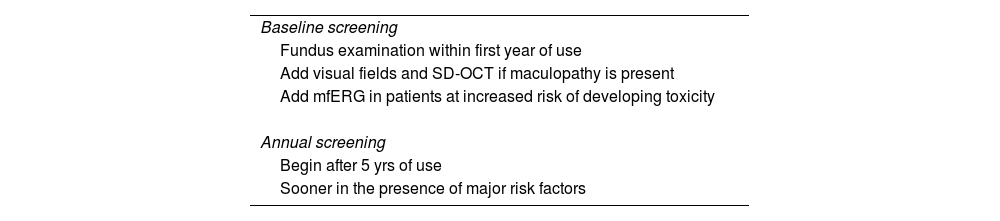
Screening frequencyThe American Academy of Ophthalmology (AAO)26 provides recommendations for the early detection of antimalarial retinopathy (Table 2).
- 1.
Baseline exam. All patients initiating long-term HCQ or CQ therapy should undergo an initial ophthalmologic examination within the first year of treatment in order to document any previous eye conditions and to establish a fundus and condition record. Most important when evaluating the fundus is to evaluate the macula to rule out any underlying disease that may interfere with interpretation of screening tests or make the use of these medications reckless due to pre-existing tissue damage. Although visual fields and spectral domain optical coherence tomography (SD-OCT) are always helpful, it is not essential to perform them early, unless there are abnormalities that may affect screening (e.g., focal macular lesion, glaucoma). On the other hand, the multifocal electroretinogram (mfERG) should possibly be added further as a reference test in patients at increased risk of developing toxicity. The baseline examination also provides an opportunity to counsel patients on the appropriate dose and to stress the importance of regular follow-ups if they continue to take long-term medication.
- 2.
Annual monitoring. Given the low initial risk of HCQ or CQ retinopathy with an appropriate dose and absence of significant risk factors, annual screening may be postponed until 5 years of drug exposure have elapsed. Screening should start earlier if the risk is high (Table 1). Annual review is considered sufficient because toxicity develops slowly and there is time to repeat tests or perform additional tests when results are suspect but not definitive.26 The frequency of visits can be increased in those patients with significant risk factors. It is important to check the dose in relation to weight at each visit and to ask about any changes in fitness, such as significant weight loss, kidney disease, or tamoxifen use.
Screening frequency.26
| Baseline screening |
| Fundus examination within first year of use |
| Add visual fields and SD-OCT if maculopathy is present |
| Add mfERG in patients at increased risk of developing toxicity |
| Annual screening |
| Begin after 5 yrs of use |
| Sooner in the presence of major risk factors |
SC-OCT: spectral-domain optical coherence tomography.
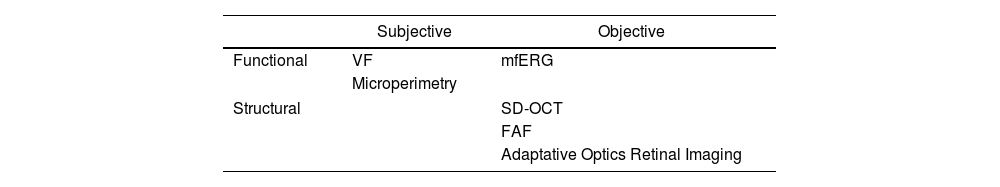
Antimalarial retinal toxicity screening tests can be classified into those that detect morphological abnormalities, such as spectral domain optical tomography (SD-OCT) or fundus autofluorescence (FAF), and those that detect functional abnormalities, such as the visual fields (VF) or the multifocal electroretinogram (mfERG)30 (Table 3).
Classification of the clinical examination techniques.
| Subjective | Objective | |
|---|---|---|
| Functional | VF | mfERG |
| Microperimetry | ||
| Structural | SD-OCT | |
| FAF | ||
| Adaptative Optics Retinal Imaging | ||
FAF: fundus autofluorescence; VF: visual fields; mfERG: multifocal electroretinogram; SD-OCT: spectral-domain optical coherence tomography.
Although each is of value in identifying early retinopathy before clinically evident structural changes are seen,29 none are 100% sensitive47 and they are generally used in a complementary fashion.29 There are limited studies comparing the sensitivity and specificity of these tests with the automated visual field test 10–2.30
The ophthalmological study consists, then, of the demonstration of permanent retinal pigmentary deposits in the study of the eye fundus.22 The early detection tests23 are VF together with SD-OCT. The mfERG can provide objective corroboration for visual fields, and the FAF can show the topography of lesions. The goal of all these examinations is to detect retinopathy before it is visible in the fundus.
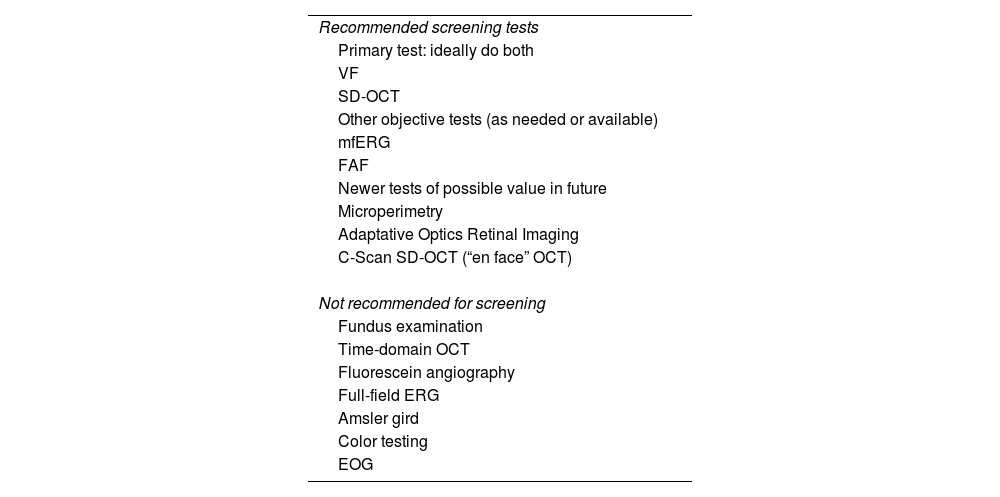
The recommended screening techniques and those that should be avoided are listed in Table 4.
Clinical examination techniques.26
| Recommended screening tests |
| Primary test: ideally do both |
| VF |
| SD-OCT |
| Other objective tests (as needed or available) |
| mfERG |
| FAF |
| Newer tests of possible value in future |
| Microperimetry |
| Adaptative Optics Retinal Imaging |
| C-Scan SD-OCT (“en face” OCT) |
| Not recommended for screening |
| Fundus examination |
| Time-domain OCT |
| Fluorescein angiography |
| Full-field ERG |
| Amsler gird |
| Color testing |
| EOG |
EOG: electro-oculogram; ERG: electroretinogram: FAF: fundus autofluorescence; VF: visual fields; mfERG: multifocal electroretinogram; SD-OCT: spectral-domain optical coherence tomography.
The use of VF and SD-OCT is recommended for routine primary screening because they are widely available. VFs are potentially more sensitive, but subjective, and patients differ in the reliability of their responses; SD-OCT is objective, very specific, and generally sensitive to levels of damage that can be visually significant. Unless toxicity is advanced and obvious, at least one objective test should be done to confirm subjectively found findings before toxicity is diagnosed.
- 1.
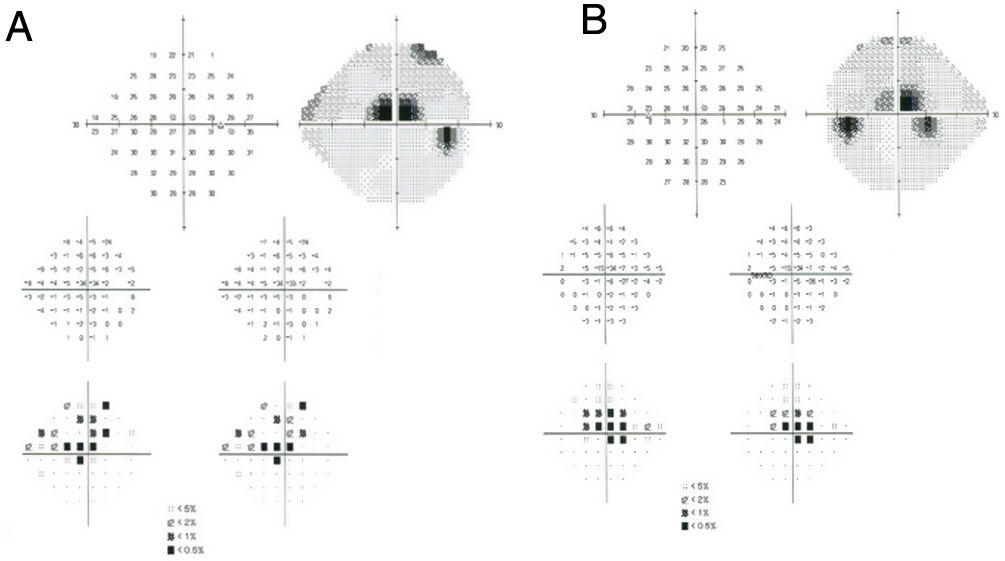
Subjective and functional test: VF. Automated central 10° perimetry (e.g., 10–2 VF) is the most common adjunct test performed for HCQ retinopathy screening (Fig. 3). The classic finding is a partial or complete annular defect between 2° and 6°, with central preservation.30 Parafoveal scotomas are typical of HCQ retinopathy: superonasal scotomas are the most common abnormality as the inferotemporal paramacular region is often affected first.48 Although perimetry is highly dependent on the patient's attitude to the test, in some patients it may be the first modality to detect toxicity, even earlier than mfERG or SD-OCT.
- 2.
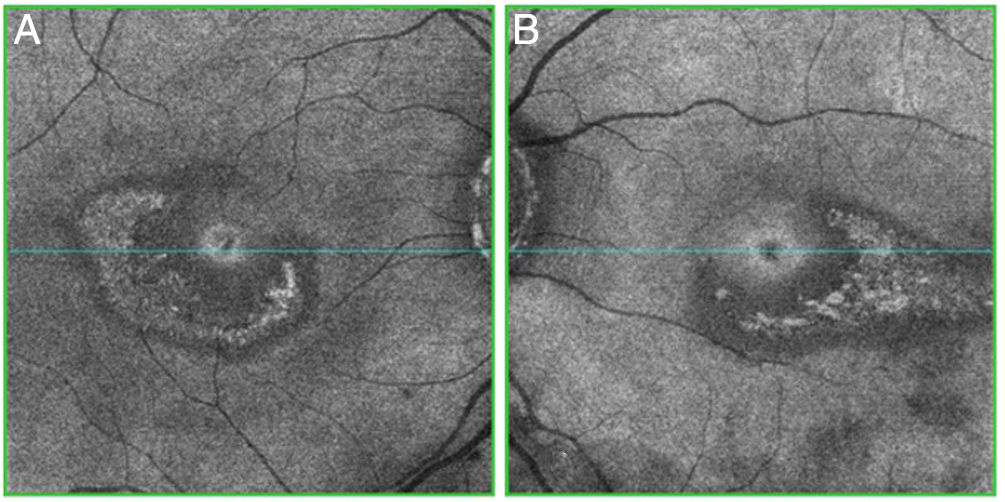
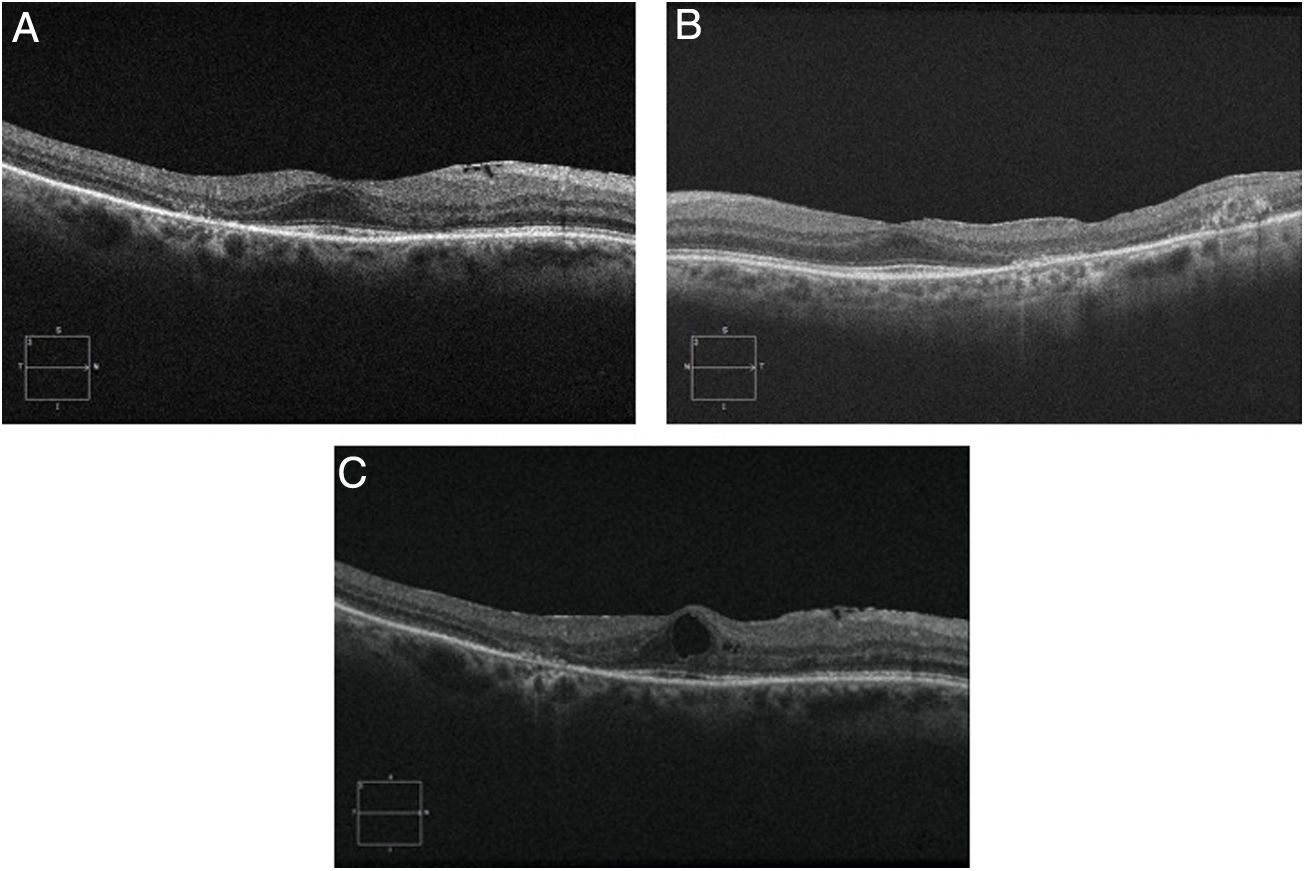
Objective and structural test: SD-OCT. Optical coherence tomography is a noninvasive interferometric optical imaging modality that provides detailed cross-sectional images of tissue morphology (Fig. 4).30 HCQ toxicity is manifested first by thinning of the inner layers of the retina,30 followed by loss of union between the inner/outer segment of the parafoveal photoreceptor with central foveal preservation. The RPE and the outer limiting membrane are preserved, and the downward displacement of the overlying inner retinal layers has sometimes been described as the flying saucer sign. In most cases, SD-OCT findings correspond with VF 10–2 findings and fundus examination abnormalities, although in some cases SD-OCT changes may precede any other anomaly. SD-OCT alterations persist on image after HCQ/CQ cessation, despite evidence of visual field recovery.49
- 1.
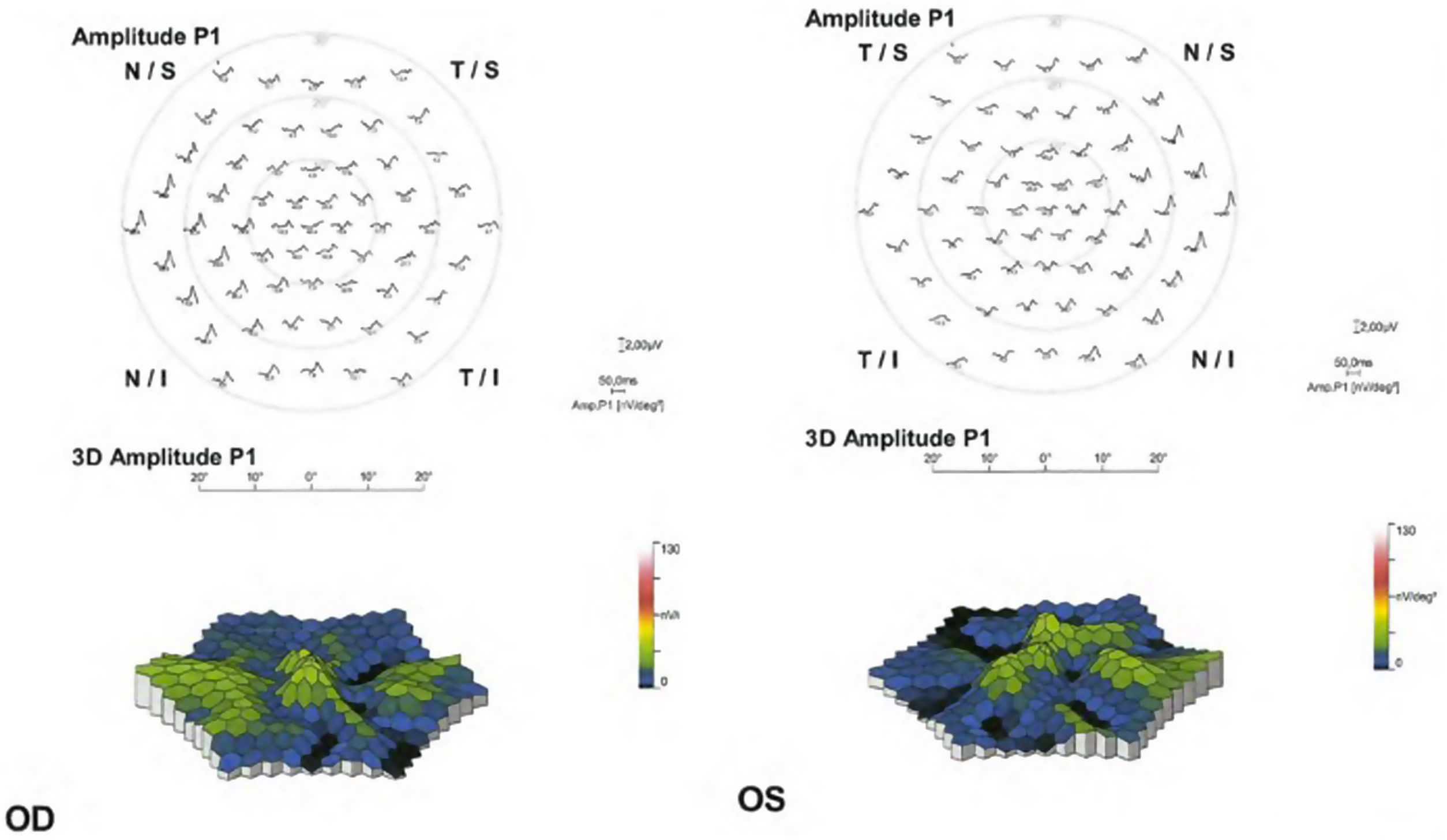
Objective and functional test: mfERG. The mfERG generates local electroretinogram responses topographically through the posterior pole and can objectively document the depression of the parafoveal or extramacular electroretinogram in early retinopathy (Fig. 5). The mfERG is similar in sensitivity to visual fields and can provide objective confirmation of suspected loss of field.30 The mfERG shows a topographic representation of retinal responses (local areas of depressed retinal sensitivity due to HCQ). It is the gold standard test for the detection of suspected HCQ retinopathy.50 The mfERG can detect the HCQ-induced retinopathy earlier than other tests. However, the mfERG requires well-calibrated suitable equipment and experienced personnel to function and interpret well and is not available in all ophthalmic units.
The patterns that can be found in the mfERG are50: parafoveal pattern (involvement of the 2 to 6 ring from the center of the fovea); pericentral pattern (with damage of the 8 or more rings from the center of the fovea); and mixed pattern (retinal changes in both the parafoveal and pericentral areas together with a relatively normal central area of the retina).
Then, the mfERG should be indicated in patients with visual field alterations without SD-OCT or FAF alterations, to establish objective evidence of HCQ retinopathy. The mfERG should possibly be added as a baseline test in patients at increased risk of developing toxicity.
- 2.
Objective and structural test: FAF. Autofluorescence imaging can reveal early damage to parafoveal or extramacular photoreceptors as an area of increased autofluorescence that may precede thinning in SD-OCT.26 The late loss of RPE appears as a dark area of reduced autofluorescence (Fig. 6). Fundus autofluorescence is especially valuable in providing a topographic view of damage through the fundus, and wide-field images can show extramacular patterns of damage in Asian eyes.
Fig. 6.Ultra-wide field FAF image (A: Right eye; B: Left eye) demonstrates mottled hypofluorescence in all mid-periphery distribution in an advanced case of toxicity. FAF macular images (C: Right eye; D: Left eye) show parafoveal distribution in both eyes with mottled hypoautofluorescence.
(0.12MB).
- 1.
Subjective and functional test: microperimetry. This procedure locates the visual field test flashes on the retina with precision. In concept, it should be useful and more reliable than automated perimetry, but in practice, it is complicated by fatigue and the length of the test. Microperimetry is a novel modality, and its potential as a detection tool is still under evaluation.
- 2.
Objective and structural test: Adaptive Optics Retinal Imaging. Special cameras with improved optics to reduce wavefront distortion can image the cone array directly and show cone damage in early disease. However, distinguishing artifact damage remains difficult with current instruments, and as of this writing, this remains a matter for investigation.
- 3.
Objective and structural test: C-Scan SD-OCT (”en face” OCT).51 Among other recent imaging techniques, C-Scan OCT allows to visualize different layers of the retina in a frontal plane. The correlation between the C-Scan OCT findings and mfERG is still under evaluation in patients treated with antimalarials drugs.
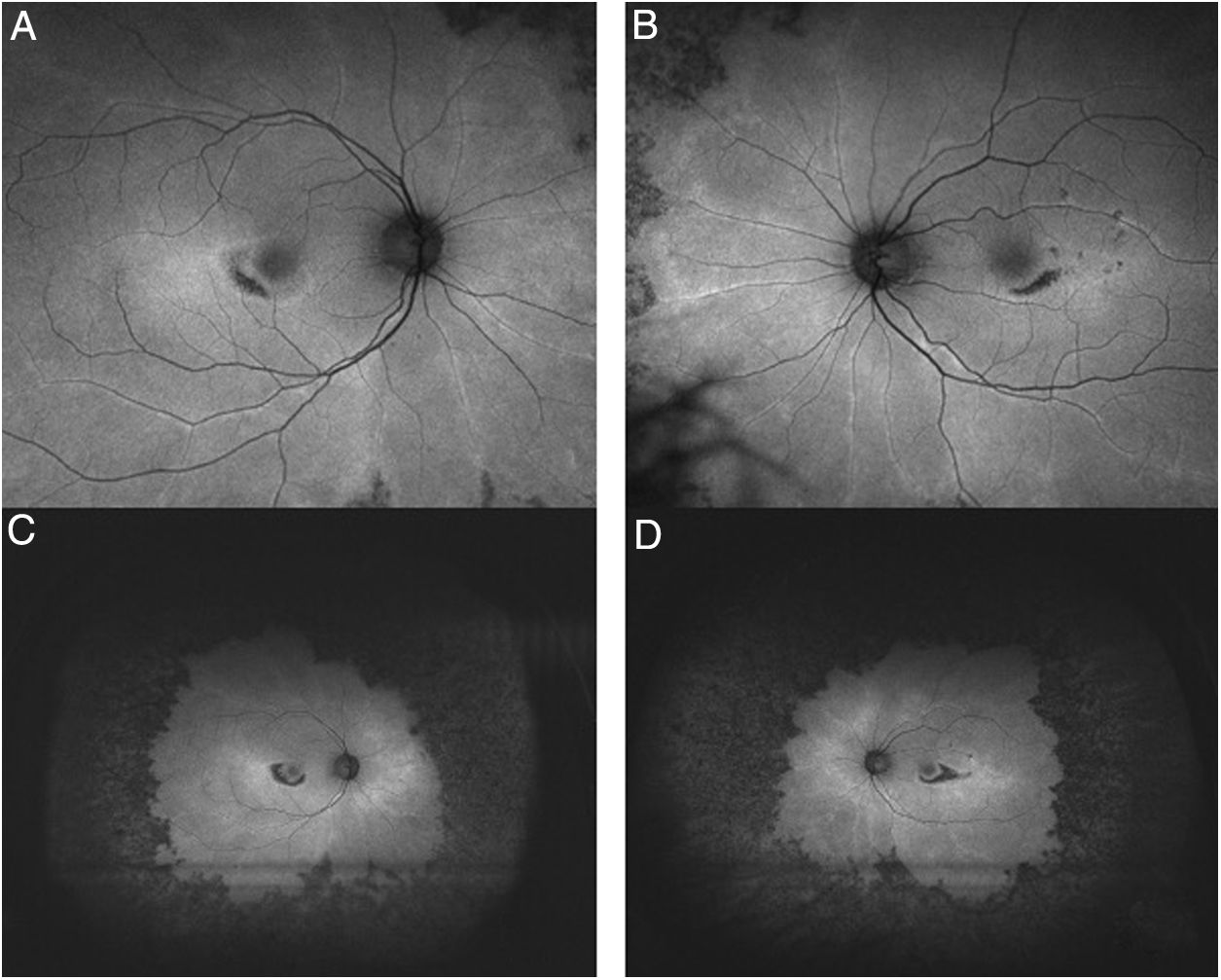
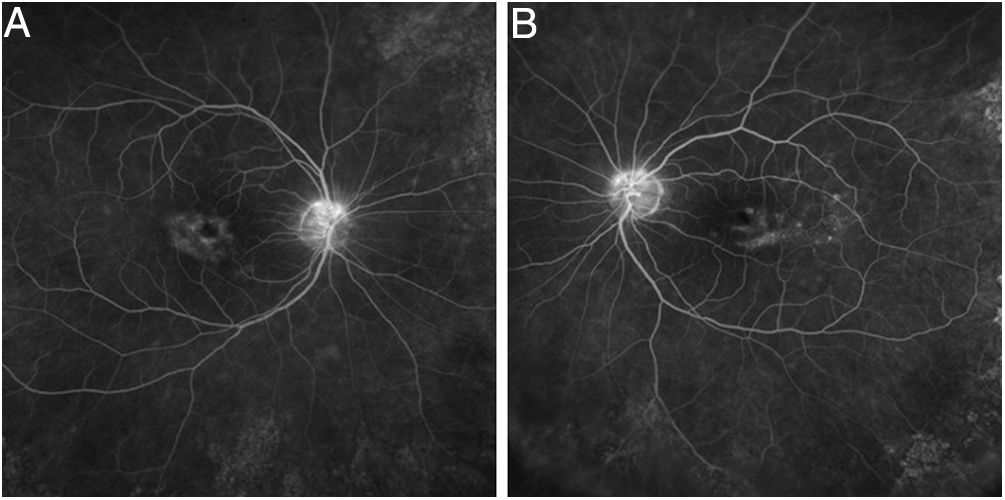
Tests that are no longer recommended26 are the Amsler grid test, color vision tests, fundus photography, time domain OCT, fluorescein angiography (Fig. 7), and full-field ERG, as they are not sensitive enough to detect the first signs of toxicity.
Management of the risk of retinopathy or antimalarial-induced retinopathy26Other than drug cessation, no diet or medical treatment has so far been shown to be effective in preventing, treating, or reducing the risk of HCQ or CQ retinopathy. Even drug discontinuation does not prevent the progression of retinopathy, although this is usually mild if toxicity is recognized before RPE damage occurs.
Patients with age-related maculopathy or macular dystrophy are sometimes advised to avoid excessive sun exposure and maintain their intake of lutein and zeaxanthin (which are foveal protectors). However, the value of such recommendations is unknown for patients at risk from exposure to HCQ or CQ, or in cases after retinopathy is detected and the drug is discontinued.
Early detection of toxicity and drug discontinuation before advanced toxicities develop may be associated with possible visual functional improvement.52 On the other hand, late detection may be associated with the progression of structural and functional visual impairment. The subtle findings observed in these imaging modalities described should lower the clinician's threshold for suspecting toxic effects and justify the use of additional tests, with the goal of achieving an early diagnosis before visual loss is irreversible.
The Royal College of Ophthalmologists recently published a summary of the key components of the guideline for retinal screening in users of hydroxychloroquine and chloroquine in the United Kingdom.53Table 5 summarizes the interpretation of screening results and Tables 6 and 7 the management of patients with possible and definite toxicity respectively.
Interpretation of screening results.53
| Grade | ||
|---|---|---|
| No toxicity | No abnormalities suggestive of retinal toxicity detected on any test. | B |
| Possible toxicity | One test result (which in case of VF should be reproducible) typical of HCQ retinopathy, but typical abnormalities not present in other tests. | GPP |
| Definite toxicity | Two test results (one subjective test and one objective test) with abnormalities typical of HCQ retinopathy. | B |
HCQ: Hydroxychloroquine; VF: visual fields.
Grade B: A body of evidence including studies rated as 2+, directly applicable to the target population and demonstrating overall consistency of results; or extrapolated evidence from studies rated as 2++. Grade GPP: Good practice points based upon consensual expert opinion where the evidence base does not support A–C grading.
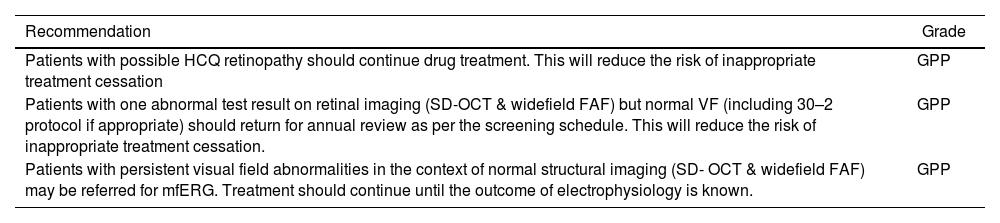
Management of patients with possible retinopathy.53
| Recommendation | Grade |
|---|---|
| Patients with possible HCQ retinopathy should continue drug treatment. This will reduce the risk of inappropriate treatment cessation | GPP |
| Patients with one abnormal test result on retinal imaging (SD-OCT & widefield FAF) but normal VF (including 30–2 protocol if appropriate) should return for annual review as per the screening schedule. This will reduce the risk of inappropriate treatment cessation. | GPP |
| Patients with persistent visual field abnormalities in the context of normal structural imaging (SD- OCT & widefield FAF) may be referred for mfERG. Treatment should continue until the outcome of electrophysiology is known. | GPP |
FAF: fundus autofluorescence; HCQ; hydroxychloroquine; mfERG: multifocal electroretinogram; SD-OCT: spectral-domain optical coherence tomography; VF: visual fields.
Grade GPP: Good practice points based upon consensual expert opinion where the evidence base does not support A–C grading.
Management of patients with definite toxicity.53
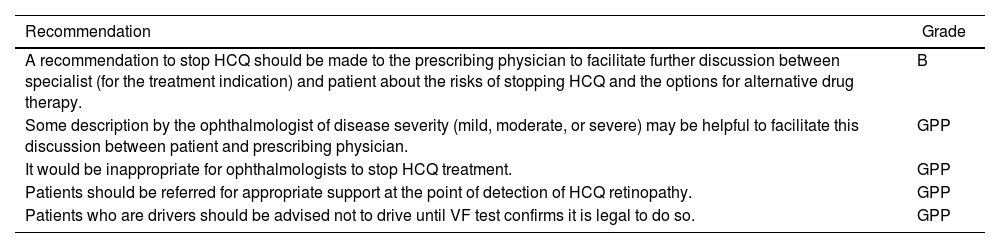
| Recommendation | Grade |
|---|---|
| A recommendation to stop HCQ should be made to the prescribing physician to facilitate further discussion between specialist (for the treatment indication) and patient about the risks of stopping HCQ and the options for alternative drug therapy. | B |
| Some description by the ophthalmologist of disease severity (mild, moderate, or severe) may be helpful to facilitate this discussion between patient and prescribing physician. | GPP |
| It would be inappropriate for ophthalmologists to stop HCQ treatment. | GPP |
| Patients should be referred for appropriate support at the point of detection of HCQ retinopathy. | GPP |
| Patients who are drivers should be advised not to drive until VF test confirms it is legal to do so. | GPP |
HCQ: hydroxychloroquine; VF: visual fields.
Grade B: A body of evidence including studies rated as 2+, directly applicable to the target population and demonstrating overall consistency of results; or extrapolated evidence from studies rated as 2++. Grade GPP: Good practice points based upon consensual expert opinion where the evidence base does not support A–C grading.
When the definitive signs of retinopathy have been detected, the decision to discontinue the medication should be made between the patient and the prescribing physician to ensure that the medical risks of such a decision (for example, a possible outbreak of SLE) are controlled. Depending on the severity of the retinopathy, the patient can be warned about the risk of further visual loss. This risk is minimal if the retinopathy was detected early, but significant if the bull's eye maculopathy and some reduction in central foveal thickness already exist, because damage can progress over several years.32
All patients with antimalarial-induced retinopathy should undergo regular check-ups with their reference ophthalmologist, regardless of the degree of involvement.
The choice of Qn, an alternative antimalarial, can be considered in patients with skin manifestations and HCQ-induced retinal toxicity.54
Conflict of interestsThe authors declare that they have no conflicts of interest concerning this article.
The authors would like to thank Sofia Luengo-Woods for her help editing the translation.