Antiphospholipid antibodies syndrome is a recently described condition. The diagnosis of this condition is based on the presence of thrombotic events without previous predisposition and the positivity of anti-phospholipid antibodies. Its clinical presentation includes a variety of patterns, some of which are not included within the clinical criteria of the diagnosis, but must be known. Dermal involvement as livedo is common. However, dermal necrosis is not usual. Thus, a case of antiphospholipid syndrome with dermal necrosis is presented as a primary manifestation of the disease.
El síndrome de anticuerpos antifosfolípidos es una condición de reciente descripción, cuyo diagnóstico se basa en la presencia de eventos trombóticos sin predisposición previa, con positividad de anticuerpos antifosfolípidos. Su presentación clínica incluye gran variedad de patrones, algunos de ellos no incluidos dentro de los criterios clínicos de diagnóstico, pero que deben ser conocidos. El compromiso dérmico es usual como livedo, sin embargo, la necrosis dérmica no es usual. Se presenta un caso de síndrome de anticuerpos antifosfolípidos con necrosis dérmica como manifestación primaria de la enfermedad.
Antiphospholipid antibodies syndrome (APS) is a condition initially described by Hughes in 1986 as anticardiolipin syndrome.1 It obtained its current name a year later, coined by Harris et al.2
It corresponds to a complex of thrombotic phenomena with a history of gestational losses in women in the presence of antiphospholipid antibodies.3 In its initial approach, the manifestations of deep vein thrombosis and pulmonary thromboembolism are usually the most common,4 and they even form part of the diagnostic criteria. However, there are other less common clinical manifestations that are not part of the diagnostic criteria, but which can be suggestive, such as dermatological, neurological, renal and hematological lesions that should be known, since in many cases they are the first manifestation of the syndrome or the only presentation of the cases which are called seronegative.4,5
Next, we present a case whose first manifestation of APS was the dermal commitment with progression to necrosis, an unusual involvement within the spectrum of this disease.
Case presentationWe present the case of a 66-year-old woman of mixed race, unemployed, who enters referred from a primary care center with a clinical picture of 2 months of evolution characterized by the presence of initially livedoid lesions, with some papules and crusts predominantly located in the lower extremities, with commitment of the soles of the feet, not painful, that the last 10 days evolved with the formation of ulcers with a necrotic base accompanied by general malaise and fever. Initially she received treatment with acetaminophen and sultamicillin, with progressive deterioration, and therefore she was referred to a higher level of complexity for study.
On the anamnesis of the review by systems, the patient indicated that she had presented similar lesions of less severity for 4 years, which she related especially to cold and that resolved spontaneously. The only known pathological antecedent was hypothyroidism on replacement therapy.
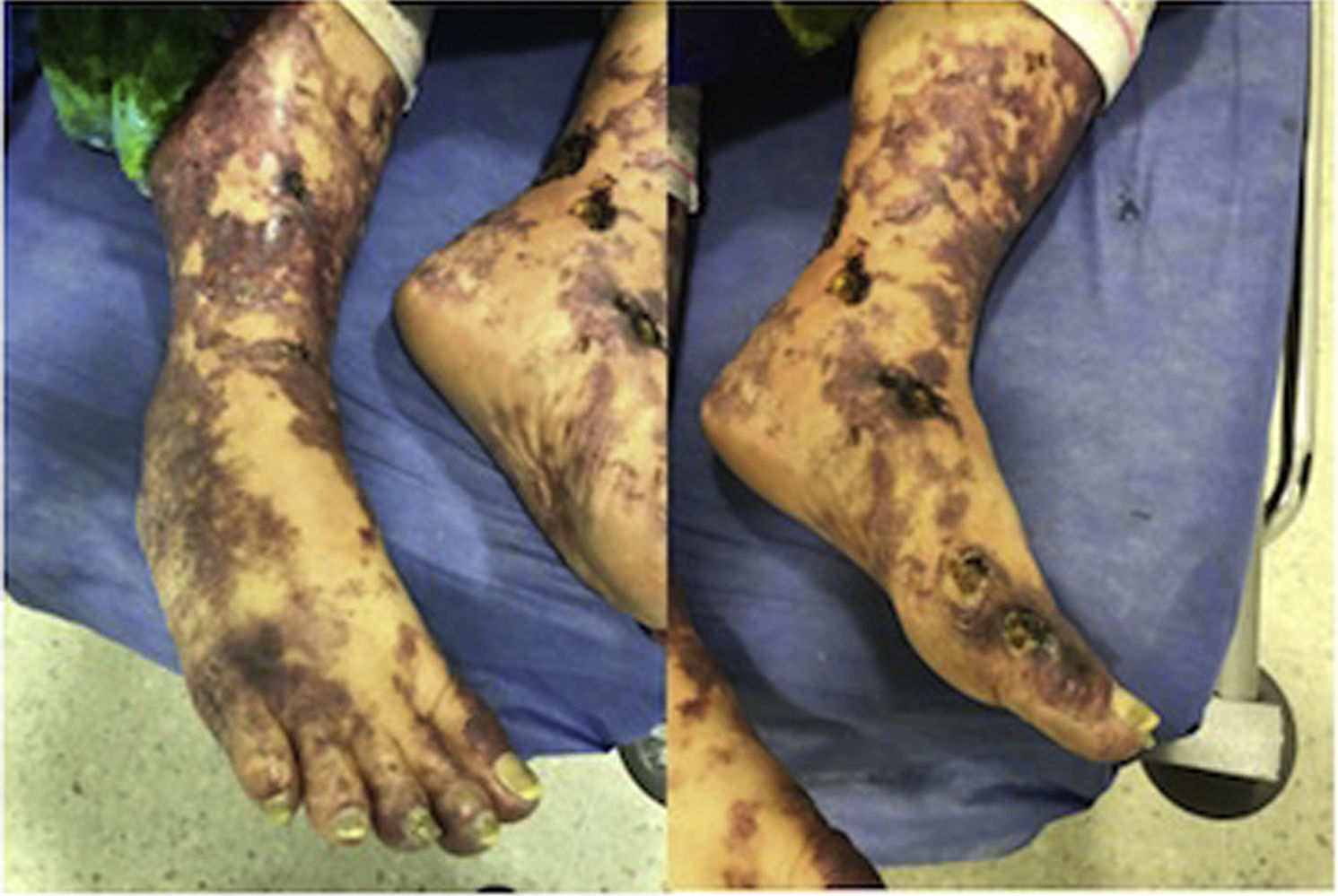
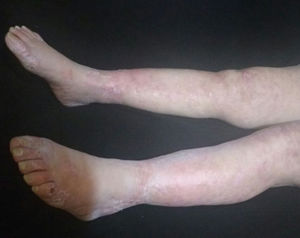
At physical examination the patient was febrile with livedoid commitment of racemosus appearance, and ulcerated lesions with necrotic bases that were predominantly located in the perimaleolar and anterior tibial regions of the distal third of the lower extremities (Fig. 1) that extended proximally preserving the abdomen and thorax; a similar compromise of less severity was seen in the distal third of the upper extremities. The mucous membranes and joint structures did not show any alteration.
The laboratory tests on admission showed elevation of acute phase reactants (ESR and CRP), without leukocyte reaction, with mild anemia of normal volumes. The renal function, plasma electrolytes and liver biochemistry were reported as normal. In addition, it was documented an alteration in the thromboplastin time (PTT), which was repeated, and the alteration persisted (Table 1).
Laboratories on admission.
| Variable | Result | Units |
|---|---|---|
| WBC | 6190 | Cells |
| Neutrophils | 77 | % |
| Lymphocytes | 10.8 | % |
| HCT | 32.2 | % |
| Hb | 10.8 | g/dl |
| MCV | 83.6 | Phentoliters |
| MCH | 27 | Picograms/Cell |
| Platelets | 183,000 | Cells |
| Creatinine | 0.66 | mg/dl |
| BUN | 14 | mg/dl |
| Na/K/Cl | 135/4.3/102 | mEq/L |
| CRP | 28.9 | mg/dl |
| ESR | 106 | mm/h |
| HIV Ab | Negative | |
| PT | 12.3 | Seconds |
| INR | 1.15 | |
| PTT | 40.4 | Seconds |
In the initial approach, vasculitis was first considered, taking into account both primary and secondary conditions. Without ruling out an infectious etiology, empiric broad spectrum therapy was initiated, and blood cultures, procalcitonin, immunological profile, chest radiograph and abdominal ultrasound were requested. The results (Table 2) showed negative procalcitonin, negative autoimmunity markers, except for antineutrophil cytoplasmic antibodies, which were reported in dilutions of 1/40 both for C-ANCA and P-ANCA. The results were confirmed by immunoassay (ELISA) for myeloperoxidase (MPO) and proteinase (P3), with positive results.
Some cervical lymphadenopathies were documented during hospitalization and it was raised the possibility that the picture could correspond to paraneoplastic commitment. Tomographic images of the chest and the abdomen were taken, with evidence of more lymphadenopathies. A biopsy and bone marrow study were indicated, and the 2 reports were negative for malignancy.
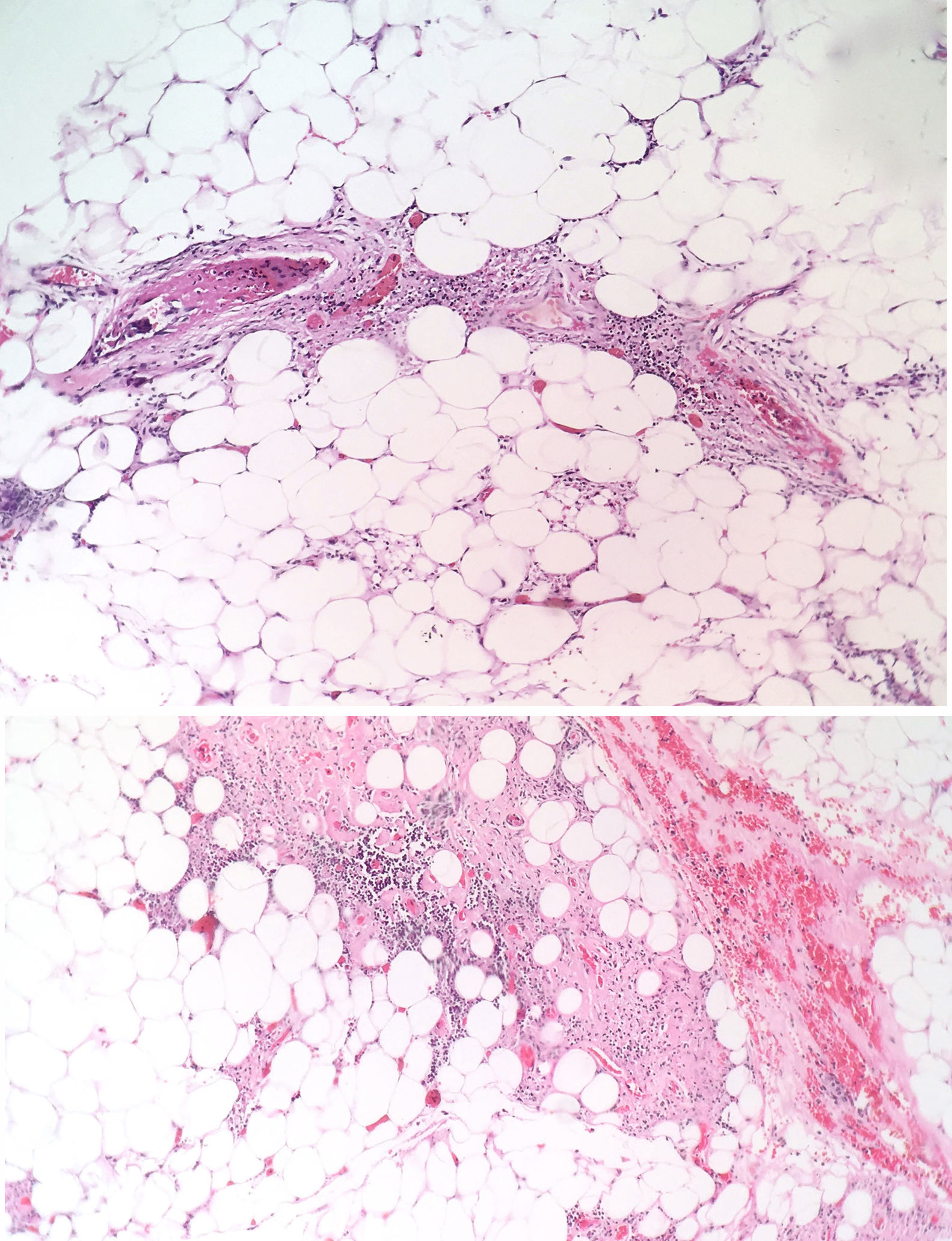
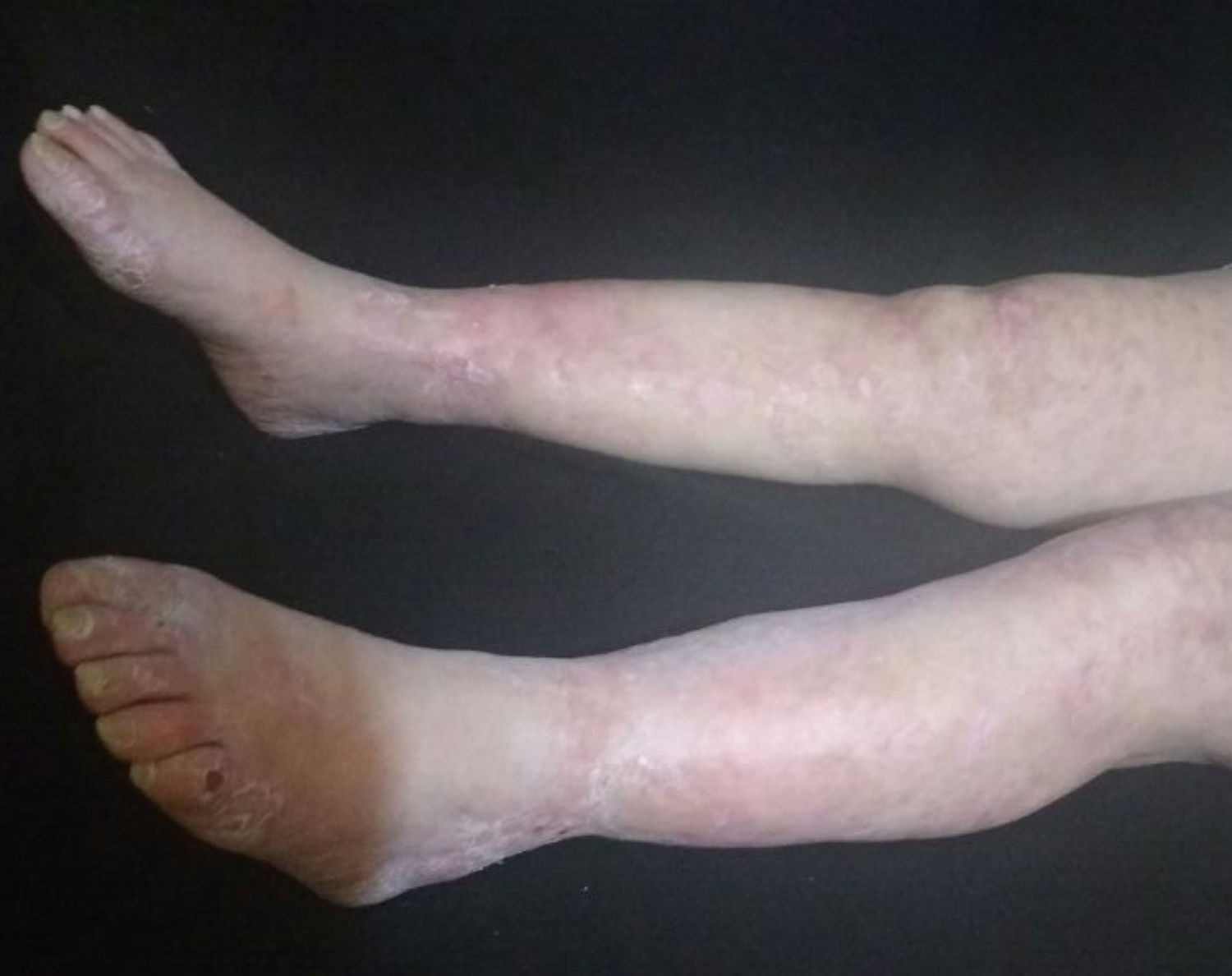
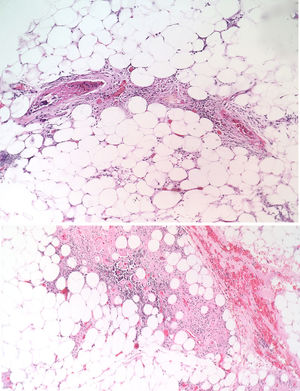
The patient was evaluated by the Dermatology group, which described a pattern of retiform purpura that indicated thrombotic involvement, with persistence of alteration in PTT. A skin biopsy and antiphospholipid antibodies screening were performed (Table 3). The results obtained indicated commitment of occlusive vasculopathy (Fig. 2), with positivity of antiphospholipid antibodies, with negative antinuclear antibodies (ANA) and anti-DNA antibodies. A diagnosis of primary PSA with dermal necrosis was made and treatment with full anticoagulation, immunomodulatory therapy with corticosteroids plus azathioprine was initiated, with a favorable clinical response. The patient was discharged, with an improvement of 90% in the lesions, and continued immunomodulatory treatment without new lesions during her outpatient follow-up (Fig. 3). The antiphospholipid antibodies were analyzed again 12 weeks after the initial event as indicated by the guidelines, and the positivity persisted (Table 4).
Control of antibodies taken 12 weeks after the initial event.
| Variable | Result | Units |
|---|---|---|
| ENA Profile (Anti-SSB-LA, anti-SM, anti-RNP, anti-SSA-RO) | Negative | U/ml |
| Anti-DNA Ab. | <12.3 (negative) | U/ml |
| ANA | 1/160 (negative) | Dilutions |
| Anticardiolipin Ab. | IgG (24.7) IgM (353) | U/ml |
| Anti-B2-glycoprotein Ab. | IgG (2.5) IgM (301) | U/ml |
| Lupus anticoagulant RVVT | Index 1.8 (++) |
RVVT: Russel viper venom time.
APS is a condition present in 1–5% of the population.6 Its diagnosis is based on the Sapporo criteria,7 which include the presence of one or more demonstrated events of arterial or venous thrombosis, with the presence of 3 or more abortions (before the 10th week of gestation), preterm births (before 34 weeks) or deaths in the presence of antiphospholipid antibodies (lupus anticoagulant, anticardiolipin and anti-B2-glycoprotein 1 antibody in 2 measurements with a difference of 12 weeks between the sample collection).
Among the possible manifestations, the deep venous thrombosis without a triggering factor is the most frequent presentation (31.9%). However, there are other manifestations not included within the criteria, but equally frequent, such as thrombocytopenia (21.9%), neurological manifestations (chorea, multiple sclerosis like), nephropathy associated with antiphospholipid antibodies or heart valve involvement, which should be taken into account at the time of giving rise to the diagnostic suspicion.5,6 For this particular case, the dermal presentation pattern is the one that can make diagnosis difficult, since it is well known that the dermal involvement in patients with APS is present in approximately 20% as livedo, which can be regular (closed lesions of circular appearance) or racemose (broken and irregular circular lesions), the latter is the most distinctive and is considered an independent risk factor for thrombosis.8,9 The presence of these lesions should give rise to diagnostic suspicion, since this semiologic pattern derives from processes of microthrombosis in the dermal vascular network.
The mechanisms of the thrombotic phenomena are poorly elucidated: studies in animal models have demonstrated that the administration of antiphospholipid antibodies does not necessarily cause spontaneous thrombotic phenomena, which gives rise to a hypothesis that involves multiple predisposing phenomena, such as infections or vascular lesions.10,11 Anti-B2 glycoprotein antibodies appear to play a fundamental role and to confer a special risk for thrombotic phenomena. It has been shown that these antibodies activate platelets, endothelial cells and monocytes by their binding to toll-like receptor 2 (TLR2), TLR4 and annexin A2 complexes, and that they generate the activation of intracellular signals which results in a greater thrombotic phenotype.12
In addition, antiphospholipid antibodies also have shown a role in thrombotic phenomena. They compete with the activated protein C for binding to phospholipids, limiting the action of this protein on its substrate.13
The skin lesions are the product of thrombotic phenomena with complete vascular occlusion, which leads to vascular damage in the skin. The angled figures reflect the thrombosed superficial vascular architecture, with hemorrhagic appearance due to extravasation of red cells.14,15 If this progresses, necrotic phenomena can be observed as happened in this particular case; this manifestation is less frequent and its presence makes diagnosis difficult due to the need to confirm other diagnostic hypotheses. In large series such as the one of Cervera et al.,8 which evaluated more than 1000 patients with APS, dermal necrosis was seen in only 2.1% of the patients and a similar frequency distribution was observed in the study conducted by Pinto-Almetida et al.9
Regarding the classification of APS into primary or secondary, it has been seen that about half are primary and, most of the remaining secondary 50% are associated with systemic lupus erythematosus.8 This ratio is the justification for research with type ANA and anti-DNA autoantibodies in these patients; negativity does not exclude a case of secondary APS, but it rules out the main associated condition (systemic lupus erythematosus).
In the report presented, the dilutions for antineutrophil cytoplasmic antibodies (ANCA) (C-ANCA and P-ANCA) were positive. In this regard, it is rescued that these studies may be positive in rheumatic diseases (systemic lupus erythematosus, systemic sclerosis, inflammatory myositis, APS, Sjögren's syndrome), especially the P-ANCA pattern, due to errors in the interpretation by the presence of ANA, since in the confirmation by immunoassay (ELISA), the results for myeloperoxidase (MPO) and proteinase 3 (PR3) were negative. That was reported in a study conducted by Merkel et al.16 In this particular case, the confirmation of the ANCAs was positive, without finding histopathological evidence of vasculitis involvement. In this regard, we did not find additional information to support the finding, since it was interpreted as an unusual pattern of presentation.
Finally, it should be highlighted the importance of the necrotic involvement in the treatment, since it is considered a major thrombotic phenomenon, which configures the need for indefinite full anticoagulation.5,9
ConclusionAPS is a condition with a wide variety of clinical manifestations that generally represent a characteristic prothrombotic state; however, some of its manifestations are not part of the criteria, but they must be known as they may correspond to the initial manifestation of the syndrome. The dermal commitment in APS is usual in the presentation of livedo however, other presentations such as dermal and digital necrosis or subungual splinter lesions are not usual, so these patterns should always be kept in mind to guide the diagnostic process early.
Conflict of interestThe authors of this manuscript declare that they do not have any conflict of interest.
Thanks to the Pathology Service of the Central Millitary Hospital, especially to Dr. María Janeth Vargas Manrique for her collaboration in the preparation, acquisition and interpretation of the histopathology images.
Please cite this article as: Vásquez JF, Carreño Jaimes M, Amador JR, Uscátegui Ruiz AC, Real Chavarro AP. Necrosis dérmica como primera manifestación del síndrome de anticuerpos antifosfolípido. Rev Colomb Reumatol. 2019;26:204–208.