The use of tumor necrosis factor (TNF) alpha inhibitors is increasing in patients with spondyloarthritis. Early identification of those that would require them, or the ability to predict their use, could lead to a more effective and timely treatment by rationalizing their use.
ObjectiveTo determine factors that better explain the indication of TNFi in the study population.
Material and methodsThe association between anti-TNFα use and categorical demographic, clinical, laboratory, radiological and treatment variables was explored using Pearson's Chi2 or Fisher's exact test. The association with the quantitative variables was evaluated using Student's t test or Mann Whitney U test, depending on their distribution. Those variables with P < .25 were entered into univariate models of explanatory logistic regression to construct crude ORs, and those with P < .25 were included in the multivariate model to construct adjusted ORs.
Results and discussionThe study population includes 181 patients. In the univariate model: reactive arthritis, urethritis, and peripheral involvement were protective factors for the use of TNFi. Axial spondyloarthritis, inflammatory lumbalgia, alternating gluteal pain, morning stiffness, sacroiliitis demonstrated by any method, treatment with COX2 inhibitors, evolution time of three years or more, and BASDAI and BASFI scores were associated with the use of TNFi. Multivariate model: reactive arthritis (P = .036), inflammatory back pain (P = .026), sacroiliitis (P = .045), use of coxibs (P = .001) and the maximum score of BASDAI (P = .022, P = 006) were independently associated with the use of TNFi. The use of coxibs was associated with the indication of using TNFi in both patients with axial (P = .001) and peripheral spondyloarthritis (P < .001).
ConclusionsThe onset of the disease in the form of reactive arthritis behaved as a protective factor for the subsequent need to use TNFi, while presenting with inflammatory back pain, sacroiliitis, demonstrated by any method, treatment with coxibs, and the maximum score of BASDAI greater than 4 associated with the use of these medications.
El uso de TNFi es cada vez más frecuente en los pacientes con espondiloartritis. Identificar tempranamente aquellos que los requerirán o poder predecir su uso puede ayudar a hacer un tratamiento más efectivo y oportuno racionalizando su uso.
ObjetivoDeterminar los factores que mejor explican la indicación de TNFi en la población de estudio.
Material y métodosLa asociación entre el uso de medicamentos anti-TNFα y las variables categóricas demográficas, clínicas, de laboratorio, radiológicas y de tratamiento se exploró por prueba exacta de Fisher. La asociación con las variables cuantitativas fue evaluada con T de Student o U de Mann Withney de acuerdo a su distribución. Aquellas variables con P < .25 fueron ingresadas a modelos univariante de regresión logística explicativa para construir los OR crudos y aquellas con P < .25 se incluyeron en el modelo multivariante para construir OR ajustados.
Resultados y discusiónLa población está constituida por 181 pacientes. Modelo univariante: la artritis reactiva, uretritis y compromiso periférico fueron factores protectores para el uso de TNFi. Espondiloartritis axial, lumbalgia inflamatoria, dolor glúteo alternante, rigidez matinal, sacroilitis demostrada por cualquier método, tratamiento con inhibidores COX2, tiempo de evolución de tres años o más y los puntajes de BASDAI y BASFI se asociaron al uso de TNFi. Modelo multivariante: artritis reactiva (OR 0.1, IC95% 0.012–0.86, P = .036), lumbalgia inflamatoria (OR 13.63, IC95% 1.36–136, P = .026), sacroilitis (OR 7,71, IC95% 1.04–57, P = .045, uso de coxibs (OR 10.1, IC95% 2.71–37.62, P = .001) y el puntaje máximo de BASDAI (4–6: OR 6.1, IC95% 1.3–28.7, P = .022, mayor de 6: OR 15.8, IC95% 2.2–113, P = .006) se asociaron independientemente con el uso de TNFi. El uso de coxibs se asoció a la indicación de usar TNFi tanto en los pacientes con espondiloartritis axial (OR 4.2, IC95% 1.74–10.11, P = .001 como periférica (OR 4, IC95% 1.85–8.62, P < .001).
ConclusionesEl inicio de la enfermedad en la forma de artritis reactiva se comportó como un factor protector para la necesidad posterior de usar TNFi mientras que presentar lumbalgia inflamatoria, sacroilitis demostrada por cualquier método, el tratamiento con coxibs y el puntaje máximo de BASDAI mayor de 4 se asociaron al uso de estos medicamentos.
Spondyloarthritis (SpA) is a group of inflammatory diseases that affect both the axial and the peripheral skeleton, presenting enthesitis and peripheral arthritis. These entities mainly affect the young population, share several clinical and radiological manifestations and have a common immunogenetic origin. As a group, SpAs affect 1% of the young adult population, especially in the Nordic countries and in Caucasian population.1–3
Until two decades ago there were few therapeutic alternatives: non-pharmacological measures such as aerobic exercise and medications such as nonsteroidal anti-inflammatory drugs (NSAIDs), sulfasalazine and methotrexate, as well as local infiltrations with corticosteroids. These treatments showed efficacy in the control of pain and stiffness of the peripheral joints and of enthesitis, but have limited effectiveness for axial involvement.4,5 In the initial stages of the disease, NSAIDs, including the cyclooxygenase 2 inhibitors (coxibs) are the drugs of choice; they have demonstrated a good control of the inflammatory low back pain and can delay the axial involvement in some patients, so they should be administered continuously. However, in some cases their efficacy is limited, there are contraindications for their use, or the adverse effects outweigh the possible benefits. In these cases, tumor necrosis factor inhibitors (TNFi) are indicated.6–8
There are currently several TNFis, including etanercept (soluble receptor), infliximab (chimeric monoclonal antibody), adalimumab (humanized monoclonal antibody), certolizumab (pegylated monoclonal antibody, without the Fc fraction) and golimumab (humanized monoclonal antibody). The use of TNFi is increasingly frequent in patients with SpA, which has changed the natural history and the quality of life of these patients.9–11 Early identification of those subjects who will require them, as well as of the factors associated with their use, can help to do a more timely, effective and rationalized treatment. In Colombia, few studies have been conducted to determine the clinical behavior of these diseases and the factors that compel the rheumatologists to use biological therapy have not been identified.12–14
The purpose of the study was to know the factors that explain or are associated with the need to use TNFi in patients with SpA at some point in their evolution.
Patients and methodsThe main objective of the study was to determine the factors that best explain the need to use TNFi in a population of patients with SpA in Northwestern Colombia. The secondary objectives were to associate demographic, clinical, laboratory, genetic, radiological and treatment factors with the use of TNFi and to determine the factors that are associated, regardless of the indication of these drugs.
It was conducted a descriptive, cross-sectional study, which included all the patients with SpA diagnosed at the Pablo Tobón Uribe Hospital in the city of Medellin, between January 1, 2005 and December 31, 2016, with a minimum follow-up of one year.
All patients over 18 years of age who fulfilled the ASAS criteria, and who consulted the services of rheumatology, hospitalization or emergencies of the institution were included and were evaluated by a rheumatologist, and those subjects with other rheumatic diseases that could interfere with the clinical evaluation such as rheumatoid arthritis, systemic lupus erythematosus, osteoarthritis of the spine, and fibromyalgia were excluded. Patients with autoimmune diseases, infections and neoplasms that contraindicate the use of TNFi were also excluded.
The independent variables were demographic (age, sex, age at onset of the symptoms and family history of spondyloarthritis, psoriasis, uveitis, inflammatory bowel disease), clinical (initial manifestation of spondyloarthritis, heel pain, uveitis, enthesitis, dactylitis, cutaneous psoriasis, ungual psoriasis, diarrhea, urethritis, arthritis, Crohn's disease, ulcerative colitis, inflammatory low back pain, gluteal pain, morning stiffness, inflammatory bowel disease and the type of spondyloarthritis), clinimetric (at baseline, maximum and average BASDAI and BASFI scores during the evolution of the disease and at the initiation of the treatment with TNFi), laboratory (erythrocyte sedimentation rate [ESR], C-reactive protein [CRP] and HLA-B27), imaging (MRI [vertebral bone marrow edema and in the sacroiliac joints], presence of erosions, syndesmophytes, fusion of the sacroiliac joints, and plain radiographs of these to apply the NY classification) and of treatment (NSAIDs, oral corticosteroids, coxib, methotrexate, sulfasalazine). The outcome variable was the indication to start TNFi by the treating rheumatologist; these drugs were analyzed as a group to facilitate the analysis.
Analysis planThe population was described according to demographic, clinical, radiological, laboratory, family and treatment characteristics. The continuous parametric variables were summarized with means and standard deviations (SD) and the non-parametric variables, with medians and interquartile ranges (IQR).
The association between the indication of TNFi and the categorical variables was explored with the Fisher’s exact test, while the distribution of the quantitative variables was evaluated using the Kolmogorov-Smirnov test. Likewise, the association of these variables with the outcome was assessed by the Student’s t test for those with normal distribution or by the Mann Withney’s U test for those with non-normal distribution.
Multivariate analysis was done by means of explanatory logistic regression using the enter method to obtain the crude and adjusted ORs. In the initial model, variables with P < .25 (Hosmer Lemeshow criterion) were included. In the final model, those with P < .25 were included in the univariate analysis, excluding collinear variables.15 The analyses were made with the SPSS® program version 22, licensed by the Pablo Tobón Uribe Hospital. P values <.05 were considered significant.
The study did not imply any risk for the patients; therefore, signing of informed consent was not required. All patients who attended the institution signed habeas data. The protocol was approved by the ethics and research committee of the hospital.
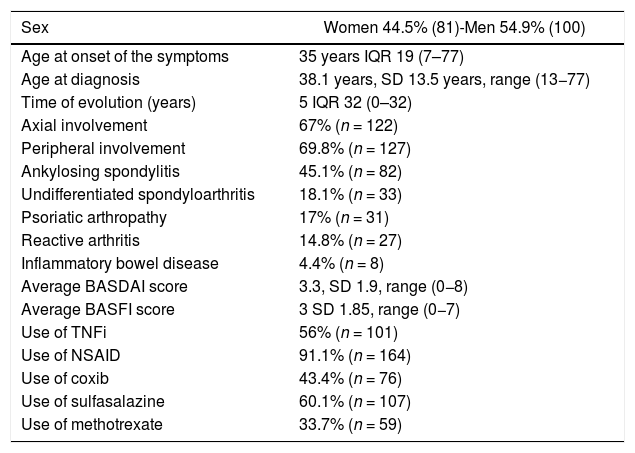
ResultsThe population is constituted by 181 patients, with an average age of 45.3 years, SD 13.39 (20−83). In 27.5% (50) the symptoms of SpA started in the first 25 years, in 40.7% (74) between the ages of 26 and 40 years and in 31.9% (57) after the age of 40 years. Table 1 shows the main clinical and demographic manifestations and the treatments of the population.
Demographic, clinical and treatment characteristics of a population of 181 patients with spondyloarthritis.
| Sex | Women 44.5% (81)-Men 54.9% (100) |
|---|---|
| Age at onset of the symptoms | 35 years IQR 19 (7–77) |
| Age at diagnosis | 38.1 years, SD 13.5 years, range (13−77) |
| Time of evolution (years) | 5 IQR 32 (0–32) |
| Axial involvement | 67% (n = 122) |
| Peripheral involvement | 69.8% (n = 127) |
| Ankylosing spondylitis | 45.1% (n = 82) |
| Undifferentiated spondyloarthritis | 18.1% (n = 33) |
| Psoriatic arthropathy | 17% (n = 31) |
| Reactive arthritis | 14.8% (n = 27) |
| Inflammatory bowel disease | 4.4% (n = 8) |
| Average BASDAI score | 3.3, SD 1.9, range (0−8) |
| Average BASFI score | 3 SD 1.85, range (0−7) |
| Use of TNFi | 56% (n = 101) |
| Use of NSAID | 91.1% (n = 164) |
| Use of coxib | 43.4% (n = 76) |
| Use of sulfasalazine | 60.1% (n = 107) |
| Use of methotrexate | 33.7% (n = 59) |
The patients had active disease. The baseline BASDAI score was 4 IQR 3 (0−8) and 89% (161) had a score of 4 points or more. The maximum BASDAI score was 4.97, SD 1.94 (4–9), and in 75.3% (137) it was higher than 4 points. Likewise, they had significant functional involvement. The baseline BASFI score was 4 IQR 3 (0−7) and 89% (162) of the subjects started with 4 points or more in this item. During the evolution they had a maximum BASFI score of 4.11, SD 1.99 (0−8), and 56.6% (103) had 4 points or more at some point.
42.9% (78) of the patients had an accelerated ESR at some point of their evolution, with a value of 33.5 mm/h, IQR 39 (2–126). The CRP was found increased in 40.7% (74) of the subjects, with a value of 6 mg/dL IQR 32 (0–32). HLA-B27 was positive in 55.7% (59/106) of the cases.
The presence of sacroiliitis on plain pelvic radiography was explored in 125 (69%) patients of the study population. 40.8% (51) of them were classified as grade 0; grade I, 6.4% (8); grade II, 8.8% (11); grade III, 28.8% (36) and grade IV 15.2% (19), according to the New York classification. A plain radiograph of the spine was obtained in 78 (43%) of the patients; lumbar syndesmophytes were observed in 23 of them (29.4%).
69.2% (54/78) of the subjects had acute changes (bone edema) and 37.2% (29/78) chronic changes in the MRI of the sacroiliac joints. In the MRI of the lumbar spine, 35% (11/31) presented acute changes and 22.5% (7/31) chronic changes (syndesmophytes).
56% (101/180) of the patients were treated with TNFi due to failure of two NSAIDs in the case of axial SpA and failure of two NSAIDs, methotrexate and sulfasalazine, in the case of peripheral SpA. 30.8% (31) of the patients received more than one TNFi. The most used was adalimumab (31.1%, n = 56), followed by etanercept (21.7%, n = 39), infliximab (13.9%, n = 25), golimumab (6.1%, n = 11) and certolizumab (0.5%, n = 1).
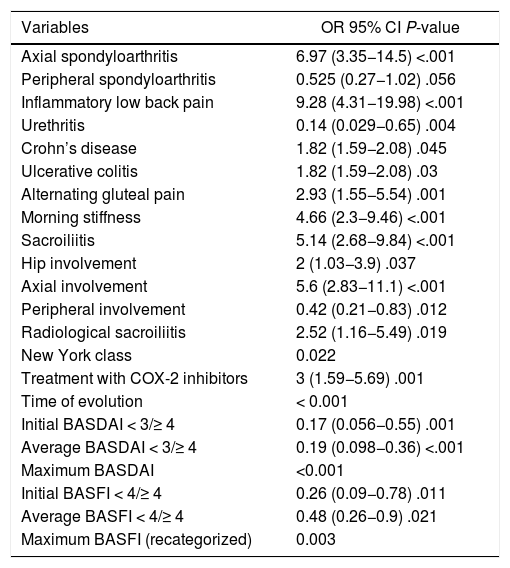
The categorical variables that were associated with the use of TNFi are described in Table 2. Those related to the involvement of the axial skeleton (axial spondyloarthritis, inflammatory low back pain, sacroiliitis, morning stiffness and alternating gluteal pain), the disease activity, the functional commitment, the need to use coxib and having inflammatory bowel disease were risk factors. The predominance of peripheral skeletal manifestations and the antecedent of urethritis behaved as protective factors for the use of TNFi.
Categorical variables associated with the use of TNFi.
| Variables | OR 95% CI P-value |
|---|---|
| Axial spondyloarthritis | 6.97 (3.35−14.5) <.001 |
| Peripheral spondyloarthritis | 0.525 (0.27−1.02) .056 |
| Inflammatory low back pain | 9.28 (4.31−19.98) <.001 |
| Urethritis | 0.14 (0.029−0.65) .004 |
| Crohn’s disease | 1.82 (1.59−2.08) .045 |
| Ulcerative colitis | 1.82 (1.59−2.08) .03 |
| Alternating gluteal pain | 2.93 (1.55−5.54) .001 |
| Morning stiffness | 4.66 (2.3−9.46) <.001 |
| Sacroiliitis | 5.14 (2.68−9.84) <.001 |
| Hip involvement | 2 (1.03−3.9) .037 |
| Axial involvement | 5.6 (2.83−11.1) <.001 |
| Peripheral involvement | 0.42 (0.21−0.83) .012 |
| Radiological sacroiliitis | 2.52 (1.16−5.49) .019 |
| New York class | 0.022 |
| Treatment with COX-2 inhibitors | 3 (1.59−5.69) .001 |
| Time of evolution | < 0.001 |
| Initial BASDAI < 3/≥ 4 | 0.17 (0.056−0.55) .001 |
| Average BASDAI < 3/≥ 4 | 0.19 (0.098−0.36) <.001 |
| Maximum BASDAI | <0.001 |
| Initial BASFI < 4/≥ 4 | 0.26 (0.09−0.78) .011 |
| Average BASFI < 4/≥ 4 | 0.48 (0.26−0.9) .021 |
| Maximum BASFI (recategorized) | 0.003 |
The quantitative variables associated with the use of TNFi were the age of onset of the symptoms (P = .036), the time of evolution (P = .006), the initial BASDAI score (P < .001) and the initial BASFI score (P = .002). Patients who required TNFi were 4.55 years younger at the onset of symptoms than those who did not need them (−8.31; −0.79, P = .018).
Patients with higher disease activity had a higher indication for TNFi; the difference in means in the maximum BASDAI score was 1.46 (95% CI 0.89; 2.02), P < .001, between those who received or did not received these medications. The difference in means in the BASDAI score between the groups during the evolution was 1.59 (95% CI 1.1; 2.08) P < .001.
Functional compromise was also associated with the need to start TNFi; the maximum BASFI score was higher in the group that received TNFi (difference in means 1.46, 95% CI 0.89; 2.02, P < .001). Likewise, during the evolution the BASFI was higher in this group (difference in means 1.59, 85% CI 1.1; 2.08, P < .001).
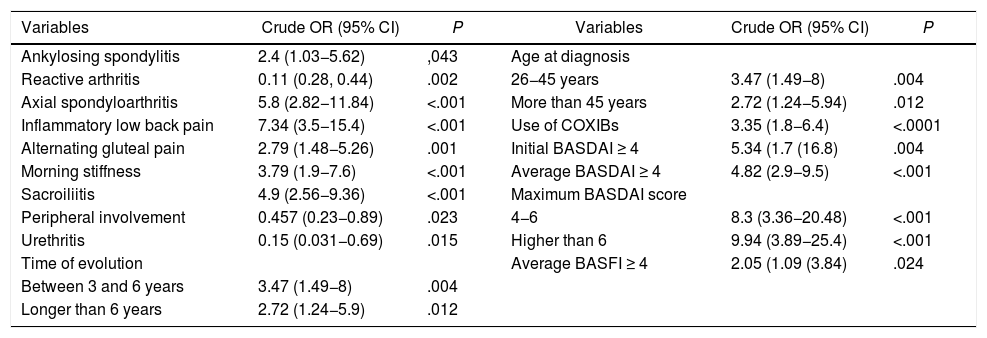
In the univariate logistic regression model, reactive arthritis, urethritis and peripheral involvement were protective factors for the use of TNFi. Axial spondyloarthritis, inflammatory low back pain, alternating gluteal pain, morning stiffness, sacroiliitis demonstrated by any method, treatment with COX-2 inhibitors, a time of evolution of three years or more and the BASDAI and BASFI scores were associated with the use of TNFi. At diagnosis, the patients over 25 years of age used more TNFi than those who were diagnosed at 25 years or less, but there were no differences between those with a diagnosis of spondyloarthritis between 26 and 45 or more years of age (Table 3).
Factors that best explain the use of TNFi in 181 patients with spondyloarthritis. Univariate analysis.
| Variables | Crude OR (95% CI) | P | Variables | Crude OR (95% CI) | P |
|---|---|---|---|---|---|
| Ankylosing spondylitis | 2.4 (1.03−5.62) | ,043 | Age at diagnosis | ||
| Reactive arthritis | 0.11 (0.28, 0.44) | .002 | 26−45 years | 3.47 (1.49−8) | .004 |
| Axial spondyloarthritis | 5.8 (2.82−11.84) | <.001 | More than 45 years | 2.72 (1.24−5.94) | .012 |
| Inflammatory low back pain | 7.34 (3.5−15.4) | <.001 | Use of COXIBs | 3.35 (1.8−6.4) | <.0001 |
| Alternating gluteal pain | 2.79 (1.48−5.26) | .001 | Initial BASDAI ≥ 4 | 5.34 (1.7 (16.8) | .004 |
| Morning stiffness | 3.79 (1.9−7.6) | <.001 | Average BASDAI ≥ 4 | 4.82 (2.9−9.5) | <.001 |
| Sacroiliitis | 4.9 (2.56−9.36) | <.001 | Maximum BASDAI score | ||
| Peripheral involvement | 0.457 (0.23−0.89) | .023 | 4−6 | 8.3 (3.36−20.48) | <.001 |
| Urethritis | 0.15 (0.031−0.69) | .015 | Higher than 6 | 9.94 (3.89−25.4) | <.001 |
| Time of evolution | Average BASFI ≥ 4 | 2.05 (1.09 (3.84) | .024 | ||
| Between 3 and 6 years | 3.47 (1.49−8) | .004 | |||
| Longer than 6 years | 2.72 (1.24−5.9) | .012 |
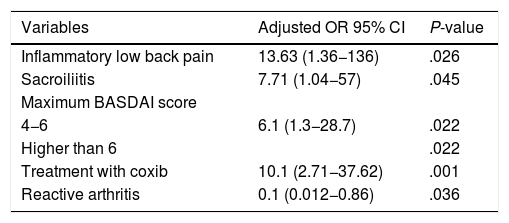
The presentation of inflammatory low back pain, sacroiliitis demonstrated by any radiological technique, the maximum BASDAI score and the requirement of coxib were associated, regardless of the use of TNFi in the population (Table 4). The odds for the use of TNFi were between 12- and 13-fold higher in the patients with inflammatory low back pain and between 6- and 7-fold higher in those with demonstrated sacroiliitis; likewise, the maximum BASDAI score was a risk factor for the use of these medications. The patients who started with reactive arthritis needed less TNFi during the evolution. Patients who required the use of coxib after two traditional NSAIDs were between 9 and 10 times more likely to require TNFi.
Factors independently associated with the use of TNFi.
| Variables | Adjusted OR 95% CI | P-value |
|---|---|---|
| Inflammatory low back pain | 13.63 (1.36−136) | .026 |
| Sacroiliitis | 7.71 (1.04−57) | .045 |
| Maximum BASDAI score | ||
| 4−6 | 6.1 (1.3−28.7) | .022 |
| Higher than 6 | .022 | |
| Treatment with coxib | 10.1 (2.71−37.62) | .001 |
| Reactive arthritis | 0.1 (0.012−0.86) | .036 |
In a population of patients with SpA, followed-up in a high-complexity hospital, 56% required the initiation of TNFi. The presentation of inflammatory low back pain, sacroiliitis demonstrated by any method, previous treatment with coxib and a maximum BASDAI score higher than 4 were associated with the use of these drugs, while the onset in the form of reactive arthritis was proven to be a protective factor for this outcome.
The onset of the disease was before the age of 40 years in approximately 73% of patients, with a higher frequency in men, while axial and peripheral involvement were present in 67% and 69% of patients, respectively. Ankylosing spondylitis, undifferentiated spondyloarthritis, and psoriatic arthritis were the most frequent, similarly to what has been described in other cohorts.16–18
Approximately 80% of the patients had elevated acute phase reactants and the positivity of HLA-B27 was 55%, lower than the described for European populations, in which it is higher than 80%. In the German GESPIC cohort, it was observed that the male gender and a high level of CRP were associated with the structural damage observed in radiographs, whereas the positivity of HLA-B27 determined the age of onset of the disease.19
Axial and peripheral involvement were present in a high percentage of patients, as well as sacroiliitis, which was evidenced both in the plain radiography and in the MRI in 59% and 69% of the subjects, respectively, findings that could explain the high BASDAI and BASFI scores at the inclusion in the cohort and during follow-up. These parameters have been considered relevant in other populations as selection criteria to start TNFi in patients.20,21 The majority of our population required rotation of at least two NSAIDs in the predominantly axial forms and of NSAIDs and immunomodulators in the peripheral forms (methotrexate and sulfasalazine).
In the univariate analysis, axial spondyloarthritis, inflammatory low back pain, alternating gluteal pain, morning stiffness, and sacroiliitis demonstrated by any method were associated with increased use of TNFi. Treatment with COX-2 inhibitors was also a factor related to the use of TNFi, possibly because in our environment the coxibs are used as a second line of treatment, since they are not included in the mandatory health plan and because most of those who receive these medications have persisted symptomatic, at least two conventional NSAIDs failed or had contraindications for their use.
Reactive arthritis, urethritis, and peripheral involvement were protective factors for the use of TNFi. This could be because these forms can be self-limited and the use of methotrexate and sulfasalazine could delay or prevent the use of TNFi in these patients.
The factors that have been associated with a poor prognosis, with the radiological progression of forms of non-radiographic axial involvement, with evident sacroiliitis and the need for the use of TNFi are: male gender, cigarette smoking, early onset of the disease, elevated C-reactive protein at the onset of the disease, elevated clinimetric parameters such as BASDAI and HAQ, as well as early involvement of the hips.22–24
Weaknesses and strengthsThe fact that the radiological evaluation of the patients is not complete and uniform, that in some of them the presence of HLA-B27 has not been explored, and that ASDAS has not been included in the clinimetry, are important weaknesses of the study. Likewise, not having at least 10 outcomes for each variable included in the multivariate logistic regression model to control confusion, takes power away from the model. This is a frequent problem in observational studies.
The main strengths of the study are the follow-up of the cohort since 2005, the inclusion of all patients diagnosed at the institution, and the analysis of all the variables associated with the need to start TNFi.
ConclusionsThe onset of the disease in the form of reactive arthritis behaved as a protective factor for the subsequent need to use TNFi, while having inflammatory low back pain, sacroiliitis demonstrated by any method, treatment with coxib and the maximum BASDAI score higher than 4, were associated with the use of these medications. The association between coxib and use of TNFi persisted after the stratified analysis by type of spondyloarthritis. Multicenter studies with a larger number of patients are required to control confounding factors, better understand the behavior of spondyloarthritis and the use of TNFi, as well as the predictive factors in our population.
FundingThis work was conducted with the support of the Colombian Association of Rheumatology.
Conflict of interestThe authors declare that they do not have any conflict of interest.
Please cite this article as: Pinto-Peñaranda LF, Echeverri-García AF, Restrepo-Escobar M, Álvarez Barreneche MF, Hurtado A, Márquez-Hernández JD. Factores asociados con el uso de inhibidores del factor de necrosis tumoral alfa en una población de pacientes colombianos con espondiloartritis. Rev Colomb Reumatol. 2021;28:184–190.