Antiphospholipid syndrome (APS) is a systemic autoimmune disease characterized by recurrent thrombosis that can affect the arterial and venous circulation.
ObjectivesTo analyze the immunological and pharmacological differences, as well as the clinical outcomes of a cohort of patients with primary antiphospholipid syndrome and secondary antiphospholipid syndrome.
Materials and methodsA retrospective cohort study was conducted that included 352 records of patients diagnosed with APS and treated between 2014 and 2018. A description is presented of the sociodemographic, clinical, and immunological profile of the population. A bivariate analysis performed using the chi-squared test to determine differences between groups with primary APS and secondary APS, and finally a multivariate analysis to search for associations with thrombotic clinical outcomes in patients with APS.
ResultsThe mean age was 42.4 ± 14 years, and 84.6% were females. Two-thirds (67.6%) of the patients had a diagnosis of primary APS, and 32.4% of secondary APS, of which 84% were associated with systemic lupus erythematosus (SLE). Among the thrombotic events, the most frequent were deep vein thrombosis (17.3%) and stroke (9.9%). Obstetric events were frequent, with a prevalence of 39.4% for miscarriages. No differences were found in the sociodemographic or immunoserological profile when comparing the group of primary vs. secondary APS. Thrombotic events were more frequent in the primary APS group, although only pulmonary embolism reached statistical significance. There were no differences between the two groups as regards obstetric events, such as miscarriages. Women were found to be 5 times more likely to have a stroke and 3 times more to have deep vein thrombosis. The anti-B2GPI type IgM increased the probability of presenting miscarriages about 3 times in women with APS.
ConclusionThe study contains one of the largest Colombian cohorts with APS reported so far, and although it is both clinically and sociodemographically similar to other cohorts, there is a higher prevalence of primary APS. There was a lower frequency of thrombotic complications compared to other cohorts. Patients with primary APS had a tendency to develop thrombosis, as has already been reported in the literature.
El síndrome antifosfolípido (SAF) es una enfermedad autoinmune sistémica caracterizada por trombosis recurrente que puede afectar la circulación arterial y venosa.
ObjetivoAnalizar las diferencias inmunológicas y farmacológicas, así como los desenlaces clínicos de una cohorte de pacientes con síndrome antifosfolípido primario y secundario.
Materiales y métodosEstudio de corte transversal que incluyó 352 pacientes con diagnóstico de SAF atendidos entre los años 2014 y 2018. Se analizaron variables sociodemográficas, clínicas e inmunológicas y se realizó un análisis univariado y un análisis bivariado mediante la prueba chi-cuadrado para determinar diferencias entre los pacientes con SAF primario y SAF secundario. Finalmente, se hizo un análisis multivariado para buscar asociaciones con los desenlaces clínicos trombóticos en los pacientes con SAF.
ResultadosLa edad promedio de la población fue de 42,4 ± 14 años, el 84,6% correspondió a sexo femenino. El 67,6% de los pacientes tenía diagnóstico de SAF primario y un 32,4% de SAF secundario, siendo el lupus eritematoso sistémico (LES) la enfermedad asociada en un 84%. Dentro de los eventos trombóticos, lo más frecuente fue trombosis venosa profunda (17,3%), seguida por el ataque cerebrovascular (9,9%). En los eventos obstétricos existió una prevalencia del 39,4% para abortos. No se encontraron diferencias en el perfil sociodemográfico ni en el perfil inmunoserológico entre los pacientes con diagnóstico de SAF primario y aquellos con SAF secundario. Los eventos trombóticos tuvieron mayor frecuencia en el grupo de SAF primario, pero solo la tromboembolia pulmonar alcanzó significancia estadística. Los eventos obstétricos como abortos no fueron diferentes entre ambos grupos. Dentro de los factores asociados a los eventos trombóticos, se encontró que el sexo femenino tiene una probabilidad 5 veces mayor de ACV y 3 veces mayor de TVP. Los anti B2GPI tipo IgM aumentaron alrededor de 3 veces la probabilidad de presentar abortos en mujeres con SAF.
ConclusiónSe presenta una de las cohortes colombianas más grandes de pacientes con SAF reportadas hasta el momento en la literatura. La población es comparable clínica y sociodemográficamente a lo encontrado en otros estudios, aunque la prevalencia de SAF primario fue mayor y las complicaciones trombóticas fueron menores. La tromboembolia pulmonar fue significativamente mayor en el grupo de SAF primario.
Antiphospholipid syndrome (APS) is a systemic autoimmune disease characterized by recurrent thrombosis that can affect the arterial and venous circulation. It is one of the most common acquired thrombophilias, with an incidence of 5 cases per 100,000 people per year. It occurs in 1% of the general population, with a predilection for the female gender and it can be primary or secondary. Approximately half of the patients with APS have a primary presentation, while the other half are associated with other pathologies,1 being systemic lupus erythematosus (SLE) the most common associated disease. One third of patients with SLE have antiphospholipid antibodies, but only 5–10 % of them will develop an APS.2 Differences have been found between primary and secondary APS, one of the most important is that in APS associated with SLE there is a higher frequency of arthritis, livedo reticularis, venous thrombosis and fetal losses.3 However, cohort data comparing primary and secondary APS are limited.
Among the most frequent clinical manifestations are: thrombosis of the deep venous system of the extremities and of the cerebral arterial circulation. Less frequently, thrombosis can occur in other sites such as the hepatic veins, visceral veins, or cerebral venous circulation. Obstetric manifestations are equally important, including fetal death without other causes, recurrent miscarriages, or preterm delivery.4 Other scenarios such as catastrophic APS are infrequent: they occur in less than 1% of patients with APS and carry a high mortality rate. Catastrophic APS is characterized by thrombotic involvement in 3 or more organs simultaneously or in rapid succession, in the presence of antiphospholipid antibodies and with histopathological confirmation of small-vessel thrombosis in the absence of inflammation at this level. It is usually triggered by infections and its main complications are brain and heart involvement, infections and multiple organ failure.5
The classification criteria establish that at least one clinical and one laboratory criteria must be met. Within the clinical criteria are episodes of thrombosis and obstetric events that include fetal death after week 10 of gestation, 3 or more abortions before week 10 and preterm delivery before week 34, related to hypertensive syndromes of pregnancy or intrauterine growth restriction.4 Other “non-criteria” clinical manifestations such as thrombocytopenia, livedo reticularis, skin ulcers and transient ischemic attacks may be found.5 Within the laboratory criteria are the persistent positivity over time (longer than 12 weeks) of lupus anticoagulant, anti-beta 2 glycoprotien I (anti-β2GPI) or IgG or IgM anticardiolipin antibodies.4
Local evidence on this topic is limited. To date, only 3 works from Colombian cohorts with APS have been reported in the literature. Two of the studies were conducted in the city of Medellín6,7 in reference centers for autoimmune diseases and the third in a population in the Southwest of Colombia.8 In Medellin, Vargas et al. described 62 patients with a diagnosis of APS by Sapporo criteria and found that those with primary APS had a later age of onset and were associated with a higher frequency of fetal losses. On the other hand, secondary APS was associated with SLE in most cases, with venous thrombotic events being the most frequent manifestation. From the immunoserological point of view, anticardiolipin antibodies were the most prevalent in this cohort of patients, being present in 93.4% of the patients.6 In 2012, Mesa et al. described another cohort of 100 patients with APS in whom they detailed the “non-criteria” clinical manifestations, that is, those that do not fit within the diagnostic criteria but are part of the spectrum of the disease. Of these, the neurological manifestations were the most prevalent (23.9%).7 The objective of this study is to compare the sociodemographic, clinical and immunoserological characteristics between primary and secondary APS in a Colombian cohort of patients with APS treated at a reference center for autoimmune diseases in Colombia.
Materials and methodsA cross-sectional study was conducted based on the registry of a retrospective cohort of 352 patients with a diagnosis of APS according to the Sapporo classification criteria, who were treated in an institution specialized in rheumatology between 2014 and 2018. The sociodemographic, clinical and immunological profile of the population is described by means of relative and absolute proportions, and subsequently a bivariate analysis is carried out to determine differences between patients with primary and secondary APS using the chi-square test. Finally, in order to identify factors associated with the clinical outcomes, a multivariate analysis was performed with a binary logistic regression. The statistical package SPSS version 21 of the CES University was used.
ResultsGeneral characteristics of the populationThe mean age of the general population was 42.4 ± 14 years, and 84.6% were women. 67.6% of the patients had a diagnosis of primary APS and 32.3% of secondary APS, of which 84% were associated with SLE. Among the thrombotic events, the most frequent was deep vein thrombosis (17.3%), followed by cerebrovascular attack (9.9%). Obstetric events were frequent, with a prevalence of miscarriages of 39.4%. From the immunoserological point of view, positivity for anticardiolipin antibodies was the most prevalent.
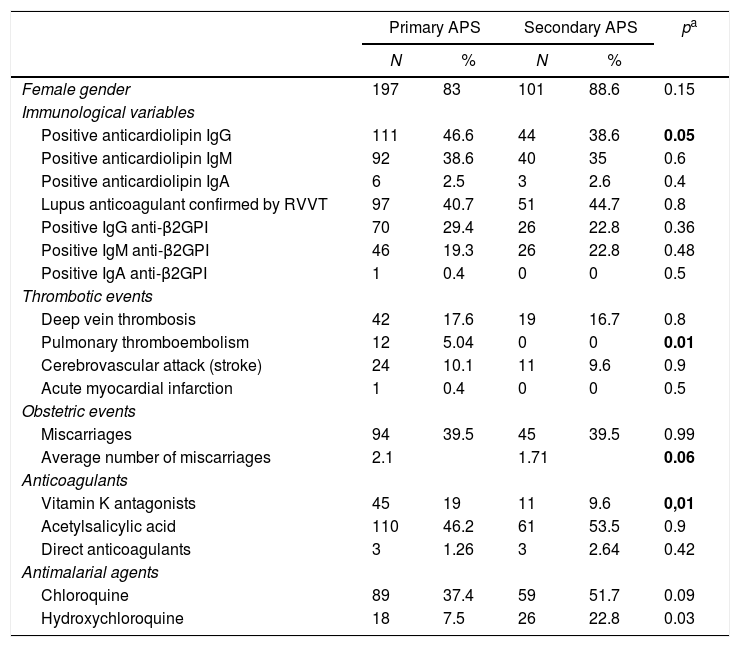
Comparison between primary and secondary antiphospholipid syndromeWhen comparing the population with a diagnosis of primary APS and that with secondary APS, no differences were found in the sociodemographic or in the immunoserological profiles. Thrombotic events were more frequent in the group with primary APS, but only pulmonary thromboembolism reached statistical significance. This suggests a more severe course in this group of patients, as has been reported in other cohorts. Obstetric events such as miscarriages were not different between the two groups (see Table 1).
Comparative analysis of patients with primary and secondary antiphospholipid syndrome.
| Primary APS | Secondary APS | pa | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Female gender | 197 | 83 | 101 | 88.6 | 0.15 |
| Immunological variables | |||||
| Positive anticardiolipin IgG | 111 | 46.6 | 44 | 38.6 | 0.05 |
| Positive anticardiolipin IgM | 92 | 38.6 | 40 | 35 | 0.6 |
| Positive anticardiolipin IgA | 6 | 2.5 | 3 | 2.6 | 0.4 |
| Lupus anticoagulant confirmed by RVVT | 97 | 40.7 | 51 | 44.7 | 0.8 |
| Positive IgG anti-β2GPI | 70 | 29.4 | 26 | 22.8 | 0.36 |
| Positive IgM anti-β2GPI | 46 | 19.3 | 26 | 22.8 | 0.48 |
| Positive IgA anti-β2GPI | 1 | 0.4 | 0 | 0 | 0.5 |
| Thrombotic events | |||||
| Deep vein thrombosis | 42 | 17.6 | 19 | 16.7 | 0.8 |
| Pulmonary thromboembolism | 12 | 5.04 | 0 | 0 | 0.01 |
| Cerebrovascular attack (stroke) | 24 | 10.1 | 11 | 9.6 | 0.9 |
| Acute myocardial infarction | 1 | 0.4 | 0 | 0 | 0.5 |
| Obstetric events | |||||
| Miscarriages | 94 | 39.5 | 45 | 39.5 | 0.99 |
| Average number of miscarriages | 2.1 | 1.71 | 0.06 | ||
| Anticoagulants | |||||
| Vitamin K antagonists | 45 | 19 | 11 | 9.6 | 0,01 |
| Acetylsalicylic acid | 110 | 46.2 | 61 | 53.5 | 0.9 |
| Direct anticoagulants | 3 | 1.26 | 3 | 2.64 | 0.42 |
| Antimalarial agents | |||||
| Chloroquine | 89 | 37.4 | 59 | 51.7 | 0.09 |
| Hydroxychloroquine | 18 | 7.5 | 26 | 22.8 | 0.03 |
Anti-β2GPI: anti-beta 2 glycoprotein I; Ig: immunoglobulins; APS: antiphospholipid syndrome; RVVT: Russell’s viper venom time.
In bold, results with p value ≤0.05.
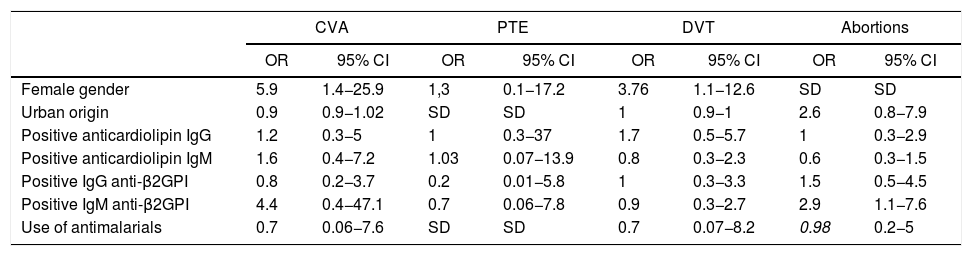
When searching for associations between the different sociodemographic, clinical, immunological and pharmacological variables, it was found that women are 5.9 times more likely to have a cerebrovascular accident (CVA) and 3.7 times more likely to have deep vein thrombosis compared with men. There were no significant differences in the use of antimalarials in any of the outcomes. It was found that the positivity of IgM anti-β2GPI antibodies increased 2.9-fold the probability of women to suffer a miscarriage (see Table 2).
Factors associated with thrombotic outcomes in patients with APS.
| CVA | PTE | DVT | Abortions | |||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Female gender | 5.9 | 1.4−25.9 | 1,3 | 0.1−17.2 | 3.76 | 1.1−12.6 | SD | SD |
| Urban origin | 0.9 | 0.9−1.02 | SD | SD | 1 | 0.9−1 | 2.6 | 0.8−7.9 |
| Positive anticardiolipin IgG | 1.2 | 0.3−5 | 1 | 0.3−37 | 1.7 | 0.5−5.7 | 1 | 0.3−2.9 |
| Positive anticardiolipin IgM | 1.6 | 0.4−7.2 | 1.03 | 0.07−13.9 | 0.8 | 0.3−2.3 | 0.6 | 0.3−1.5 |
| Positive IgG anti-β2GPI | 0.8 | 0.2−3.7 | 0.2 | 0.01−5.8 | 1 | 0.3−3.3 | 1.5 | 0.5−4.5 |
| Positive IgM anti-β2GPI | 4.4 | 0.4−47.1 | 0.7 | 0.06−7.8 | 0.9 | 0.3−2.7 | 2.9 | 1.1−7.6 |
| Use of antimalarials | 0.7 | 0.06−7.6 | SD | SD | 0.7 | 0.07−8.2 | 0.98 | 0.2−5 |
CVA: cerebrovascular accident; Anti-β2GPI: anti-beta 2 glycoprotein I; 95% CI: 95% confidence interval; Ig: immunoglobulins; OR: odds ratio; APS: antiphospholipid syndrome; SD: conflicting information due to the presence of zero in the groups; PTE: pulmonary thromboembolism; DVT: deep vein thrombosis.
APS is characterized by the development of venous or arterial thrombosis, morbidity in pregnancy and presence of antiphospholipid antibodies. The Euro-Phospholipid Project has provided a large part of the descriptive data from the sociodemographic, clinical and immunological aspects.9 The results presented in this cohort are similar to those reported, which confirms several characteristics of the disease independently of the geographic location.
As in other studies, it was found a higher frequency of primary APS when comparing it with the secondary (67.6% vs. 32.3%). SLE was the main associated pathology in the patients with secondary APS (84.2%), as reported in the literature.1 Regarding local studies, the data obtained show some differences. Osio et al. reported a prevalence of primary APS slightly lower than that of the present cohort (62% vs. 67%).8
According to the Euro-Phospholipid Project, 87.9% of the cohort had positive anticardiolipin antibodies, with a relatively homogeneous distribution between IgG, IgM and IgA.
In this cohort, 44% were positive for IgG, 37% for IgM, and 2.5% for IgA, with no statistically significant differences between primary and secondary APS. Similarly, the presence of lupus anticoagulant and anti-β2GPI did not show statistically significant differences between primary and secondary APS.
Similarly to what was published by Cervera et al. in the Euro Phospolipid Project,9 the most frequent thrombotic event in our population was deep vein thrombosis, but in a much lower percentage than in the European cohort, without significant differences between primary and secondary APS. In our study it was evidenced a statistically significant difference in the prevalence of pulmonary thromboembolism in the group with primary APS; however, this frequency is lower than the reported in the literature10 and it is possible that there is an underdiagnosis that explains this finding. Among arterial thrombosis, CVA was the most frequent manifestation, although with a lower prevalence than the reported.
Obstetric as well as thrombotic events, occurred with less frequency than the reported in literature,11 with a trend towards a higher average of miscarriages in the patients with primary APS. In our population, patients with positive IgM anti-β2GPI were more likely to have miscarriages (OR = 2.9; 95% CI = 1.1−7.6), which contrasts with that was reported by Alijotas-Reig et al. in the European Registry on Obstetrical Anti-Phospholipid Antibody Syndrome (Euroaps),12 in which there was no significant difference with the presence of this antibody or with that was reported by Silver et al., who found a higher probability of miscarriage with the presence of positive IgG β2GPI antibodies (OR = 3.03; 95% CI = 1.20–7.62).13 A local study with Colombian population showed a frequency of obstetric complications higher than the reported in this cohort (39% vs. 30%).7 In addition, it reported an association between IgM anticardiolipin antibodies and vascular access thrombosis and between positivity of lupus anticoagulant and neurological involvement (p < 0.05),7 findings that cannot be reaffirmed due to the design of the present study.
Even though the gender does not appear to be a variable that determines differences in thrombotic risk, in the multivariate analysis of the present study it was found that female gender, compared to male gender, is associated with a higher probability of CVA (OR = 5,9, 95% CI = 1.4–25.8) and deep vein thrombosis (OR = 2.9; 95% CI = 1.1−7.6). In a study in which the influence of gender was evaluated in 49 patients with primary APS, it was found a higher prevalence of pulmonary thromboembolism in women than in men (34.2% vs. 0.0%, p = 0.024).14 The association of the female gender with a higher frequency of thrombotic phenomena could be explained by a different positivity of antibodies, however, neither the sample of the present study nor of that of Carvalho are sufficient to reach this conclusion. This outcome should be more widely evaluated in future studies.
Vitamin K antagonists were the main anticoagulants administered in the study population. Direct oral anticoagulants (DOAC) were used in 1.26–2.64% of cases. The DOACs have been positioned as the first line in many clinical settings, however, their safety is questionable in APS.15 In the RAPS trial, Cohen et al.16 demonstrated that the use of rivaroxaban was associated with a 2-fold increase in the thrombin potential, suggesting a high risk of thrombosis compared with warfarin. The importance in this effect was assessed in the multicenter non-inferiority study published by Pengo et al.,17 in which patients with APS of high thrombotic risk with a triple positive immune profile (lupus anticoagulant, anticardiolipin and β2-glycoprotein), who received rivaroxaban, presented higher rates of thromboembolic events (12% vs. 0%) and of major bleeding (7% vs. 3%) compared with warfarin, which led to its early withdrawal. In the same sense, in 2019 a non-inferiority clinical trial18 reaffirmed the inferiority of rivaroxaban, compared to warfarin, reporting a nearly twice increase of recurrent thrombosis (11.6% vs. 6.3%). The low prevalence of the use of DOACs in the present study is merely a reflection that, given the current evidence, they do not appear to be a safe strategy in this group of patients.
ConclusionWe present one of the largest Colombian cohorts of patients with APS reported so far in the literature. The population is clinically and sociodemographically comparable with that found in other studies,6–8 although the prevalence of primary APS was higher and the prevalence of thrombotic complications was lower. The frequency of pulmonary thromboembolism was significantly higher in the group with primary APS, while the female gender was associated with a higher risk of CVA and deep vein thrombosis.
FundingThis work was developed with own resources of the institution.
Conflict of interestThe authors declare that they have no conflict of interest.
Please cite this article as: Díaz-Coronado JC, Herrera-Uribe S, Hernández-Parra D, Betancur-Vásquez L, Lacouture-Fierro J, González-Hurtado D, et al. Síndrome antifosfolípido (SAF): diferencias clínicas e inmunoserológicas entre SAF primario y secundario en una cohorte colombiana. Rev Colomb Reumatol. 2021;28:191–196.