The purpose of this study was to evaluate patients in clinical remission of psoriasis for at least for one year, who maintained therapeutic goals after initiating optimization of biologic therapy.
Materials and methodsA descriptive, observational study was conducted on patients with a diagnosis of moderate–severe psoriasis in treatment with biologic therapy who were started on optimization of biologic therapy.
ResultsA total of 29 patients started therapeutic optimization, of these, 27 patients were in the target range with absolute PASI less than 3. Only one patient failed therapeutic optimization with final PASI 3.6 and there was a case of a patient who lost continuity of management due to an accident and had a final PASI 3.8. Most of the patients were male, with an average age of 53 years, married, employed, residing in urban areas, with psoriasis of more than ten years of evolution, without associated morbidities, and without previous biologic treatment, the most frequently used being etanercept and adalimumab.
ConclusionOptimizing biologic therapy in patients with moderate–severe psoriasis may be viable. We seek to share this experience to propose a protocol to reduce the possibility of adverse events due to the prolonged use of this type of therapy, preserving clinical response and reducing costs to the health system.
El propósito de este estudio fue evaluar a los pacientes que, luego de al menos un año en remisión clínica de la enfermedad psoriásica, persistían en metas terapéuticas después de iniciar la optimización de la terapia biológica.
Materiales y métodosSe llevó a cabo un estudio descriptivo, observacional en pacientes con diagnóstico de psoriasis moderada-severa en tratamiento con terapia biológica a quienes se les inició optimización de la terapia biológica.
ResultadosEn total, 29 pacientes comenzaron optimización terapéutica. De estos, 27 se encontraban en rango de metas con el Psoriasis Area and Severity Index (PASI) absoluto menor a 3. Solo un paciente fracasó en la optimización con PASI final 3,6, y se menciona el caso de uno que por un accidente perdió continuidad en el manejo, por lo cual se encontró con PASI final 3,8. La mayoría de los pacientes eran de sexo masculino, con edad promedio de 53 años, casados, empleados, residentes en zonas urbanas, con psoriasis de más de 10 años de evolución, sin morbilidades asociadas y con un único biológico para su tratamiento, siendo los más frecuentemente utilizados etanercept y adalimumab.
ConclusiónOptimizar la terapia biológica en pacientes con psoriasis moderada-severa puede ser viable. Buscamos compartir esta experiencia con miras a más adelante proponer un protocolo que permita disminuir la posibilidad de presentación de eventos adversos por el uso prolongado de este tipo de terapias, conservando la respuesta clínica y adicionalmente disminuyendo costos al sistema de salud.
Psoriatic disease is a chronic, inflammatory, and immune-mediated condition that requires treatment for long periods, and has a great physical, psychological, and economic impact.1 In the pre-biological era, the clinical improvement of patients with this disease in very few cases reached 100%, but with the advent of biologic therapies, the response obtained is increasingly better and the goals in turn, more stringent because it went from having therapeutic objectives of delta PASI (Psoriasis Area and Severity Index) 75 to be more demanding, as this is what the new therapies achieve.2 Some guidelines consider an objective to obtain an improvement of 90% of PASI or an absolute PASI lower or equal to 3, since these are the values most related to an adequate quality of life in treated patients,3 highlighting that many patients achieve complete remission of their disease for long periods, which raises the question about giving continuity to the therapy received or if it is possible to optimize it (spacing frequency, adjusting doses, prolonging intervals, discontinuing medication) and sustaining the effectiveness in time.
Recently, the Colombian Rheumatology Association (ASOREUMA) published a consensus on recommendations for the reduction and discontinuation of biologic therapy in patients with rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis.4 In this context, we pose the question: is it possible to imagine a patient with moderate to severe psoriasis whose treatment can be optimized and who will continue to maintain an adequate response over time? If the answer is yes, the benefit is both for the patient, who will be completely controlled of his or her underlying pathology and without medication-associated events, and for the health system since the use of these therapies represents a high cost and the savings generated could be used to improve access to this type of therapy for other patients. Currently, there are not a relevant number of studies evaluating the optimization of biologic therapy in psoriatic disease, especially in the cutaneous domain. Therefore, the objective of this research was to describe how our population behaves with treatment with biologic therapy and what possibilities exist, in case of complete improvement, to successfully optimize it.
Materials and methodsA descriptive, observational study with analytical intent was conducted on patients with a diagnosis of moderate–severe psoriasis attended at CLIPSO (Integral Psoriasis Clinic)) of the Colombian health institution+helPharma, in treatment with biologic therapy and who had therapy optimization. The information was collected from clinical history databases, from January 2019 to December 2021.
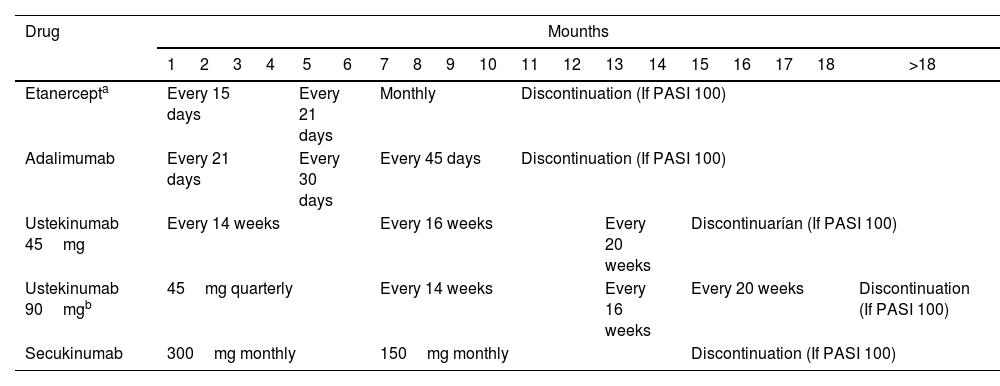
Patients with a diagnosis of psoriasis, older than 18 years of age, who had been on biologic therapy for at least one year and started the strategy of optimization of biologic therapy were included. Optimization of therapy consisted of increasing the drug application interval or reducing the administered dose and, eventually, allowing drug discontinuation, as shown in Table 1. Patients were evaluated by the CLIPSO dermatologist every 3, 6, or 12 months depending on the clinical risk of each patient. The Psoriasis Area Severity Index (PASI) and the Dermatological Life Quality Index (DLQI) were used to measure disease severity.
Description of therapy optimization.
| Drug | Mounths | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | >18 | |
| Etanercepta | Every 15 days | Every 21 days | Monthly | Discontinuation (If PASI 100) | |||||||||||||||
| Adalimumab | Every 21 days | Every 30 days | Every 45 days | Discontinuation (If PASI 100) | |||||||||||||||
| Ustekinumab 45mg | Every 14 weeks | Every 16 weeks | Every 20 weeks | Discontinuarían (If PASI 100) | |||||||||||||||
| Ustekinumab 90mgb | 45mg quarterly | Every 14 weeks | Every 16 weeks | Every 20 weeks | Discontinuation (If PASI 100) | ||||||||||||||
| Secukinumab | 300mg monthly | 150mg monthly | Discontinuation (If PASI 100) | ||||||||||||||||
Sociodemographic variables (age, sex, area of residence, occupation), clinical variables (time of disease evolution, type of psoriasis, PASI at the beginning and end of the follow-up period, DLQI, comorbidities), and pharmacological variables (medication, previous therapies received, date of treatment initiation, date of initiation and end of therapeutic optimization) were analyzed.
A univariate or descriptive analysis was performed in which absolute and relative frequencies were used for qualitative or categorical variables, and summary measures (central tendency, dispersion, position) were used for continuous quantitative variables. To establish the differences between initial and final PASI, initial and final DLQI, normality test, and T-test for paired samples (Wilcoxon test) was performed. The Jamovi Project (2021) free license statistical software was used.
Ethical considerationsThe study was approved by the scientific area of the health Institution and the insurer. This research was considered safe according to Resolution 8430 of 1993, under the Colombian regulations for research with human subjects.
ResultsWe identified 689 patients with moderate–severe psoriasis on biologic therapy, of which 29 patients were on biologic therapy optimization. Sixty-two percent were men with an average age of 53 years. Regarding the time of evolution of the psoriatic disease, 37.9% had a diagnosis of psoriasis of more than 20 years, followed by the group of 10–20 years with 34.4% and 5–10 years with 20.6%. The most common phenotype was plaque psoriasis in 51.7% of patients (Table 2).
Patient characteristics.
| Gender, n (%) | |
| Women | 11 (37.9) |
| Men | 18 (62.1) |
| Age, years | |
| Mean (SD) | 53 (15.3) |
| Range | 20–76 |
| Psoriasis duration, years | |
| Mean (SD) | 17.2 (10.2) |
| Range | 1–44 |
| Observational period, months | |
| Mean (SD) | 13.7 (10.7) |
| Range | 0.7–36 |
| Psoriasis phenotypes n (%) | |
| Vulgar | 15 (51.7) |
| Vulgar and scalp | 8 (27.6) |
| Vulgar and nails | 3 (10.3) |
| Vulgar, scalp and nails | 1 (3.4) |
| Scalp | 1 (3.4) |
| Nails | 1 (3.4) |
| Previous biological, n (%) | |
| Mean (SD) | 1.1 (0.4) |
| 1 | 27 (93.2) |
| 2 | 1 (3.4) |
| 3 | 1 (3.4) |
The most common comorbidities were arterial hypertension in 24%, coronary artery disease in 6.8%, and obesity in 6.8%. The treatments used before biologic therapy were topical treatments (steroids/vitamin D analogs) in 100% of patients, 82.7% methotrexate, and 51.7% phototherapy.
Regarding biologic treatment, 37.9% received etanercept, 31% received adalimumab (24.1% received biosimilar drugs and 6.9% received innovative medicines), 20.6% received ustekinumab and 10.3% received secukinumab. Regarding the history of previous biologic therapies, 6.8% of patients had received one and two therapies before the current biologic. 27.6% received previous topical therapy, 17.2% received systemic therapy with methotrexate, and 3.4% received another biologic. However, during the optimization of biological therapy, no patient received methotrexate or other types of psoriasis control medications. Of the patients, 75.8% had therapeutic optimization by dose spacing and 24.1% by dose tapering; at the end of the observation period, five patients were on total discontinuation of therapy. Regarding the length of time patients had been on the strategy of therapy optimized the 51.7% had been on optimization therapy for more than one year, 24.1% for 3–6 months, 13.7% for less than three months, and 10.3% for 6–9 months. The initial PASI means were 0.03 and 0.28 final and the initial DLQI was 0.03 and 0.48 final, in both cases there were no significant differences between the measurements of PASI (p=0.10) and DLQI (p=0.18) before and after the optimization biological therapy.
Additionally, 6.8% (n=2) of the patients did not meet the therapeutic goals established in the CLIPSO program (PASI≤3). The first patient was a dose frequency increase and had a final PASI of 3.6, requiring a return to his usual dose. The second patient had a final PASI of 3.8, this patient was hospitalized due to an accident and had to be suspended medication for a prolonged time, which resulted in the reappearance of the lesions. In addition, two patients died, one due to metastatic hepatic cancer and the other one no information could be obtained as to the cause of death.
DiscussionOver the years there has been debate as to whether it is favorable to treat psoriasis on a continuous or intermittent treatment regimen. Traditionally, psoriasis has been managed intermittently with non-biologic systemic therapy (methotrexate, cyclosporine, acitretin, among others), due to the possible associated adverse effects such as hepatotoxicity, nephrotoxicity, and hematological alterations.1
Biological therapies offer better safety profiles than most conventional therapies and have been contemplated for an indefinite duration of use. Currently, there are no controlled studies available that report the definitive suspension of biologic therapy, however, this situation should be considered, given the high economic cost that this generates to health systems, mainly in less developed countries. Now, there is little evidence about the optimization of biologic therapy in patients with psoriasis, but there is an increasing experience of this practice in real life, due to the need to impact patient safety and reduce costs for the health system.5
We report the experience of 29 patients, in whom optimization of biologic therapy was implemented by decreasing the dose or increasing the application interval, the latter being the most used strategy. Like other studies where the variation of the dosing interval is recommended over the modification of the dose.6,7 A study conducted on patients with psoriatic arthritis receiving biologic therapy showed that prolonging the treatment interval for optimization may be a better strategy compared to decreasing the dose in those individuals who have achieved minimal disease activity.8
Optimization of biologic therapy in our patients was performed in those who were in disease remission for at least one year. Similar studies suggest that the eligibility criteria for considering the optimization of therapy in patients with psoriasis are minimum time on biologic therapy of one year and absolute PASI≤5 or ≤3 and delta of PASI75 or PASI90-100.9,10
The 37.9% of our patients had a history of psoriasis of more than 20 years of evolution. A study evaluating dose modification of biologics in patients with moderate–severe psoriasis found that those who had successful optimization therapy with a strategy of increased frequency of application were those with psoriasis of longer evolution.11 Furthermore, like our study, optimization of tumor necrosis factor inhibitor therapy has been described the most. That is probably explained by the fact that these are the drugs that have been in use the longest, being more frequent studies with adalimumab.6,7,12
Most of our patients were naïve to biologic therapies, only two patients had received one or two previous biologic therapies, without this affecting the results of success. Having received only one biologic and reaching therapeutic goals early might suggest a tendency to succeed in therapeutic optimization,13 but having other previous biologics does not disprove this, since as Carrascosa et al. state in their study, the history of previous biologic therapy and longer duration of treatment showed a favorable association toward the possibility of dose reduction of the current biologic.5
Concerning the sociodemographic variables, most of the patients in whom success is achieved with optimization of biologic therapy are married or cohabiting, suggesting that family support acts as a factor that promotes adequate response to treatment. These results are consistent with a study conducted by Stefano Vinaccia in which the relationship between social support and the disease was evaluated in 55 patients diagnosed with mild psoriasis in the city of Medellin. It was concluded that emotional support from friends, caregivers, and family members acts as a buffer that mitigates the negative emotions generated by the disease and the treatment.14 Regarding comorbidities, the most common was arterial hypertension in 24% of the patients, followed by obesity and coronary heart disease, both in the same proportion (6.8%), data similar to those reported in other studies, where the most frequent comorbidities were associated with cardiovascular risks, such as hypertension and diabetes mellitus, in 31.4% of their population15 and where the adjustment of therapy could be carried out without difficulty and preserving therapeutic goals, as was the case in our population.
This study has some limitations, since it is retrospective, there may be risks of access to complete information on the variables analyzed and problems in measuring and defining exposure, and there may also be selection bias.
Although the present study had a sample of 29 patients, we emphasize that it is the first time that the optimization of biologic therapy in patients with psoriasis is reported in a health care center in Colombia. This could serve as a starting point for future work that would allow us to propose protocols for the successful management of patients and at the same time optimize economic resources in health care for their benefit.
ConclusionWe present the experience of 29 patients where optimization of biologic therapy was implemented, obtaining satisfactory results demonstrated by a sustained PASI0 for more than 12 months in most patients.
Having clear treatment goals and management based on guidelines when therapies are initiated, allows patients to obtain complete remission of their disease in a sustained manner, and subsequently, in these patients, the possibility of optimizing the treatment progressively could be considered if the therapeutic objective continues to be met. In this way, in addition to having the patient with a completely satisfactory response, side effects derived from the therapy that could generate cost overruns such as hospitalization or disability would be avoided. In addition, savings are generated for the health system and with this, it would be possible to impact other populations with difficulties in accessing this type of therapy.
FundingThe present study did not receive funding.
Conflicts of interestThe authors have no conflict of interest to declare.