Comorbidities contribute to the development of PsA and worsen disease severity. The aim of the study is to describe the clinical characteristics of Mexican PsA patients focusing on comorbidities and medications. The primary objective of our study was to investigate the clinical and epidemiological characteristics of patients with Psoriatic Arthritis (APs) who were undergoing treatment with biological disease-modifying antirheumatic drugs (bDMARDs).
Materials and methodsWe conducted a cross-sectional observational study in a secondary care clinic in northern Mexico.
ResultsOf the total sample, 38/66 (57.5%) were women, with a mean age of 47.8 (SD 11.5) years, a mean weight of 83.8 (SD 19) kg, and BMI of 31.5 (SD 6.5) kg/m2. Smoking and alcoholism were reported in 10/66 (15.1%) and 2 (3%) of patients, respectively.
Obesity occurred in 41 (62.1%) patients, followed by diabetes mellitus in 23 (34.8%) patients, and systemic arterial hypertension in 19 (28.7%) patients. Ischaemic heart disease, heart failure, and cancer were not present in any of the patients.
Conclusions0ur study revealed a higher prevalence of chronic metabolic diseases among patients with psoriatic arthritis (PsA) compared to other cohorts without inflammatory arthropathy and the Mexican population.
Las comorbilidades contribuyen al desarrollo de la artritis psoriásica (APs) y empeoran la gravedad de la enfermedad. El objetivo del estudio es describir las características clínicas de pacientes mexicanos con APs, centrándose en las comorbilidades y los medicamentos. El objetivo principal de nuestro estudio fue investigar las características clínicas y epidemiológicas de los pacientes con APs que estaban en tratamiento con fármacos antirreumáticos modificadores de la enfermedad biológicos (bDMARDs).
Materiales y métodosRealizamos un estudio observacional transversal en una clínica de atención secundaria en el norte de México.
ResultadosDe la muestra total, 38/66 (57,5%) eran mujeres, con una edad media de 47,8 (desviación estándar [DE] 11,5) años, peso medio de 83,8 (DE 19) kg y un índice de masa coporal (IMC) de 31,5 (DE 6,5) kg/m2. Se informó tabaquismo en 10/66 (15,1%) y alcoholismo en dos (3%) de los pacientes, respectivamente. La obesidad se presentó en 41 (62,1%) pacientes, seguida de diabetes mellitus en 23 (34,8%) pacientes e hipertensión arterial sistémica en 19 (28,7%) pacientes. La enfermedad cardiaca isquémica, la insuficiencia cardiaca y el cáncer no estuvieron presentes en ninguno de los pacientes.
ConclusionesNuestro estudio reveló una mayor prevalencia de enfermedades metabólicas crónicas entre los pacientes con APs)en comparación con otras cohortes sin artropatía inflamatoria y con la población mexicana.
Psoriatic arthritis (PsA) is a chronic inflammatory disease characterized by arthritis, which may be accompanied by skin and/or nail psoriasis, enthesitis, and other extra-articular manifestations. The prevalence of PsA in the general population ranges from 0.10% to 0.25% and varies among different ethnicities and geographical locations.1 20-30% psoriasis patient eventually developing PsA.2
The impact of the comorbidities in PsA development and patient outcomes is of utmost importance. Alajmi et. al. reports that 41.2% of PsA patients had two or more comorbidities.3 Previous studies have reported an association between PsA and metabolic disorders, such as obesity, hypertension, hyperlipidemia, and diabetes mellitus.4,5 These comorbidities contribute to the development of PsA and worsen disease severity, reduce the likelihood of achieving remission, and predict a poorer response to treatment, particularly to TNFi (tumor necrosis factor inhibitors).6,7 Early recognition and management of these comorbidities have been shown to lead to better long-term outcomes, including improved disease progression and treatment response.8
While comorbidities in rheumatoid arthritis have been extensively described in the Mexican population, studies focusing on the prevalence of comorbidities in Mexican PsA patients are lacking. Thus, investigating the co-morbidities and their impact on PsA patients in this population is crucial for providing comprehensive care and optimizing treatment strategies.9
The aim of the study is to describe the clinical characteristics of Mexican Psoriatic Arthritis patients in terms of comorbidities and medications.
MethodsWe conducted a cross-sectional observational study in a secondary care clinic of the Mexican Social Security Institute, located in northern Mexico in the period from 2017-2021. The primary objective of our study was to investigate the clinical and epidemiological characteristics of patients with Psoriatic Arthritis (PsA) who were on treatment with biologic disease-modifying antirheumatic drugs (bDMARDs) where the use of biosimilars is evaluated, which is why only patients on biologic therapy were included. We focused on examining comorbidities and prescribed medication in these patients.
Eligibility criteria included participants of both sexes,>18 years of age at the time of Psoriatic Arthritis diagnosis classified according to CASPAR classification criteria confirmed by a certified rheumatologist. Additionally, participants must have received bDMARDs treatment for a minimum of one year. For data collection, institutional medical records were thoroughly reviewed, which were available for academic and research purposes.
The protocol was reviewed and approved by our local RBI with the number PI-2021-3337.
We collected demographics, such as sex, age, weight, body mass index (BMI), disease duration, and severity, as well as details regarding tobacco smoking and alcohol intake. We collected data on comorbidities from the medical record, based on clinical history. We looked for diagnosis of diabetes mellitus, hypertension, chronic renal and heart failure, dyslipidemia, hepatic steatosis and cirrhosis, obesity, malignancy, asthma, hypothyroidism, depression, and fibromyalgia. Additionally, we recorded the medications prescribed to the patients, which included PsA-related medications like csDMARDs and non steroidal anti inflammatory drugs (NSAIDs), and non-PsA-related, such as antidiabetics, statins, angiotensing-converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARBs), beta-blockers, among others.
We used descriptive statistics to summarize the data. We presented numerical variables with mean or median, with standard deviation or interquartile range –depending on normality test results- Categorical variables were summarized using frequency and percentages.
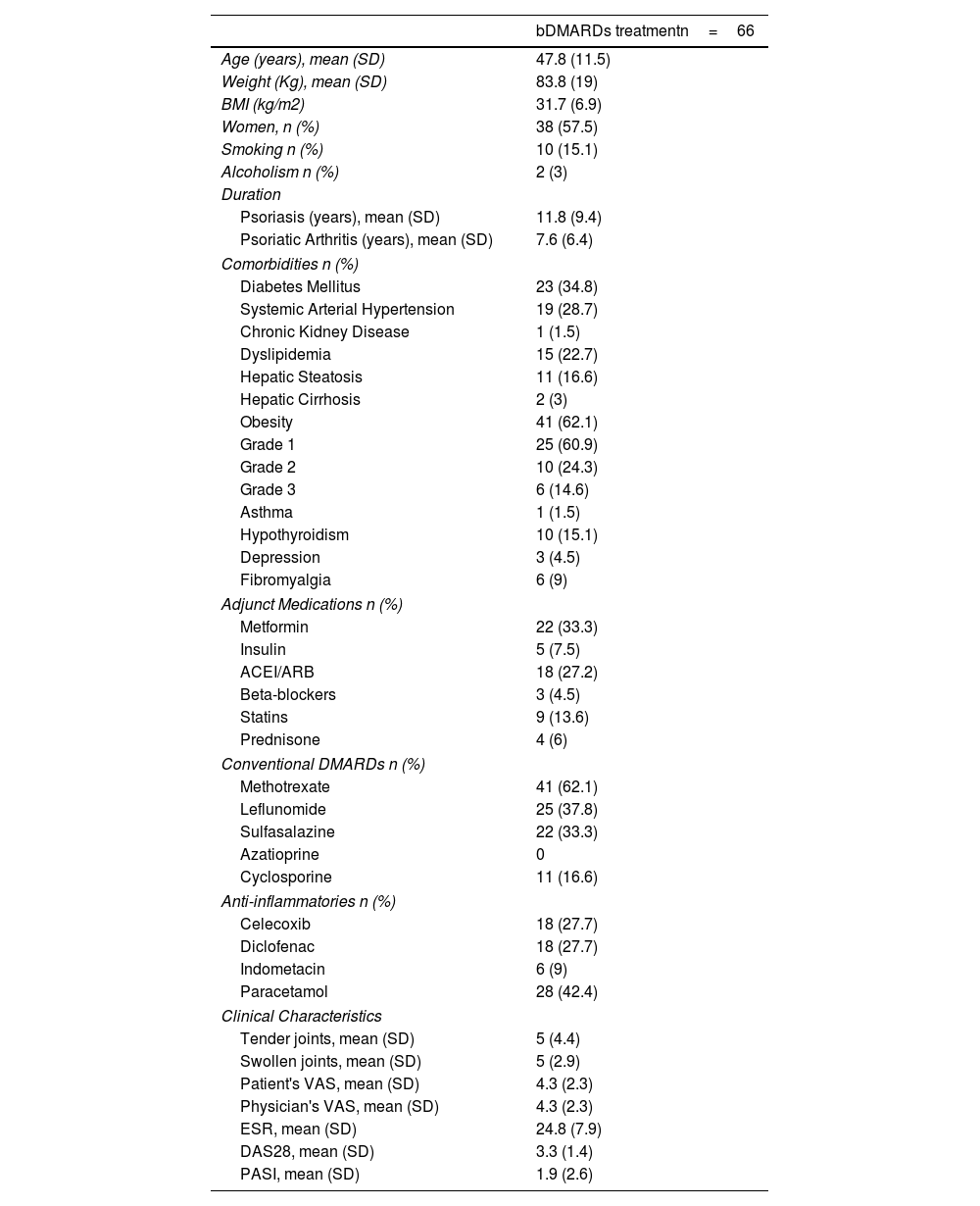
ResultsA total of 636 patients with inflammatory arthritis were identified, of whom 87 were diagnosed with Psoriatic Arthritis (APs). Among the 87 patients who met the inclusion criteria, 21 patients with incomplete medical records were excluded during the file review and variable collection, resulting in a total sample of 66 patients.
Of the total sample, 38/66 (57.5%) were women, with a mean age of 47.8 (SD 11.5) years, a mean weight of 83.8 (SD 19) kg, and BMI of 31.5 (SD 6.5) kg/m2. Smoking and alcoholism were reported in 10/66 (15.1%) and 2 (3%) of patients, respectively.
Obesity occurred in 41 (62.1%) patients, followed by diabetes mellitus in 23 (34.8%) patients, and systemic arterial hypertension in 19 (28.7%) patients. Table 1. Ischemic heart disease, heart failure, and cancer were not present in any of the patients.
Demographic and Clinical Characteristics of Patients with Psoriatic Arthritis.
| bDMARDs treatmentn=66 | |
|---|---|
| Age (years), mean (SD) | 47.8 (11.5) |
| Weight (Kg), mean (SD) | 83.8 (19) |
| BMI (kg/m2) | 31.7 (6.9) |
| Women, n (%) | 38 (57.5) |
| Smoking n (%) | 10 (15.1) |
| Alcoholism n (%) | 2 (3) |
| Duration | |
| Psoriasis (years), mean (SD) | 11.8 (9.4) |
| Psoriatic Arthritis (years), mean (SD) | 7.6 (6.4) |
| Comorbidities n (%) | |
| Diabetes Mellitus | 23 (34.8) |
| Systemic Arterial Hypertension | 19 (28.7) |
| Chronic Kidney Disease | 1 (1.5) |
| Dyslipidemia | 15 (22.7) |
| Hepatic Steatosis | 11 (16.6) |
| Hepatic Cirrhosis | 2 (3) |
| Obesity | 41 (62.1) |
| Grade 1 | 25 (60.9) |
| Grade 2 | 10 (24.3) |
| Grade 3 | 6 (14.6) |
| Asthma | 1 (1.5) |
| Hypothyroidism | 10 (15.1) |
| Depression | 3 (4.5) |
| Fibromyalgia | 6 (9) |
| Adjunct Medications n (%) | |
| Metformin | 22 (33.3) |
| Insulin | 5 (7.5) |
| ACEI/ARB | 18 (27.2) |
| Beta-blockers | 3 (4.5) |
| Statins | 9 (13.6) |
| Prednisone | 4 (6) |
| Conventional DMARDs n (%) | |
| Methotrexate | 41 (62.1) |
| Leflunomide | 25 (37.8) |
| Sulfasalazine | 22 (33.3) |
| Azatioprine | 0 |
| Cyclosporine | 11 (16.6) |
| Anti-inflammatories n (%) | |
| Celecoxib | 18 (27.7) |
| Diclofenac | 18 (27.7) |
| Indometacin | 6 (9) |
| Paracetamol | 28 (42.4) |
| Clinical Characteristics | |
| Tender joints, mean (SD) | 5 (4.4) |
| Swollen joints, mean (SD) | 5 (2.9) |
| Patient's VAS, mean (SD) | 4.3 (2.3) |
| Physician's VAS, mean (SD) | 4.3 (2.3) |
| ESR, mean (SD) | 24.8 (7.9) |
| DAS28, mean (SD) | 3.3 (1.4) |
| PASI, mean (SD) | 1.9 (2.6) |
DMARDs: Disease-Modifying Antirheumatic Drugs, BMI: Body Mass Index, ACEI: angiotensing-converting enzyme inhibitors, ARB: angiotensin receptor blockers, VAS: Visual Analog Scale, DAS: Disease Activity Score, ESR: Erythrocyte Sedimentation Rate, PASI: Psoriasis Area and Severity Index
Among used TNF inhibitors, adalimumab was reported used in 40 patients (60.6%), followed by etanercept used by 15 patients (22.7%), infliximab in 8 patients (12.1%), and certolizumab by 3 patients (4.5%).
In addition to bDMARD treatment, 41 (62.1%) patients used methotrexate, followed by leflunomide used in 25 (37.8%). Regarding non-APs related medications, metformin and antihypertensives (ACEI/ARB) were the most frequently used in 22 (33.3%) and 18 (27.2%) patients, respectively.
In terms of the duration, the mean years at the first consultation were 11.8 (SD 9.4) years for psoriasis diagnosis and 7.6 (SD 6.4) years for APs diagnosis, suggesting that most patients initially presented with psoriasis. The approximate time from the first consultation to the initiation of TNF inhibitor was 1.7 years.
DiscussionThe prevalence of comorbidities found in our study, including hypertension, dyslipidemia, obesity, diabetes, and cardiovascular disease, closely aligns with the findings reported by Albrecht et al. for the German population.10 Another observational retrospective analysis involving 186,552 PsA patients from the United States reported a prevalence of 47.5% for hyperlipidemia, 47.3% for hypertension, 21.2% for depression, 20.2% for type 2 diabetes mellitus, and 16.6% for fibromyalgia.11 These comorbidities were also associated with a reduction in quality of life. Ross et al. evaluated a similar group of comorbidities along with health disparities among African American and Caucasian PsA patients in the United States, demonstrating differences among the prevalence of comorbidities and treatment received between the two groups.1 This consistency among studies indicates a similar pattern of comorbidity occurrence in patients with PsA, though with certain variations due to various factors, across different ethnicities.
Arterial hypertension was present in almost 30% of patients. This takes relevance since according to the results obtained by Kamata, patients with psoriasis presented a higher prevalence of coronary artery stenosis>70% and the use of biologic therapy was shown to reduce coronary artery plaque by 6%.12 At the moment there is insufficient evidence to support the use of biologic therapy to decrease the risk of cerebral vascular disease (CVD), while for RA the literature suggests that TNFi and methotrexate could decrease this risk.13
While our study did not directly assess polypharmacy, defined as the concomitant use of five or more drugs,14 previous research has shown that nearly half of all patients with PsA (49%) are affected by polypharmacy, exceeding the medication burden observed in individuals without inflammatory arthritis (17%).10 Moreover, compared to patients with rheumatoid arthritis, it was found that after six months of treatment initiation, 83% of patients with PsA had polypharmacy vs. 93.5%.15 In a retrospective cohort study analyzing 2-year longitudinal data, a cohort of 6,132 adults over 21 years with arthritis was examined. The study identified an association between polypharmacy and lower physical component summary (PCS) scores in adults living with arthritis.16 Considering the potential risks associated with drug-drug and drug-disease interactions, healthcare providers should adopt a cautious approach when prescribing multiple medications to manage chronic conditions, with the goal of improving the health-related quality of life for adults affected by arthritis.
In PsA patients, polypharmacy encompasses a combination of PsA-specific, pain-related, and comorbidity-related medications.10 To address this issue, increased prescription of biologic disease-modifying antirheumatic drugs (bDMARDs) and ensuring a favorable treatment response can help reduce the reliance on nonsteroidal anti-inflammatory drugs (NSAIDs) and prednisone.17
These findings highlight the importance of a holistic approach to PsA management that considers both the disease itself and its associated comorbidities. By addressing comorbidities and optimizing treatment strategies, we can potentially improve patient outcomes, reduce medication burden, and enhance overall quality of life for individuals with PsA.
Several studies have provided valuable insights into the relationship between PsA, rheumatoid arthritis (RA), and their associated comorbidities. Bhole et al. reported that individuals with PsA had a higher mean BMI compared to those with psoriasis (PsO), RA, and the general population, similar to findings found in our cohort. Specifically, the mean BMI values for individuals with PsA, PsO, RA, and the general population were 29.6, 27.9, 27.3, and 26.1, respectively. The prevalence of obesity followed a similar trend, with PsA individuals exhibiting a higher proportion of obesity (37%) compared to those with PsO (29%), RA (27%), and the general population (18%). Notably, the difference in BMI between PsA and PsO was found to correlate with physical health outcomes.18
Galarza-Delgado et al. conducted a study specifically focusing on Mexican patients with RA. Their findings revealed that the most frequently observed comorbidities in this patient group were hypertension (29.8%), dyslipidemia (27.1%), osteoporosis (19.1%), diabetes (12.4%), hypothyroidism (6.2%), and solid malignancies (4.4%). Additionally, risk factors such as overweight (44.9%) and obesity (31.6%) were prevalent among the participants.9
Furthermore, the National Survey of Health and Nutrition (ENSANUT) conducted in Mexico provides valuable population-level data for healthcare decision-making and public health interventions. According to the 2021 ENSANUT data, the national prevalence rates of obesity, diabetes, and hypertension were 16.8% (vs 61.2%), 10.2% (vs 33.3%), and 15.7% (vs 28.8%), respectively. Importantly, all of these comorbidities were found to be more prevalent among PsA patients compared to the general population in Mexico, except for dyslipidemia, which had a higher prevalence (38.8%) in the general population compared to PsA patients (22.7%).19
When considering our study findings in conjunction with the results reported by Bhole, Galarza-Delgado et al., and the National Survey of Health and Nutrition (ENSANUT), a consistent pattern emerges. Specifically, our study reveals a notable similarity to Bhole's findings, indicating that individuals with PsA have a higher mean BMI compared to those with RA and the general population. This collective evidence underscores the consistent association between PsA and elevated BMI levels.
In our study, several limitations need to be acknowledged. Firstly, the inclusion of patients solely from a single institution restricts the generalizability of our findings, as their treatment options were limited to the resources available within that particular institution. Variations in treatment patterns and outcomes may exist in other healthcare settings with different resource capacities, emphasizing the need for caution when extrapolating our results to the broader population with PsA.
Secondly, the relatively small sample size, attributed to the uncommon nature of PsA, limits the precision and comprehensiveness of our study. Inclusion of a larger number of patients from multiple clinics or health services would enable a more robust analysis, enhancing the generalizability and reliability of our findings.
Moreover, the inclusion of patients relied on the availability and quality of medical records and consultations, introducing the potential for selection bias. The heterogeneity of these records and consultations may have excluded certain individuals who could have otherwise contributed valuable data. The implementation of standardized data collection and record-keeping practices is crucial to ensure comprehensive inclusion and representation of the patient population.
Additionally, our focus on medication utilization was primarily centered on the known associated comorbidities of PsA. However, there is scope for further expansion to encompass other drug groups and explore the utilization of alternative or complementary medical treatments. It is important to acknowledge that the assessed comorbidities might not capture all potential sources of pain experienced by individuals with PsA, such as osteoarthritis, which has a known association with obesity. Future research endeavors should strive to investigate a broader range of treatment modalities and consider the impact of additional sources of pain in this patient population.
Careful consideration of these limitations is essential when interpreting the results of our study, as they may influence the general applicability and validity of our findings. Future research endeavors should aim to address these limitations, expand the scope of investigation, and contribute to a more comprehensive understanding of PsA and its management.
ConclusionOur study revealed a higher prevalence of chronic metabolic diseases among patients with psoriatic arthritis (PsA) compared to other cohorts without inflammatory arthropathy and the Mexican population. Comorbidities grouped under metabolic syndrome were the most prevalent.
Ethical considerationsThe protocol was reviewed and approved by our local RBI with the number PI-2021-3337. Author has the informed consent from patients.
FundingNo external funding was received during the development of this study.
Conflict of interestThe authors declare no conflicts of interest regarding this research.
The authors express their gratitude to Dr. Paloma Rodríguez and Dr. Teresa Sánchez for their valuable contributions to this research project.