Rheumatoid arthritis (RA) is an autoimmune inflammatory disorder characterized by immunobiological homeostasis. The recently discovered cytokine interleukin-41 (IL-41) is among the immunobiological components suggested to have modulatory effects in RA and has shown up-regulated levels in patients. However, IL-41 has not been explored during the pre- (PRM) and post-menopausal (POM) periods in women with RA, and its relationship to disease activity and medications has not been well studied.
Materials and methodsIn this case–control study, serum IL-41 concentrations were quantified in 120 women with RA (70 PRM and 50 POM) and 110 control women using an enzyme-linked immunosorbent assay kit. Thirty patients were newly diagnosed (ND) and 90 patients were on treatment with etanercept (a tumor necrosis factor inhibitor; TNF) plus methotrexate (MD).
ResultsMedian IL-41 concentrations (interquartile range) were significantly lower in RA patients than in control women (49.8 [32.5–79.5] vs. 104.7 [76.9–134.6]pg/mL; probability <.001). As indicated by the area under the curve, .827, IL-41 showed reliable discrimination between RA patients and HC. IL-41 concentrations stratified by menopausal status (PRM vs. POM), disease activity score 28 (<3.2 vs. ≥3.2), and medication (ND vs. MD) showed no significant difference in each stratum.
ConclusionsIn contrast to previous studies, serum IL-41 concentrations were significantly decreased in the present cohort of women with RA. These concentrations were not affected by menopausal status, disease activity, or medication. Data from the current study suggest that IL-41 is involved in the pathophysiology of RA.
La artritis reumatoide (AR) es un trastorno inflamatorio autoinmune caracterizado por una homeostasis inmunobiológica. La citocina interleucina-41 (IL-41), descubierta recientemente, se encuentra entre los componentes inmunobiológicos que se sugiere que tienen efectos moduladores en la AR y ha mostrado niveles regulados al alza en los pacientes. Sin embargo, la IL-41 no ha sido estudiada durante los periodos pre (PRM) y posmenopáusico (POM) en mujeres con AR, y su relación con la actividad de la enfermedad y los medicamentos no se ha estudiado bien.
Materiales y métodosEn este estudio de casos y controles se cuantificaron las concentraciones séricas de IL-41 en 120 mujeres con AR (70 PRM y 50 POM) y 110 mujeres de control, utilizando un kit de ensayo inmunoabsorbente ligado a enzimas. Treinta pacientes fueron diagnosticados recientemente (ND) y 90 estaban en tratamiento con etanercept (un inhibidor del factor de necrosis tumoral; TNF) más metotrexato (MD).
ResultadosLas concentraciones medianas de IL-41 (rango intercuartil) fueron significativamente más bajas en pacientes con AR que en mujeres de control (49,8 [32,5-79,5] vs. 104,7 [76,9-134,6]pg/mL; probabilidad <0,001). Como lo indica el área bajo la curva, 0,827, la IL-41 mostró una discriminación confiable entre pacientes con AR y HC. Las concentraciones de IL-41 estratificadas por estado menopáusico (PRM vs. POM), puntuación de actividad de la enfermedad 28 (<3,2 vs. ≥3,2) y medicación (ND vs. MD) no mostraron diferencias significativas en cada estrato.
ConclusionesA diferencia de los estudios anteriores, las concentraciones séricas de IL-41 disminuyeron significativamente en la presente cohorte de mujeres con AR. Estas concentraciones no se vieron afectadas por el estado menopáusico, la actividad de la enfermedad o la medicación. Los datos del estudio actual sugieren que la IL-41 está implicada en la fisiopatología de la AR.
Rheumatoid arthritis (RA) is among the most important rheumatic autoimmune disorders with an estimated global prevalence of 460/100,000 population during 1980–2019. The disease affects females more than males in a ratio of 3:1 and peaks between the ages of 30 and 50 years.1 RA is characterized by a marked inflammatory response that targets the peripheral joints and causes chronic synovial hyperplasia.2 Although RA etiology is largely undefined, experimental, observational, and epidemiological studies have revealed that the disease is multifactorial with genetic, environmental, hormonal, metabolic, and immunological determinants playing an interactive role in its pathogenesis.2–5 Furthermore, the menopausal status is suggested to have an impact on the immunobiological homeostasis and response to these risk factors in RA patients.3,6
Cytokines are glycoprotein molecules that participate in cellular signaling to coordinate immune functions and through both pro- and anti-inflammatory effects, they play an important role in regulating inflammatory reactions.7 Accumulating data indicated that a number of pro-inflammatory cytokines are connected to the pathophysiology of RA, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1 and IL-17A, and there is almost conclusive evidence that pro-inflammatory cytokines correlate with disease activity and severity.8,9 In addition, anti-inflammatory/regulatory cytokines also show dysregulated levels in RA. In fact, the balance between pro- and anti-inflammatory/regulatory cytokines is disturbed and this may determine disease outcome, activity, and severity.10 However, some of the more recently discovered cytokines, such as IL-41, have not been well explored in RA and their role in the disease pathophysiology has not been well defined particularly during the pre-menopausal (PRM) and post-menopausal (POM) periods.
Metrnl/IL-41, a novel cytokine discovered in 2004, is encoded by the METRNL gene that maps to human chromosome 17 (17q25.3).11 Although the cells that produce IL-41, the target cells of IL-41, and the signaling pathways for activating IL-41 are still being explored, many tissues have been shown to express this cytokine, particularly the barrier tissues of the skin, intestines, and respiratory tract. Besides, IL-41 has been linked to innate and adaptive immunity as alternatively activated macrophages and M2-like macrophages have been demonstrated to express IL-41.12 The expression of IL-41 is affected by a variety of cytokines. For example, IL-4, IL-12, IL-17A, and TNF-α up-regulate IL-41 expression, while interferon (IFN)-γ and transforming growth factor (TGF)-β are associated with down-regulated expression.13 Further evidence indicated that METRNL knockout mice showed dysregulated production of cytokines involved in the progression of inflammatory lesions, and thus immune regulatory properties of IL-41 were suggested, particularly in inflammatory reactions.13,14 Additional recent data implicate IL-41 in the pathogenesis of several inflammation-mediated diseases, such as type 2 diabetes mellitus, psoriatic arthritis, Kawasaki disease, Graves’ disease, and inflammatory bowel disease.15–19 With respect to RA, two recent studies have disclosed that IL-41 concentrations are up-regulated in the serum/plasma of RA patients and associated with disease activity. In light of this, it has been proposed that IL-41 may be considered as a potential predictive biomarker for RA.20,21 However, the relationship between IL-41 and menopausal status has not been explored. Besides, the effects of RA therapies on IL-41 concentrations and the association of this cytokine with disease activity have been not clear.
In this study, serum IL-41 levels were analyzed in women with RA with the aim of determining whether these levels are affected by menopausal status, disease activity, and medication. Furthermore, the relationship between IL-41 levels and patients’ basic clinical and laboratory data was evaluated. Available data in this regard are either limited or non-existent and this study may pave the way for a greater understanding of IL-41 in the pathophysiology of RA.
Materials and methodsPatients and controlsA case–control study was conducted on 120 women with RA (age range: 25–68 years) and 110 healthy women (HC group; age range: 24–66 years). Patients were registered and diagnosed in the rheumatology referral unit at the Medical Education Complex in Baghdad during the period from December 2021 to June 2022. Diagnosis was made by rheumatologists according to the 2010 diagnostic guidelines established by the American College of Rheumatology/European League Against Rheumatism collaborative initiative (ACR/EULAR).22 The included patients were only women over 18 years of age who did not suffer from other chronic inflammatory diseases or cancer. Patients with juvenile idiopathic arthritis and pregnant women were excluded. Ninety patients (75.0%) were receiving regular treatment with a TNF inhibitor, a single weekly subcutaneous dose of etanercept (50mg), plus a single weekly oral dose of 2.5–7.5mg of methotrexate (medicated group; MD). The remaining 30 patients (25.0%) were newly diagnosed (ND). Seventy patients (58.3%) were in the PRM period, while 50 patients (41.7%) were in the POM period. ESR-based disease activity score 28 (DAS28) was used to evaluate RA activity. DAS28 is a continuous scale from 0 to 10, and accordingly, disease activity is classified as low (<3.2; n=21) or moderate/high (≥3.2; n=99).23 Patients were also tested for anti-cyclic citrullinated peptide antibody (ACCP), hemoglobin (Hb), white blood cell count (WBC), erythrocyte sedimentation rate (ESR), liver function parameters (alanine aminotransferase [ALT] and aspartate aminotransferase [AST]), kidney function parameters (blood urea nitrogen; BUN, and serum creatinine; SCR), estrogen (ES), and vitamin D (VitD). The HC group included blood donors for the age range 24–40 years and university employees for the age range 41–66 years (89 PRM and 21 POM). They had no chronic diseases and the ESR was taken as an indicator for evaluating the inflammatory status. The only women included in the HC group were those with an ESR ≤20mm/h.
Laboratory methodsHb, WBC, and ESR tests were performed using conventional laboratory methods. Serum concentrations of AST, ALT, BUN, and SCR were measured using a fully automated clinical chemistry analyzer (Cobas c311, Roche, Germany). Enzyme-linked immunosorbent assay (ELISA) kits were used to measure serum concentrations of IL-41 (meteorin-like protein; MyBioSource, USA), ACCP antibody (AESKU, Germany), ES (SunLong Biotech, China), and VitD (1,25-dihydroxyvitamin D3; Elabscience, USA), following instructions of manufacturer.
Statistical analysisContinuous variables were tested for normal distribution using the Shapiro–Wilk test. Mean and standard deviation (SD) were used to describe normally distributed (parametric) variables and significant differences were assessed using one-way analysis of variance test. Median and interquartile range (IQR: 25–75%) were used to describe non-parametric (skewed) variables and Mann–Whitney test was used to assess significant differences. The discriminatory potential of IL-41 was assessed using receiver-operating characteristic (ROC) curve analysis, which estimated the area under the curve (AUC) and its 95% confidence interval (CI). Spearman's rank-order correlation analysis was performed to estimate the correlation coefficient (rs). A probability (p) value of less than 0.05 was considered statistically significant. GraphPad Prism version 9.4.1 (San Diego, CA, USA) was used to perform statistical analysis. G*power software version 3.1.9.7 was used to assess power of sample size.24 The data required to calculate the sample size power were entered into the G*power software as follows: effect size d=0.5, two-tailed α error p=0.01, RA sample size=120, and HC sample size=110. The estimated power for sample size (1−β error p) was 0.88. The accepted power for sample size is 0.8.25 Therefore, the current sample size of RA patients and HC is suggested to be statistically valid.
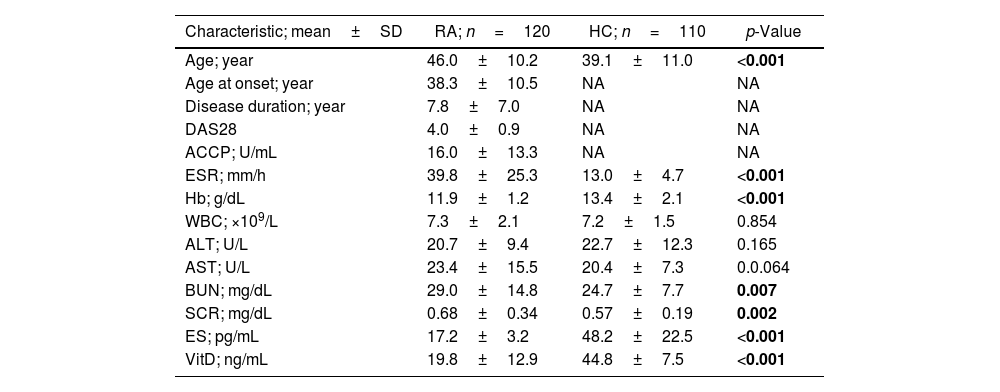
ResultsBaseline dataData describing baseline characteristics of women with RA and HC are illustrated in Table 1. It should be noted that RA patients and controls were not matched for age because the mean age of the patients was significantly higher than the mean age of the HC group (46.0±10.2 vs. 39.1±11.0 years; p<0.001). This difference was due to the difficulty of obtaining healthy women over the age of 50. Other parameters that showed significant differences between RA patients and HC were ESR, Hb, BU, SCR, ES, and VitD. Of particular interest are ES (17.2±3.2 vs. 48.2±22.5pg/mL; p<0.001) and VitD (19.8±12.9 vs. 44.8±7.5ng/mL; p<0.001), which showed significantly lower levels in RA patients compared to HC (Table 1).
Data describing baseline characteristics of women with rheumatoid arthritis and control women.
| Characteristic; mean±SD | RA; n=120 | HC; n=110 | p-Value |
|---|---|---|---|
| Age; year | 46.0±10.2 | 39.1±11.0 | <0.001 |
| Age at onset; year | 38.3±10.5 | NA | NA |
| Disease duration; year | 7.8±7.0 | NA | NA |
| DAS28 | 4.0±0.9 | NA | NA |
| ACCP; U/mL | 16.0±13.3 | NA | NA |
| ESR; mm/h | 39.8±25.3 | 13.0±4.7 | <0.001 |
| Hb; g/dL | 11.9±1.2 | 13.4±2.1 | <0.001 |
| WBC; ×109/L | 7.3±2.1 | 7.2±1.5 | 0.854 |
| ALT; U/L | 20.7±9.4 | 22.7±12.3 | 0.165 |
| AST; U/L | 23.4±15.5 | 20.4±7.3 | 0.0.064 |
| BUN; mg/dL | 29.0±14.8 | 24.7±7.7 | 0.007 |
| SCR; mg/dL | 0.68±0.34 | 0.57±0.19 | 0.002 |
| ES; pg/mL | 17.2±3.2 | 48.2±22.5 | <0.001 |
| VitD; ng/mL | 19.8±12.9 | 44.8±7.5 | <0.001 |
SD: standard deviation; RA: rheumatoid arthritis; HC: healthy controls; DAS28: disease activity score 28; ACCP: anti-cyclic citrullinated peptide antibody; Hb: hemoglobin; WBC: white blood cell count; ESR: erythrocyte sedimentation rate; ALT: alanine aminotransferase; AST: aspartate aminotransferase; BUN: blood urea nitrogen; SCR: serum creatinine; ES: estrogen; VitD: vitamin D; NA: not applicable; p: probability (significance was detected using one-way analysis of variance test; significant p-value is indicated in bold).
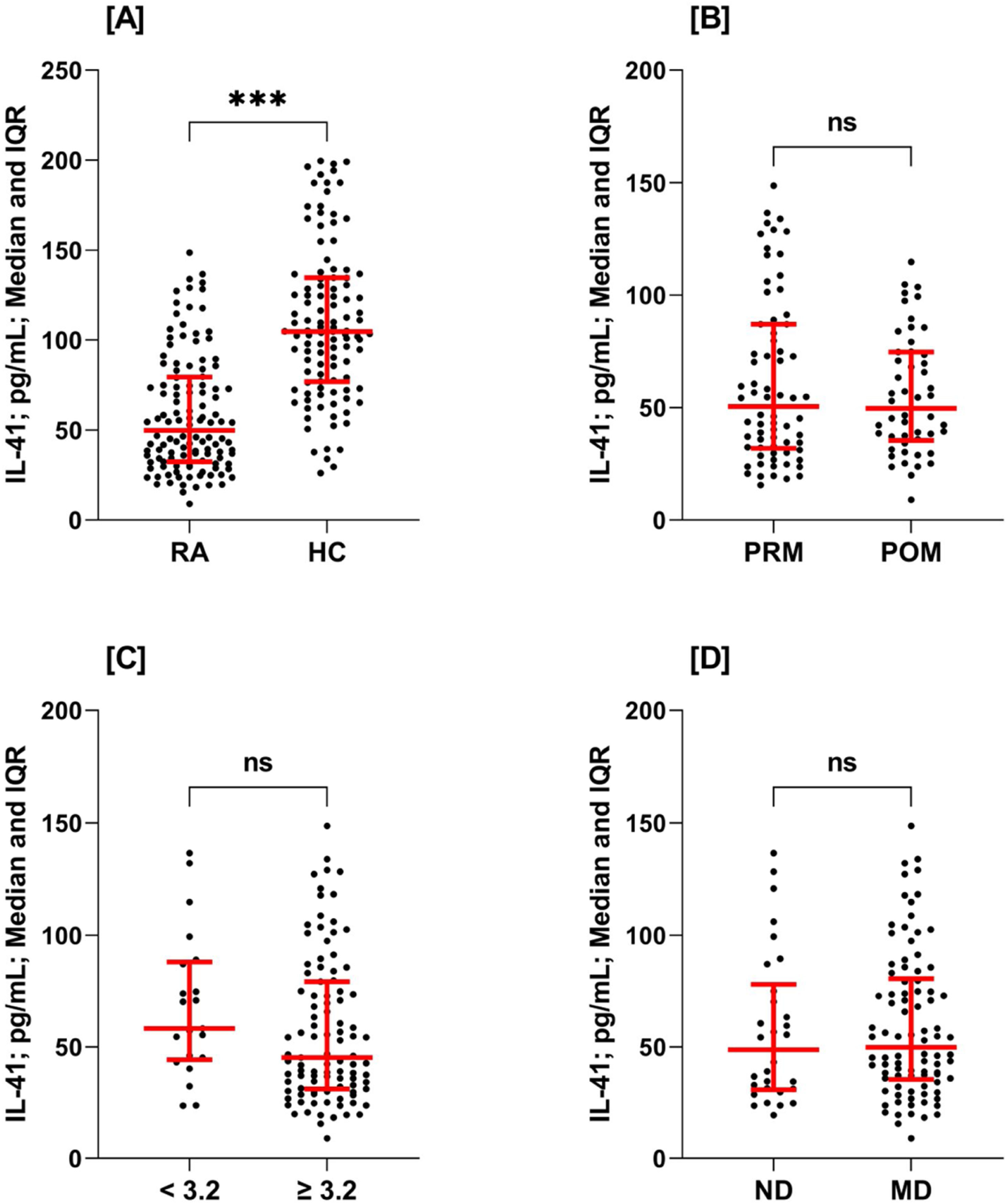
Median concentrations (IQR: 25–75%) of IL-41 were significantly decreased in RA patients compared to HC (49.8 [32.5–79.5] vs. 104.7 [76.9–134.6]pg/mL; p<0.001). When IL-41 concentrations were stratified by menopausal status (PRM vs. POM), DAS28 (<3.2 vs. ≥3.2) and medication (ND vs. MD) in RA patients, no statistically significant difference was found in each stratum (Fig. 1 and Supplementary Table 1).
Scatter-dot plots of serum interleukin-41 (IL-41) concentrations. Plot A: rheumatoid arthritis (RA) patients vs. healthy controls (HC); plot B: pre-menopausal (PRM) RA patients vs. post-menopausal (POM) RA patients; plot C: disease activity score 28 (DAS28) <3.2 vs. DAS28 ≥3.2; plot D: newly diagnosed (ND) RA patients vs. medicated (MD) RA patients. The horizontal line indicates median. The vertical line indicates interquartile range (IQR: 25–75%). Significance was detected using Mann–Whitney U test (***p<0.001; ns: not significant). The IL-41concentrations were significantly decreased in RA patients compared to HC (49.8 [IQR: 32.5–79.5] vs. 104.7 [IQR: 76.9–134.6]pg/mL; p<0.001). When IL-41 concentrations were stratified by menopausal status, DAS28 and medication in RA patients, no significant differences were found.
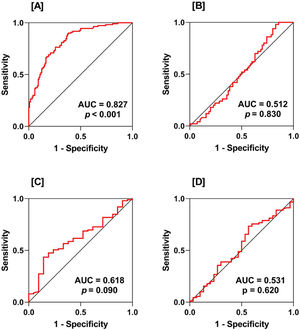
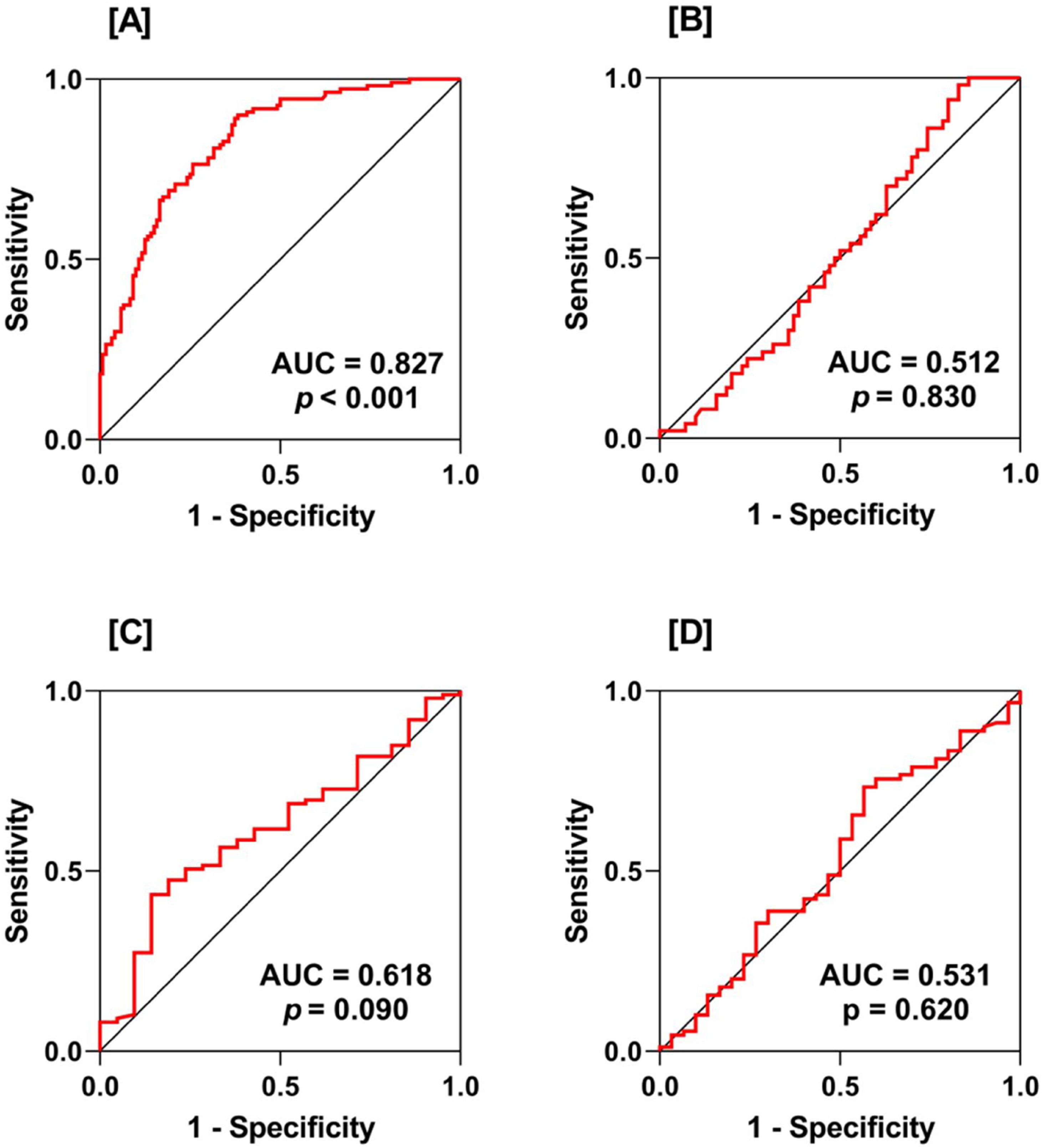
ROC curve analysis demonstrated that IL-41 was a reliable marker in distinguishing between RA patients and HC (AUC=0.827). However, the discriminatory power of IL-41 was poor when ROC curve analysis was performed in PRM vs. POM (AUC=0.512), DAS28 <3.2 vs. DAS28 ≥3.2 (ROC=0.618), and ND vs. MD (ROC=0.531) (Fig. 2).
Receiver-operating characteristic (ROC) curve analysis of interleukin-41 (IL-41) showing area under the curve (AUC) and probability (p). Plot A: rheumatoid arthritis (RA) patients vs. healthy controls (HC); plot B: pre-menopausal (PRM) RA patients vs. post-menopausal (POM) RA patients; plot C: disease activity score 28 (DAS28) <3.2 vs. DAS28 ≥3.2; plot D: newly diagnosed (ND) RA patients vs. medicated (MD) RA patients. As indicated by the AUC value, IL-41 showed excellent discrimination between RA patients and HC (AUC=0.827). However, the discriminatory power of IL-41 was poor when ROC curve analysis was performed between PRM and POM, DAS28 <3.2 and DAS28 ≥3.2, and ND and MD.
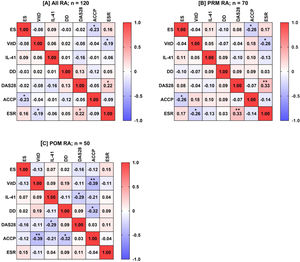
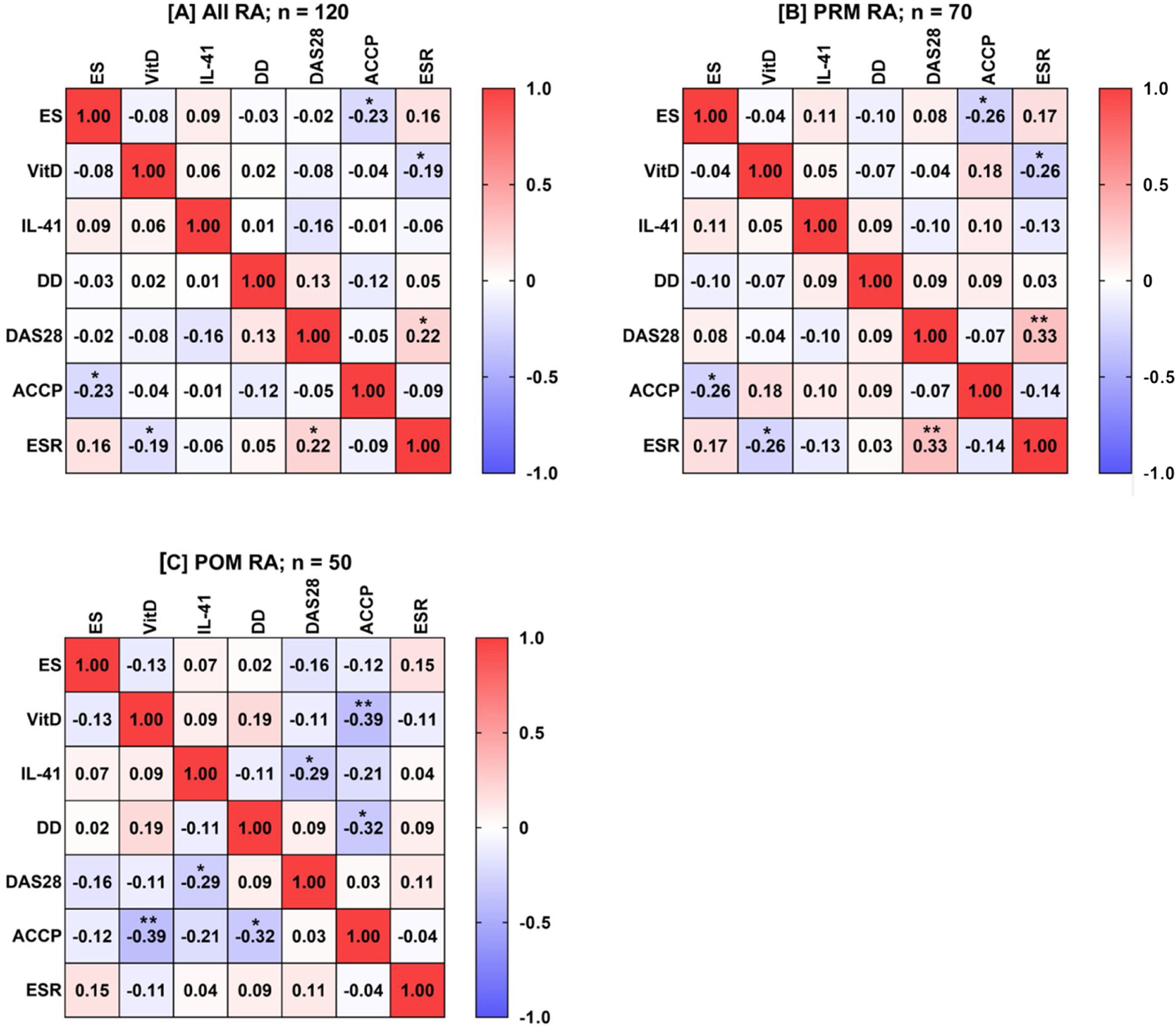
Spearman's rank-order correlation analysis was applied to calculate the rs between ES, VitD, IL-41, age, disease duration, DAS28, ACCP antibodies, and ESR for all possible combinations in RA patients as well as in PRM and POM patients. In all RA patients, ES negatively correlated with ACCP antibodies (rs=−0.23; p=0.013), VitD negatively correlated with ESR (rs=−0.19; p=0.04), and DAS28 positively correlated with ESR (rs=0.22; p=0.015). Likewise, this pattern of correlations was also observed in PRM patients. In POM patients, a different pattern of correlations was found. VitD negatively correlated with ACCP antibodies (rs=−0.39; p=0.005), IL-41 negatively correlated with DAS28 (rs=−0.29; p=0.041), and disease duration negatively correlated with ACCP antibodies (rs=−0.32; p=0.021) (Fig. 3).
A heat-map matrix of Spearman's correlation coefficient (rs) between estrogen (ES), vitamin D (VitD), interleukin-41 (IL-41), disease duration (DD), disease activity score 28 (DAS28), anti-cyclic citrullinated peptide (ACCP) antibody, and erythrocyte sedimentation rate (ESR) among all women with rheumatoid arthritis (RA), as well as in women in the pre-menopausal (PRM) and post-menopausal (POM) periods (plots A, B, and C, respectively). The value inside the boxes denotes rs, and a significant correlation (two-tailed probability; p) is indicated by an asterisk (*p<0.05; **p<0.01). Red indicates a positive correlation. Blue indicates a negative correlation.
In the current study, a novel cytokine (IL-41) was analyzed in the serum of women with RA and HC. Next, IL-41 concentrations were stratified by menopausal status (PRM vs. POM), DAS28 (<3.2 vs. ≥3.2), and medication (ND vs. MD) to evaluate their effects on IL-41 concentrations. This study may be the first to adopt this stratification regarding the role of IL-41 in RA. Significantly lower concentrations of IL-41 were found in the serum of RA patients compared with HC. ROC curve analysis demonstrated the discriminatory potential of IL-41 in RA vs. HC (AUC=0.827). IL-41 concentrations were not affected by menopausal status, disease activity or medication. In addition, IL-41 did not correlate with clinical indicators of RA in all patients or PRM patients, while in POM patients, IL-41 showed a significant negative correlation with DAS28.
IL-41 is a novel immune-modulating cytokine with high expression in macrophages and barrier tissues, such as skin and mucosa. In addition, IL-4, IL-12, IL-17A, and TNF-α have been shown to enhance IL-41 expression in macrophages, while IFN-γ and TGF-β reduce this expression.13 Although the functional effect of IL-41 still needs to be fully elucidated, IL-41 knockout mice showed dysregulated cytokine production and the development of spontaneous inflammatory lesions, and IL-41 has accordingly been proposed to be involved in the control of inflammatory responses.13 Subsequent studies have considered IL-41 to be an anti-inflammatory cytokine that plays an important role in regulating inflammatory reactions in several inflammatory and autoimmune diseases.14 With regard to RA, two recent studies have explored serum IL-41 concentrations in patients with RA. In the first, Zhang and colleagues analyzed serum IL-41 (metrnl) concentrations in 159 patients with RA, 28 patients with osteoarthritis, and 50 HC. Their results indicated that IL-41 concentrations were significantly higher in RA patients than in osteoarthritis patients or HC, and there was no significant difference between osteoarthritis patients and HC. Besides, IL-41 concentrations showed a positive correlation with DAS28, rheumatoid factor, and C-reactive protein concentrations, while no correlation was found with ESR and ACCP antibodies.21 In the second, Gong and colleagues examined IL-41 in 46 patients with RA and 32 HC and demonstrated that IL-41 concentrations were significantly elevated in the serum of RA patients compared to HC and positive correlations were found between IL-41, DAS28 and ESR.20 The present study results were inconsistent with these observations and instead, IL-41 concentrations decreased significantly in RA patients. Besides, our data indicated that IL-41 was not correlated with ESR or DAS28, except for its negative correlation with DAS28 in POM patients. Consistent with our findings, down-regulation of serum IL-41 concentrations has been reported in two inflammatory and autoimmune diseases, Graves’ disease and inflammatory bowel disease.15,17 However, a meta-analysis of nine studies revealed that circulating IL-41 concentrations did not show significant changes in patients with type 2 diabetes.18 Despite these conflicting findings, it may be too early to fully understand the role of IL-41 in the pathogenesis of these diseases, and further studies are warranted to dissect the mechanisms involved.
Serum ES concentrations were significantly decreased in women with RA. This is consistent with previous observations recognizing the relationship between ES and inflammation in RA, where pregnancy (accompanied by higher ES concentrations) is associated with disease amelioration, whereas relapse occurs during the post-partum period in which ES concentrations drop significantly. Accordingly, a hypothesis was formulated that ES deficiency, particularly in POM women, is associated with an increased risk of RA.5,26 ES is a steroidal sex hormone that coordinates the development of the female reproductive system, but it has also become clear that ES regulates/affects a wide spectrum of biological functions including inflammation and immunity.27 Recent evidence has indicated that ES exerts immunomodulatory effects by inhibiting T helper (h)1 and Th17 responses and enhancing Th2 and T regulatory responses, thus pro-inflammatory cytokines are down-regulated (for instance IL-1, IL-6, IL-17A, and TNF-α) while anti-inflammatory cytokines (for instance IL-4 and IL-10) are up-regulated.28 Therefore, the ameliorative effects of ES in RA are likely related to the down-regulation of pro-inflammatory cytokines, which exhibit elevated concentrations in RA patients and play a crucial pathogenic role in the disease.3 Furthermore, ES showed a negative correlation with ACCP antibodies in RA cases. There is increasing evidence supporting a role for ACCP antibodies in the pathogenesis of RA, and it is widely accepted that ACCP antibodies are an indispensable tool for the early diagnosis, treatment and management of patients with RA.29 An association between ACCP antibody-positivity and ES has also been suggested in women at risk of RA,30 but further studies are warranted to understand this association.
Regarding VitD, this study showed that median concentrations of this vitamin in RA patients were <20ng/mL (30–60ng/mL is the normal optimal range). In light of this, most of the RA patients in the current study were considered to be VitD insufficient/deficient. Thus, a causal relationship between VitD status and the onset/progression of RA cannot be ruled out. Low concentrations of VitD were also negatively correlated with the inflammatory marker ESR. There is accumulating evidence indicating that VitD insufficiency is associated with an increased incidence of inflammatory and autoimmune diseases, including RA.31 As revealed recently, cells involved in the innate and adaptive immune response, such as macrophages, dendritic cells, T cells, and B cells, express VitD receptor and are therefore functionally affected by VitD insufficiency.32 It has been shown that VitD is involved in regulating the differentiation and maturation of these cells, as well as their ability to produce cytokines and chemokines, and thus the immunomodulatory functions of VitD have been proposed.31 Consistent with our observation, VitD insufficiency has been shown to exhibit a high prevalence in RA patients, particularly in female patients, and VitD deficiency has been considered to be an important risk factor associated with RA.5
The low sample size of ND cases is a major limitation of the study along with the lack of synovial fluid analysis for IL-41. In addition, other cytokines (IL-1, IL-6, IL-17A, IL-21, IL-22, TNF-α, and IFN-γ) affected by and/or associated with IL-41 were not studied.
ConclusionsIn contrast to previous studies, serum IL-41 concentrations were significantly decreased in the present cohort of women with RA. These concentrations were not affected by menopausal status, disease activity, or medication. Data from the current study suggest that IL-41 is involved in the pathophysiology of RA.
Ethical approvalInstitutional approval was obtained from the Ethics Committee of the Medical Education Complex in Baghdad (No. 46731 on December 14, 2021). Written consent was provided by all participants to participate in the study.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestThe authors declare no conflict of interest.
The cooperation of the medical staff of the rheumatology unit at Baghdad Teaching Hospital is highly appreciated. We also express our appreciation to rheumatologist Mohammed Hadi Munshed Alosami for his role in diagnosing RA.





![Scatter-dot plots of serum interleukin-41 (IL-41) concentrations. Plot A: rheumatoid arthritis (RA) patients vs. healthy controls (HC); plot B: pre-menopausal (PRM) RA patients vs. post-menopausal (POM) RA patients; plot C: disease activity score 28 (DAS28) <3.2 vs. DAS28 ≥3.2; plot D: newly diagnosed (ND) RA patients vs. medicated (MD) RA patients. The horizontal line indicates median. The vertical line indicates interquartile range (IQR: 25–75%). Significance was detected using Mann–Whitney U test (***p<0.001; ns: not significant). The IL-41concentrations were significantly decreased in RA patients compared to HC (49.8 [IQR: 32.5–79.5] vs. 104.7 [IQR: 76.9–134.6]pg/mL; p<0.001). When IL-41 concentrations were stratified by menopausal status, DAS28 and medication in RA patients, no significant differences were found. Scatter-dot plots of serum interleukin-41 (IL-41) concentrations. Plot A: rheumatoid arthritis (RA) patients vs. healthy controls (HC); plot B: pre-menopausal (PRM) RA patients vs. post-menopausal (POM) RA patients; plot C: disease activity score 28 (DAS28) <3.2 vs. DAS28 ≥3.2; plot D: newly diagnosed (ND) RA patients vs. medicated (MD) RA patients. The horizontal line indicates median. The vertical line indicates interquartile range (IQR: 25–75%). Significance was detected using Mann–Whitney U test (***p<0.001; ns: not significant). The IL-41concentrations were significantly decreased in RA patients compared to HC (49.8 [IQR: 32.5–79.5] vs. 104.7 [IQR: 76.9–134.6]pg/mL; p<0.001). When IL-41 concentrations were stratified by menopausal status, DAS28 and medication in RA patients, no significant differences were found.](https://static.elsevier.es/multimedia/01218123/unassign/S0121812323001068/v1_202402020428/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)