To analyze initial and follow-up features of patients with systemic lupus erythematosus (SLE) diagnosed during hospitalization.
MethodsRetrospective analysis of medical records: two groups were studied, a) SLE diagnosed during hospitalization (SLEin), b) SLE diagnosed on an outpatient basis (SLEout).
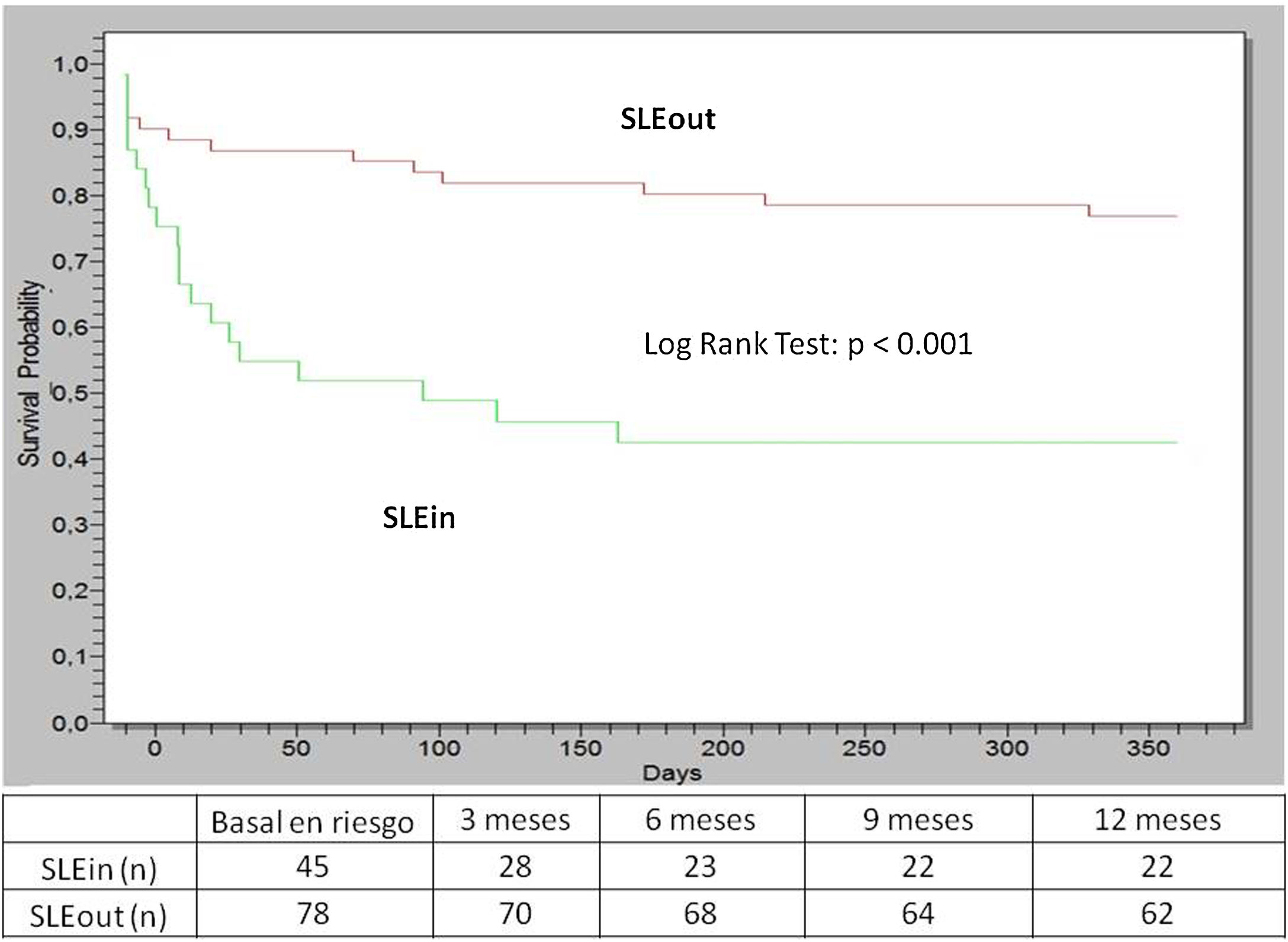
Results123 patients were assessed, 87% female, mean age at diagnosis was 34 years and 45 (37%) of them were SLEin. Patients in the SLEin group had a median of 144 days from the onset of symptoms to diagnosis of SLE vs. 287 days in the SLEout group (p = 0.04). Initially, SLEin had an average SLEDAI of 10 vs. 8 in SLEout (p = 0.004) and anti-dsDNA was positive in 71% vs. 53% in SLEout (p = 0.07). Within the first 6 months, the average cumulative glucocorticoid dose was 6493 mg in SLEin patients vs. 3563 mg in SLEout (p < 0.001) and immunosuppressant usage was higher in SLEin: 62% vs. 26% in SLEout (p < 0.001). Within the first year, SLEin’s kidney biopsies showed lupus nephritis III or IV in 31% vs. 12% in SLEout (p = 0.003, log-rank test). Within the first 2 years, 6 SLEin patients died vs. 1 SLEout patient (p = 0.02) and SLEin patients had more damage as measured by SLICC/ACR Damage Index (median 0, range 25%–75% 0–1 vs. median 0, range 25%–75% 0–0 in SLEout; p = 0.04).
ConclusionsSLEin are initially more active, require higher doses of glucocorticoids and immunosuppressants, have more significant kidney involvement, and present more damage and greater mortality in the short term.
Analizar las características de los pacientes con lupus eritematoso sistémico (LES) diagnosticados durante una hospitalización.
MétodosAnálisis retrospectivo de historias clínicas. Se estudiaron dos grupos: a) LES diagnosticado durante la hospitalización (SLEin) y b) LES diagnosticado de forma ambulatoria (SLEout).
ResultadosSe evaluaron 123 pacientes (87% mujeres); edad promedio al diagnóstico 34 años; el 37% de ellos era SLEin. Los pacientes del grupo SLEin tuvieron una mediana de 144 días desde el inicio de los síntomas hasta el diagnóstico, vs. 287 días en SLEout (p = 0,04). Inicialmente, los pacientes SLEin tenían un SLEDAI promedio de 10, vs. 8 en SLEout (p = 0,004) y anti-dsDNA positivo en el 71%, vs. el 53% en SLEout (p = 0,07). A los 6 meses, la dosis acumulada de glucocorticoides (promedio) fue de 6.493 mg en SLEin vs. 3.563 mg en SLEout (p < 0,001), y el uso de inmunosupresores fue mayor en SLEin: 62% vs. 26% en SLEout (p < 0,001). Al año se halló nefritis lúpica clase III o IV en el 31% de SLEin vs. el 12% en SLEout (Log Rank Test: p = 0,003). A los 2 años, 6 pacientes de SLEin murieron, vs. un paciente de SLEout (p = 0,02). Los pacientes con SLEin tuvieron más daño (índice de daño SLICC/ACR: mediana 0, rango 25–75%: 0-1, vs. mediana 0, rango 25–75%: 0–0 en SLEout; p = 0,04).
ConclusionesLos pacientes SLEin fueron inicialmente más activos, requirieron mayores dosis de glucocorticoides e inmunosupresores, tuvieron una afectación renal más significativa y presentaron más daño y mayor mortalidad a corto plazo.
Systemic lupus erythematosus (SLE) is a relatively frequent autoimmune disorder that occurs predominantly in women during the reproductive years. The hallmark of SLE is its diversity of presentation, the accumulation of manifestations, the damage over time, and the undulating course of the disease. Virtually any organ or system can be affected by SLE.1
The usual presentation of the disease includes arthralgia, arthritis and cutaneous manifestations,2 which is why, in most cases, the diagnosis is usually made on an outpatient basis.
In the context of multisystem disease, patients are frequently hospitalized for the treatment of certain clinical manifestations of SLE or for treatment complications, usually infections.3,4
There is a group of patients who receive the diagnosis of SLE during a hospitalization. In some cases, it may be due to the initial presence of non-specific manifestations and delay in the diagnosis (due to low clinical suspicion by the treating physicians) and is associated with more episodes of activity, hospitalizations and health expenditure.5 In other cases, it is due to the existence of barriers to early access to the rheumatologist, either for socioeconomic reasons, or because patients live far from the most densely populated areas, which are where specialists usually work.6,7
Finally, given the severity of certain initial clinical manifestations—for example, significant renal involvement, hemolytic anemia or severe thrombocytopenia—hospitalization is logically inevitable.
It is known that the higher the disease activity, the greater the requirement for immunosuppressive therapy and, therefore, the probability of infections, damage and death is higher.8–11
Then, the question arises whether the need for hospitalization at the beginning of the disease is just a casual event within the evolutionary course of the illness of each patient, or if, on the contrary, identifies a phenotype of patient with a more active disease and a worse short-term prognosis.
For this reason, it was decided to carry out the following work, with the aim of evaluating whether patients with SLE diagnosed during hospitalization indicate a group with higher basal activity, worse prognosis and greater damage and short-term mortality.
Materials and methodsA retrospective analysis of the medical records of the patients with a diagnosis of SLE established in the center from January 1, 1997 to December 31, 2017 was carried out. The day when the patients met at least 4 ACR/SLICC 2012 criteria for SLE was taken as the date of diagnosis.12
The patients were distributed into two groups: a) patients with SLE diagnosed during a hospital stay (SLEin), and b) patients with SLE diagnosed on an outpatient basis (SLEout).
In all cases, the demographic data: age, gender, whether they lived in the City of Buenos Aires (CABA) or not at the time of diagnosis, and the baseline serological findings (ANF, antiDNA, C3 and C4) were recorded. The date of the first symptom related to SLE, in the opinion of the researchers, was recorded, and the time from the first symptom of SLE to diagnosis was calculated.
The disease activity at the time of diagnosis was calculated using the Lupus Disease Activity Index (SLEDAI).13 The cumulative dose of glucocorticoids (the different doses are expressed in mg of prednisone), the use of hydroxychloroquine, the use of methotrexate, the use of corticosteroid pulses, the use of immunosuppressants (cyclophosphamide, mycophenolate mofetil, azathioprine, belimumab and rituximab) and the use of plasmapheresis or gamma globulin during the first 6 months of evolution were calculated.
The clinical manifestations of SLE accumulated during the first year of evolution were determined and the cases in which a renal biopsy was performed within the first year of evolution and those in which the renal biopsy showed lupus glomerulonephritis (LGN) III or IV were analyzed in particular.
The cumulative damage by the SLICC/ACR Damage Index14 and the mortality within the two first years of diagnosis were assessed. The patients were followed-up until 2 years after diagnosis, or until their death or loss to follow-up. In the cases in which the 2 years of follow-up were not reached, the damage observed in the last visit to the center was completed.
Patients younger than 18 years at the time of the diagnosis of SLE, patients who were treated in another institution at the time of the diagnosis of SLE, those with incomplete data in the medical record or whose initial diagnosis of SLE was modified during the evolution were excluded. The patients of the SLEout group were considered ambulatory patients even when they were hospitalized after the diagnosis was established, that is, during the first 2 years of follow-up.
Descriptive statistics were used for the general analysis and the Student’s t-test was used to compare means. Mann–Whitney and Kruskal–Wallis tests were applied to compare medians as appropriate. The nominal variables were analyzed using chi square or Fisher’s exact test, accordingly. In order to explore survival for two groups, the Log Rank Test, Kaplan–Meier curves and Cox proportional hazards model were used. A p < 0.05 was considered statistically significant in a two-tailed test. The Epi Info version 3.5.4 was used for the statistical analysis.
The study was approved by the Bioethics Committee of the Hospital. As it was a retrospective study, the signed informed consent of the patients was not required.
ResultsA total of 292 medical records were reviewed, of which 169 (58%) were excluded: 75 (44%) because the diagnosis was made in another center, 63 (37%) because the diagnosis was made before 18 years of age, 23 (14%) for having the diagnosis before or after the date of opening or closure of the database, and the rest for other causes. Of 169 patients excluded, 152 (90%) were women and 45 patients (27%) lived in the CABA.
123 patients were analyzed, of whom 107 (87%) were women and 16 (13%) were men, with a mean age of 34 years (SD: 12) at diagnosis of SLE. 32 patients (26%) lived in the CABA and the rest lived outside of it. In 45 patients (37%) the diagnosis was established during hospitalization (SLEin) and in the rest it was on an outpatient basis (SLEout). 6 patients (13%) in the SLEin group vs. 11 (14%) in the SLEout were lost to follow-up (p = 0.9). The median follow-up was 730 days for SLEin (range 25%–75%: 730–730) vs. 730 days for SLEout (range 25%–75%: 730–730; p = 0.9). All patients were positive for the antinuclear antibody (ANA) test by IIF.
There were no statistically significan differences regarding gender: 41 patients (91%) were women in SLEin vs. 66 (85%) in SLEout (p = 0.5), nor in the mean age at diagnosis: 33 years (SD: 12) in SLEin vs. 34 years (SD: 12) in SLEout (p = 0.8). 16 patients (36%) of the SLEin group vs. 16 (21%) in the SLEout lived in the CABA (p = 0.1). The patients of the SLEin group had a median of 144 days (range 25%–75%: 61–394) from the onset of the symptoms until the diagnosis of SLE vs. 287 days (range 25%–75%: 120–624) in SLEout (p = 0–04).
32 patients (71%) in SLEin vs. 41 patients (53%) in SLEout had positive antiDNA at diagnosis (p = 0.07). At the time of diagnosis, the mean SLEDAI score was 10 (SD: 5) for SLEin vs. 8.0 (SD: 4) for SLEout (p = 0.004). The levels of C3 were decreased in 32 patients (74%) with SLEin vs. 26 patients (36%) with SLEout (p < 0.001), while the levels of C4 were below normal in 37 (86%) patients of SLEin vs. 49 (68%) of SLEout (p = 0.05).
In the first 6 months from the diagnosis of SLE the average cumulative dose of prednisone was 6493 mg for SLEin (SD: 3404) vs. 3563 mg (SD: 2985 mg) for SLEout (p < 0.001) and corticosteroids in pulses were used in 14 patients (31%) in SLEin vs. 11 (14%) in SLEout (p = 0.04). The average cumulative dose of prednisone continued to be statistically significant when the pulses of corticosteroids were disaggregated (5582 mg for SLEin vs. 3190 mg for SLEout; p < 0.001).
Hydroxychloroquine was indicated in 30 patients (67%) in SLEin vs. 73 patients (94%) in SLEout (p < 0.001) and methotrexate in five patients (11%) in SLEin vs. 18 (24%) in SLEout (p = 0.1).
In addition, immunosuppressants were used in 28 patients (62%) of the SLEin vs. 20 patients (26%) of SLEout (p < 0.001). Plasmapheresis was used in 2 cases (4.4%) in SLEin vs. 2 cases (2.6%) in SLEout and gamma globulin in 3 cases (6.7%) in SLEin vs. 3 cases (3.8%) in SLEout. In both cases the differences were not statistically significant.
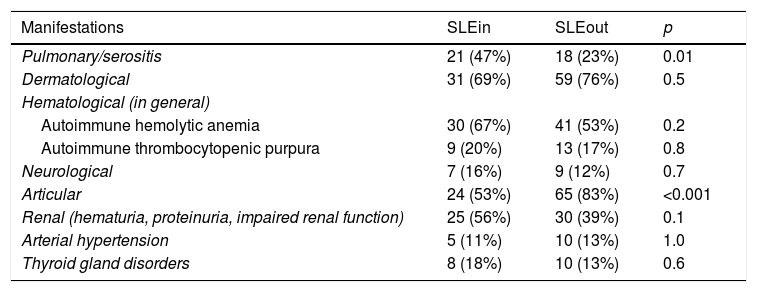
Within the first year of follow-up the patients of the SLEin group had greater lung/serosal involvement and less articular involvement (Table 1) than those of the SLEout group. 19 patients (42%) of SLEin vs. 13 patients (17%) of SLEout had kidney involvement that justified a renal biopsy (Log Rank Test: p < 0.001) (Fig. 1). The finding of a biopsy compatible with lupus glomerulonephritis (GNP) class III or IV was observed in 14 patients (31%) of the SLEin group vs. 9 patients (12%) of the SLEout group (Log Rank Test: p = 0.003). The results were maintained when adjusting for the use of hydroxychloroquine within the first 6 months (Hazard Ratio GNF III–IV: 2.6; 95% CI: 1.04–6.24; p = 0.04).
Cumulative clinical manifestations observed during the first year of follow-up.
| Manifestations | SLEin | SLEout | p |
|---|---|---|---|
| Pulmonary/serositis | 21 (47%) | 18 (23%) | 0.01 |
| Dermatological | 31 (69%) | 59 (76%) | 0.5 |
| Hematological (in general) | |||
| Autoimmune hemolytic anemia | 30 (67%) | 41 (53%) | 0.2 |
| Autoimmune thrombocytopenic purpura | 9 (20%) | 13 (17%) | 0.8 |
| Neurological | 7 (16%) | 9 (12%) | 0.7 |
| Articular | 24 (53%) | 65 (83%) | <0.001 |
| Renal (hematuria, proteinuria, impaired renal function) | 25 (56%) | 30 (39%) | 0.1 |
| Arterial hypertension | 5 (11%) | 10 (13%) | 1.0 |
| Thyroid gland disorders | 8 (18%) | 10 (13%) | 0.6 |
Within the first two years of follow-up the median damage (measured by the SLICC/ACR Damage Index) was 0 (range 25%–75%: 0–1) in SLEin vs. 0 (range 25%–75%: 0–0) in SLEout (p = 0.04). 6 deaths (13%) were recorded in SLEin: 3 patients within the first 5, 90 and 104 days after diagnosis and the rest after the first day of follow-up: two cases due to infections, one case due to respiratory failure, one case due to kidney failure, one case due to complications of diabetes mellitus and one case due to multiple organ failure attributed to the baseline disease vs. one case (1.3%) in SLEout (after the first year of follow-up), due to kidney failure generated by the underlying disease (Fisher’s exact test: p = 0.02).
DiscussionIt is estimated that between 20 and 25% of the patients with SLE are admitted to hospitals in the United States each year.15 Between the years 2006 and 2009, 3300 discharges due to SLE were registered in the hospitals of the Public Health Subsector in Argentina.16 Several works published in America have studied the hospitalizations in patients with SLE. While the intrahospital mortality was described between 1.3 and 3.7% in the United States17–19 and 3.4% in Argentina,16 higher values (between 5.8 and 10%) can be seen when the intrahospital mortality in a single center is described, according to studies carried out in reference hospitals from Canada,20 Argentina,21 Colombia,22 Peru23 and Paraguay.24
Aspects related to renal involvement or hematological manifestations of SLE are usually mentioned as frequent causes of hospitalization related to the disease, and they also worsen the short-term prognosis of patients with early SLE.25–27
In 2010, the findings of an international cohort of 200 patients with a diagnosis of early SLE were published: the mean age was 34.8 years, positive antiDNA was observed in 75% and the baseline SLEDAI was 12.2 (±9.8). During the first year of the disease, the use of antimalarials was 51%, the use of glucocorticoids was 83%, the use of pulse therapy with glucocorticoids was 33%, and the use of cytotoxic drugs reached 51% of cases. Lupus nephritis confirmed by biopsy was observed in 25% of the cases and the patients with lupus nephritis more frequently received cyclophosphamide, azathioprine, pulses of corticosteroids, and less frequently, hydroxychloroquine and antiinflammatory agents.28
In an international multicenter study published in 2016 on 1827 patients with recent-onset SLE, the occurrence of lupus nephritis was observed in 38.3%, and frequently as an initial manifestation. The presence of lupus nephritis was associated with a substantial risk of kidney failure and death.29
In a study published by the Gladel group in 2017 on 1268 patients with early SLE, a mean age at diagnosis of 29.2 years and a baseline SLEDAI of 10.9 (SD: 8.4), the basal use of antimalarials and doses of corticosteroids between 15 and 60 mg of prednisone were protective factors against a high disease activity.30
In 2019, the Spanish Registry of Systemic Lupus Erythematosus published the data of 223 patients: the average SLEDAI score at the time of diagnosis was 9.8, and within the first year of follow-up, the diagnosis of lupus nephritis was reached in 21% of the cases, the use of hydroxychloroquine reached 80%, the use of methylprednisone was 15% and the use of immunosuppressants was 40%.31 In addition, the dose of corticosteroids received during the first month of illness was an independent predictive factor of the load of prednisone received throughout the year.
An Argentinian multicenter study published in 2007 on 197 patients with SLE estimated a damage of 0.52 (measured by SLICC/ACR Damage Index) for the first year of evolution, being the kidney the most frequently affected organ.32 According to a work conducted by Petri et al.9 published in 2012, which analyzed a large cohort of more than 2000 patients, the damage caused by SLE increases by around 0.13 per year, due to (among other factors) the greater disease activity, the presence of positive antiDNA, the consumption of complement and the lower socioeconomic level, although after adjusting for different variables, the age, hypertension, and the use of corticosteroids seem to be the most determining factors.
Another study published in 2015 on more than 1700 patients with SLE from an international cohort pointed out SLEDAI and the use of steroids (among others) as the factors associated with the transition from no damage to damage. The damage was associated with higher mortality, and the use of hydroxychloroquine was associated with a smaller increase in the pre-existing damage.11
Thus, it is proposed that patients with SLE diagnosed during hospitalization set up a perfect storm: they present higher baseline activity, positive antiDNA with consumption of complement, accumulate a higher dose of corticosteroids and early use of immunosuppressants, they present early nephritis and have a greater progression of the damage and a greater risk of death in the short term. The data from this study also suggest that these patients appear to have a more aggressive disease phenotype, namely: while more than 70% of the patients hospitalized in the CABA due to SLE live outside this city33; conversely, the patients diagnosed with SLE during hospitalization lived there in a higher percentage and also showed a shorter time since the onset of symptoms until the diagnosis of the disease.
Regarding the limitations of this study, the analysis is retrospective and belongs to a single hospital, public, of the CABA, and associated with the University, and for this reason it receives, in general, patients without medical coverage and of greater complexity. Furthermore, many patients under follow-up could not be included in the analysis because they had been diagnosed in other centers. Although the use of hydroxychloroquine reached 83% in the first 6 months, it was significantly higher in the ambulatory patients. However, to the best of our knowledge, there are no other published studies with similar characteristics.
In conclusion, patients diagnosed with SLE during hospitalization appear to form a group with a worse prognosis and should have, during the initial stages of the disease, strict follow-up by the professionals in charge, with the aim of controlling the activity of the disease and implement all necessary measures to minimize the damage.
FundingThis research did not receive a specific grant of any kind.
Conflict of interestThe authors declare that they have no conflicts of interest for the preparation of this article.
Please cite this article as: Porta S, Hassan R, Aquino V, Estrella N, Micelli ML, Sequeira G, et al. Lupus eritematoso sistémico diagnosticado durante una internación: mayor actividad basal de la enfermedad, daño y muerte a corto plazo. Rev Colomb Reumatol. 2022;29:101–106.