There is a complex, dynamic, and bidirectional connection between autoimmunity and cancer. The underlying mechanisms of carcinogenesis and physiopathological aspects of autoimmune diseases are not fully understood. However, there is a common immunological basis related to the expression of autoantigens by tumor cells that cause an anti-cancer immune response, which triggers the development of paraneoplastic rheumatic syndromes and autoimmune rheumatic diseases in the genetically predisposed population. This article discusses the case of a 57-year old man presenting with pulmonary renal syndrome, and who was simultaneously diagnosed with lung cancer and systemic lupus erythematosus.
Existe una relación compleja, dinámica y bidireccional entre la autoinmunidad y el cáncer. Si bien los mecanismos carcinogénicos y fisiopatológicos de las enfermedades autoinmunes (EAI) no están claramente dilucidados, existe una base inmunológica común relacionada con la expresión de autoantígenos por parte de las células tumorales que desencadenan una respuesta antitumoral, facilitando el desarrollo de síndromes paraneoplásicos reumáticos (SPaR) y enfermedades autoinmunes reumáticas (EAR) en población genéticamente susceptible. Se presenta un caso de un hombre que debutó con un síndrome pulmón riñón (SPR) y se diagnosticó cáncer de pulmón (CP) y lupus eritematoso sistémico (LES) de forma simultánea.
There is a complex, dynamic, and bidirectional relationship between cancer and autoimmunity, which has not yet been fully elucidated. On the one hand, it has been said that malignant disease may induce a loss of immune tolerance, a process characterized by the generation of autoantibodies against tumor-associated autoantigens.1 On the other hand, autoimmune disease may be predisposing for the development of cancer,2 or may manifest as a paraneoplastic syndrome (PNS).2–4 The latter would be related with malignant transformation due to immune dysregulation, accounting for the increased incidence of leukemia and lymphoma in patients with rheumatoid arthritis, systemic lupus erythematosus (SLE) and systemic sclerosis (SS).2 When both conditions—i.e., autoimmune disease and cancer—are simultaneously diagnosed in the clinic, this gives rise to a very interesting diagnostic challenge.
A PNS is defined as a set of signs and symptoms presenting in patients with cancer, with systemic manifestations in areas remote from the tumor.5 The syndrome comprises a diverse clinical spectrum, with the endocrinological manifestations being the most frequently reported, and less frequently hematological, neurological and rheumatic signs. The rheumatic manifestations are called paraneoplastic rheumatic syndromes (PRS)6 and represent the joint and cutaneous clinical manifestations; the lupus-like syndrome is included in this category.7
Following is a discussion of a case of man who initially presented with alveolar hemorrhage associated with renal involvement, with a simultaneous diagnosis of squamous cell lung cancer.
Clinical caseThis is a 57-year old male patient, from Santander, Colombia, with a 3-month evolution of stabbing chest pain in the right hemithorax of pleural characteristics, with 8/10 pain intensity (0 to 10 verbal pain scale, with 0 being no pain, and 10 excruciating pain), associated with progressive dyspnea from 1 to 4/4 based on the modified Medical Research Council, and hemoptoic cough. The only personal history reported was that the patient smoked for 10 years (15 packs/year and smoking cessation 17 years before his current hospital admission). The physical examination and assessment showed unintentional weight loss of 8 kg in the month prior to admission. The patient had previously consulted a local hospital where a high resolution CT (HRCT) documented a lung mass in the right lower lobe.
The vital signs during the physical examination at admission were: BP: 124/78 mmHg, HR: 101 x´, RR 20 x´, pulse oximetry (SaO2): 92%; fraction of inspired oxygen (FiO2): 21% and temperature (T): 37.0 °C. Mucocutaneous pallor was observed; in terms of the cardiopulmonary assessment, there was louder S2 at the mitral apex and softer murmur at the base of the right lung, with no aggregates.
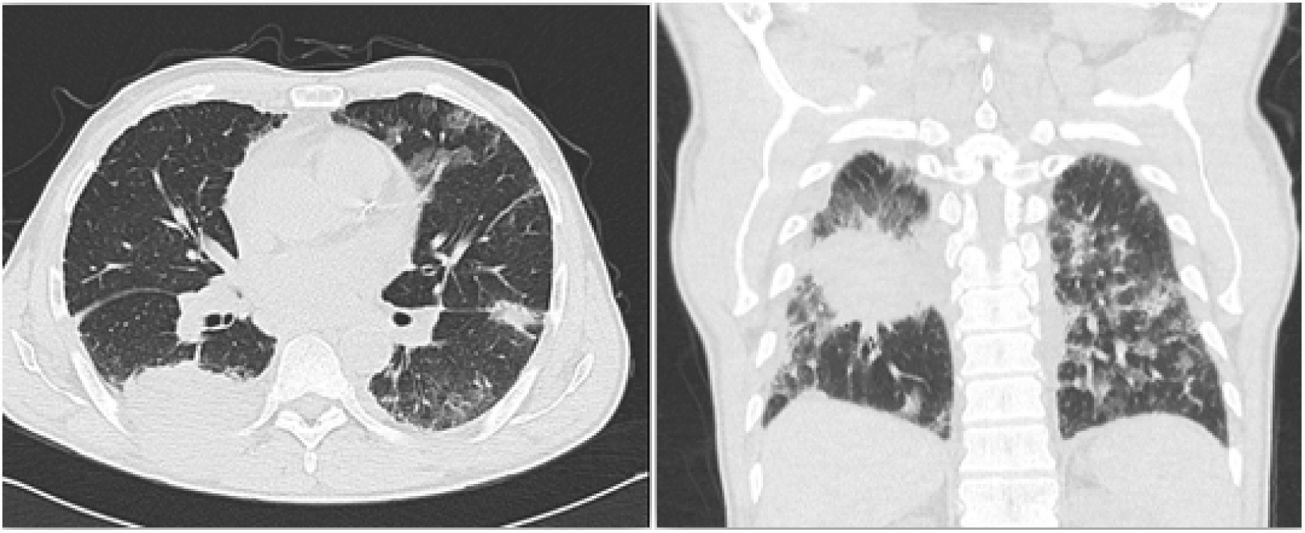
Based on the past history of smoking, the finding of a lung mass, and the current pleural pain, hemoptysis, and unintentional weight loss, the clinical approach was to screen for lung cancer. Other differential diagnoses were considered, including pulmonary thromboembolism and chronic mycobacterial infection. A second chest CT was conducted (Fig. 1) which confirmed the presence of a 63 × 57 × 64 mm mass in the right lower lobe, with extra-thoracic extension and neoplastic features.
Chest CT depicting a loop in the right lower lobe, with extra-thoracic extension and neoplastic appearance. Thickening of the pleura and of the right lung fissures, with potential secondary involvement. Bilateral apical fibroatelectatic changes and calcified right apical mass. Bilateral interstitial lung disease.
Complementary laboratory tests evidenced normochromic normocytic anemia (Hb 9.45 mg/dL, MVC 88 fL), elevated acute phase reactants (erythrocyte sedimentation rate –ESR–: 107 mm/h and C-reactive protein –CRP–: 8,49 mg/dL), elevated D-dimer (11.1 mg/L) and hyperazotemia (creatinine: 1.9 mg/dl; BUN 40 mg/dl). The urinalysis showed pyuria (75 Leu/ul), proteinuria (500 mg/dL) and hematuria (50 erythrocytes/ul), with active urinary sediment showing leukocyturia (157 leukocytes/ul), hematuria (141 erythrocytes/ul) and pathological casts. The microbiology of the sputum was negative for acid-alcohol fast bacilli and the ventilation / perfusion scintigraphy (V/Q) was negative for pulmonary thromboembolism. The chronicity studies for kidney disease were also negative (renal ultrasound with no atrophic changes or loss of the corticomedullary index), suggesting acute renal involvement.
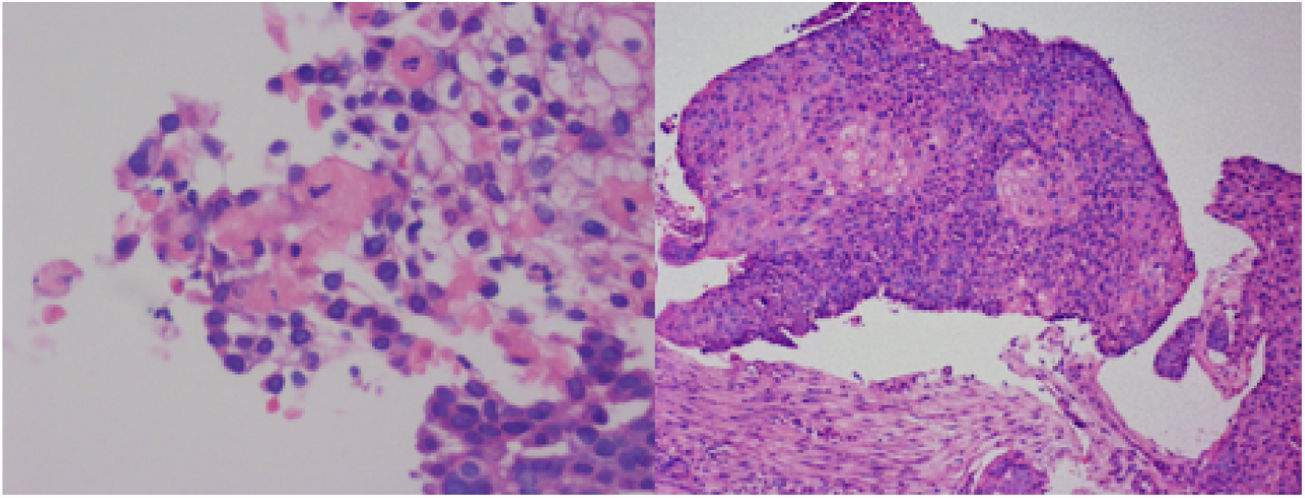
In view of the clinical context of a constitutional syndrome with hemoptysis, anemia, acute kidney injury, and active sediment, the diagnostic approach was reoriented to a pulmonary-renal syndrome (PRS). The patient underwent fibrobronchoscopy and bronchoalveolar lavage, (Fig. 2) which produced progressively hemorrhagic fluid, suggestive of alveolar hemorrhage. The cytology evidenced a predominance of erythrocytes, with abundant pigmented macrophages, lymphocytes and neutrophils. The hematoxylin – eosin stain, Ziehl-Neelsen, periodic acid Schiff, and Grocott stains, and the culture, were all negative. The iron stain confirmed the presence of hemosiderophages with a 25% index. The immune profile reported: antineutrophil cytoplasmic antibodies (ANCA), anti-glomerular basement membrane antibodies, total extractable nuclear antibodies (ENA) and antiphospholipid syndrome profile, all negative. Antinuclear antibodies (ANA) were positive in 1:2560, with a homogeneous pattern, and the anti-DNA in 1:80 (positive). Renal and pulmonary biopsies were ordered, and due to a presumptive diagnosis of immune origin PRS, 3 doses of methylprednisolone 1 g/day treatment was indicated.
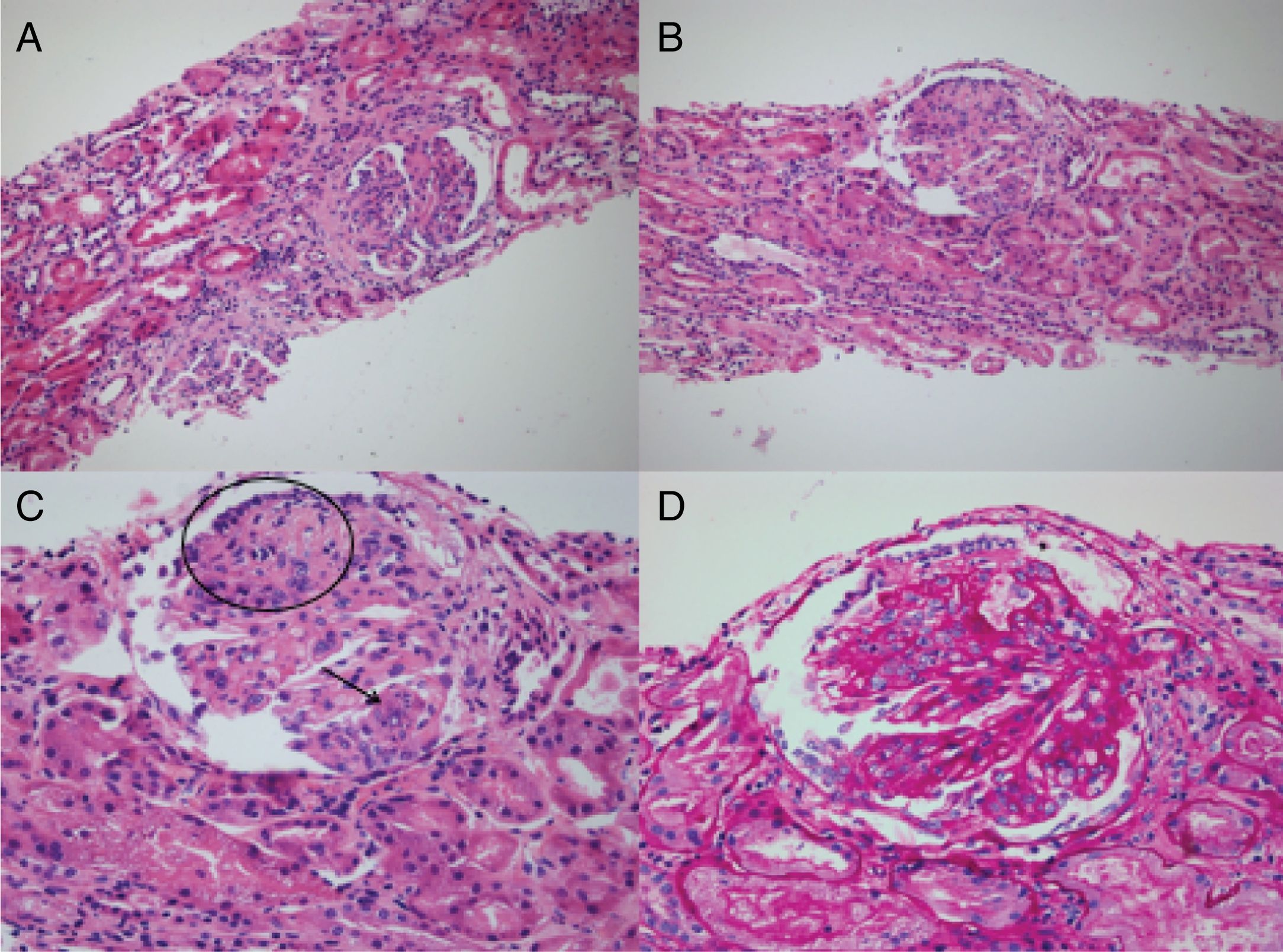
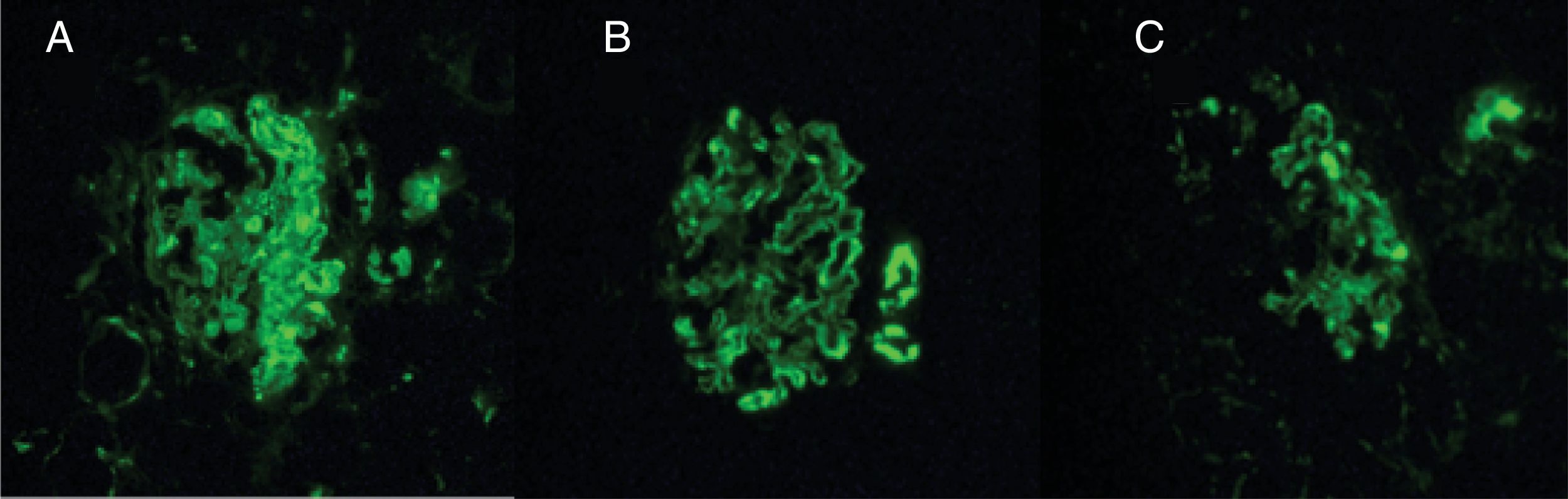
During the hospital stay, the patient had a torpid evolution and was admitted to the intensive care unit with severe hypoxemic respiratory failure. The pulmonary biopsy showed keratinizing squamous cell carcinoma (Fig. 3), and the renal biopsy reported necrotizing glomerulonephritis (NGM) with extra-capillary proliferation (26%) associated with global diffuse lupus nephritis (LN) class IV, with activity index of 14/24 and chronicity index of 4/12 (Fig. 4). The indirect immunofluorescence (IIF) identified the presence of IgG, IgA and C1q reactive deposits on the glomerular capillary basement membrane and mesangium (Fig. 5), compatible with a full-house pattern.
A and B) H-E 20× stain. Visible glomeruli with membranoproliferative pattern, with evidence of segmented lesions and their corresponding attachment to Bowman’s capsule. Segmental destruction of the parietal epithelium and its glomerular capillary basement membrane. C) H-E 40×. Glomerulus with membranoproliferative changes, collapse (circle) with prominent visceral epithelium, with no evidence of patent capillary lumen. Presence of hematoxylin bodies (arrow) characteristic of lupus nephropathy, with cariorexys. D) Periodic acid-Schiff staining 40×. The same glomerulus described in C, with notable thickening of the glomerular capillary basement membranes with double contours and the presence of subendothelial deposits.
The suggested diagnosis was PRS with lupus-like manifestation which accounts for the syndrome, and the renal histopathology. There was no indication for palliative chemotherapy, and surgical resection was not feasible; so, in the light of the poor clinical condition of the patient, the decision was made to limit any further treatment. The patient died 12 days after hospital admission.
DiscussionThis clinical case discusses two different approaches with regards to the primary clinical condition. The first one considers the possibility of two severe and potentially lethal diseases, and the second one attempts to explain the renal pulmonary symptoms based on the presence of a paraneoplastic rheumatic syndrome in the context of lung cancer.
The clinical evolution was subacute, and during the systems assessment, there were no symptoms suggesting autoimmunity. Weight loss is a constitutional symptom that could have been more related to the lung cancer. Before the patient was hospitalized, a lung mass of neoplastic characteristics had been previously documented, which confirms that the lung cancer was present before the development of SLE. In fact, the initial clinical approach focused on confirming the diagnosis of lung cancer (considering that the patient used to smoke) and only after the active urinary sediment was documented, autoimmunity was suspected.
PRS is defined as the coexistence of diffuse alveolar hemorrhage (DAH) and GMN; this condition usually has a poor prognosis, with a mortality of around 70%.8 95% of the cases are secondary to primary vasculitis associated with ANCA (56%–77,5%) and anti-glomerular basement membrane antibodies disease (12.5%–17.5%). The remaining 5% is due to other non-ANCA vasculitis and systemic autoimmune diseases, where SLE is the prototype pathology in this group.8,9 In this particular case, the immunological and histopathological diagnosis of SLE was confirmed, and this was finally the cause of the PRS, and hence, the clinical event leading to the demise of the patient.
DAH is a rare manifestation of vasculitis in SLE with a frequency of presentation ranging between 2 and 5.4%.10,11 Histologically, the most frequent findings are: soft pulmonary hemorrhage, capillaritis similar to that in systemic vasculitis, and diffuse alveolar injury.12 In this case, while the clinical presentation was consistent with DAH, the histology reported squamous cell carcinoma of the lung, which became the key element in the clinical approach to consider other differential diagnoses, as discussed hereunder.
Paraneoplastic rheumatic syndromes associated with lung cancerPRS refers to the number of distant clinical manifestations unrelated with contiguous tumor spread, presence of metastasis or side-effects from treatment.13 The clinical course is usually parallel to the development of the tumor, but may precede the tumor.14 The clinical spectrum of PRS is variable, but the most frequent are endocrinological (30%), while the less frequent are hematological, neuromuscular, and rheumatic.15
In the case of lung cancer, the frequency of presentation of PRS has been described at 15% at the time of diagnosis, and up to 70% during the course of the disease.16 Paraneoplastic rheumatic syndromes are associated with the release of cytokines by the tumor cells that result in an abnormal immune response involving cell damage and cross-reactions leading to the deposit of immune complexes and subsequent inflammatory response.17 The most frequent presentations include: hypertrophic osteoarthropathy, carcinoma-related polyarthritis, myositis and vasculitis.16 The lupus-like syndrome is rare and has been described in other neoplasms besides lung cancer—breast, ovarian, Hodgkin disease and leukemia hairy cells leukemia. It is characterized by polyserositis, Raynaud’s phenomenon, inflammatory arthritis, leucopenia and positive ANA.18,19 Whilst the onset of the immune manifestations is temporarily associated with a diagnosis of lung cancer, the clinical presentation of a lupus-like syndrome is very different from the clinical presentation in this patient.
Paraneoplastic glomerulonephritisThese are glomerular lesions which are not directly related to the tumor burden, invasion or metastasis, but are induced by tumor cell products. In solid tumors, membranous nephropathy (MGN) is most commonly reported, and less frequently the minimal changes disease, membranoproliferative glomerulonephritis, rapidly progressing glomerulonephritis and IgA nephropathy.20,21 The optic microscopy of the patient identified a membranoproliferative pattern with thickening of the glomerular basement membrane, double contours, subendothelial deposits, and karyorrhexis; the indirect immunofluorescence showed a «Full House» pattern; consequently, in view of the histopathological characteristics and the clinical presentation, the diagnosis was lupus nephritis and not membranous GMN associated with malignancy.
Therefore, the patient did not present with a lupus-like syndrome but certainly met all the SLICC criteria for SLE22: 1) elevated positive ANA titers; 2) positive anti-DNA; 3) Lupus nephritis histologically confirmed with compatible IIF.
The presence of elevated ANA titers was another conclusive paraclinical finding for a definite diagnosis of SLE. ANA positivity has been described in malignant disease, not just in lung cancer, but also in breast and colon cancer, and in lymphoproliferative disorders.23 A study published by Solans-Laque et al., showed that 27.7% of the patients diagnosed with cancer, had positive ANA, with titers ranging from 1:80 and 1:640.24 However, its presence has been considered an epiphenomenon with no clear clinical relevance and its interpretation with regards to malignancy is not yet clearly defined.1,24 In that same trial, more elevated titers—between 1:320 and 1:640—were identified in patients with clinical symptoms of rheumatic disease or connective tissue disease, but no relationship was shown with the risk of subsequently developing autoimmune disease.24 In this specific case, the very high titers of ANA and the homogeneous pattern are of diagnostic value and support the idea of an actual systemic autoimmune disease (SLE) rather than a random clinical occurrence.
Combining the clinical, histological, and laboratory findings, it is clear that there was an autoimmunity outbreak characterized by the presence of SLE on top a preexisting malignant disease, with a pulmonary-renal syndrome (PRS) as the primary manifestation.
Consequently, the obvious question is: Is cancer able to induce autoimmunity?
Relationship between cancer and autoimmunityCancer and autoimmunity share a common immunological origin which is the aberrant activation of the immune system that results in chronic inflammation and the accumulation of tissue damage. However, it is yet unclear at which point in the process do they take different immunopathologic pathways. In cancer, a tumor cells-mediated anti-inflammatory microenvironment prevails, and these cells lose their antigenic capacity, favoring the progression and spread of the tumor. Moreover, in autoimmune disease, the tolerance mechanisms fail, autoantigens are expressed and are misrecognized, facilitating the activation of an excessive inflammatory response against the host. This immunological background gives rise to two opposing pathophysiological scenarios: 1) autoimmunity-induced cancer; and 2) cancer-induced autoimmunity. In this case, the mechanism is consistent with the phenomenon of induction of carcinogenesis-mediated autoimmunity.
The mechanisms leading to autoimmunity from the starting point of a tumor disease, are poorly known. Some have suggested the presence of genetic events associated with oncogene activation, the inhibition of tumor suppressor genes, and the induction of inflammatory proteins.
Systemic Lupus Erythematous and lung cancerPatients with SLE have 1.28 times the risk of developing cancer, as compared to the general population, particularly hematologic tumors (non-Hodgkin lymphoma, [RR: 5.4 95% CI 3.75–7.77], Hodgkin Lymphoma [RR: 3.26; 95% CI 2.17–4.88], leukemia [RR: 2.01; 95% CI 1.61–2.52] and multiple myeloma [RR: 1.45; 95% CI 1.04-2-03]). In the case of non-hematological cancer, the associations more frequently reported are laryngeal tumors, lung, liver, vagina/vulva, bladder and thyroid.4
In 2016, Wu et al., published a meta-analysis including 12 trials and 57,890 patients. They found that the patients with SLE had 1.6 times more risk of developing lung cancer (LC).25 In terms of histopathological distribution, no differences were reported between patients con lung cancer/SLE comorbidity and isolated lung cancer. Smoking was also a risk factor for the development of LC in SLE, in contrast to the exposure to immunosuppressive agents which were unrelated.26 A genetic basis has also been identified for this association. Shared loci have been described, involved with the genesis of LC in the general population, and also present in SLE, such as 4p15.1–15.3 and 6p21.27,28
ConclusionThere is a bidirectional and dynamic relationship between cancer and autoimmunity but it is still difficult to determine whether it is a real association or a coincidence. These two groups of diseases share a complex immunological mechanism; this is an undeniable fact and, from the biological perspective, it is reasonable that these two groups of diseases coexist. The broad range of clinical manifestations, the high morbidity, and the medical challenge involved in treating these patients, demands a multidisciplinary approach to these patients, based on detailed clinical judgement and the comprehensive review of all the clinical and laboratory findings to foster timely diagnosis and treatment.
Conflicts of interestThe authors have no conflict of interests to disclose.
Please cite this article as: Padilla-Ortiz D, Sánchez-Esquivel F, Londoño J, Merayo-Chalico J, Reyes-Martínez V, Florez-Vargas A, et al. Carcinoma de pulmón como disparador de una respuesta autoinmune: un desafío médico. Rev Colomb Reumatol. 2019. https://doi.org/10.1016/j.rcreu.2019.10.004