Osteoarticular manifestations (OAM) are frequently present in patients with systemic sclerosis (SSc). These OAM are related to important functional disability and a severe impact on patient’s life quality, therefore, they require special attention from clinicians.
ObjectiveDetermining the frequency of reported OAM in SSc patients, the general impact of osteoarticular manifestations in patient’s condition and the tools available for diagnosis and treatment.
MethodologyA systematic review of the literature was performed for information published between January 1970 and December 2018 in the medical research databases of: Medline, Embase, Lilacs, Scielo, Cochrane and clinicaltrials.gov.
Results and ConclusionsA total of 116 articles were included in this review. The type and prevalence of the different OAM reported in the literature were determined. In addition to the evolution of their diagnostic methods, there are more validated methods to assess the impact of OAM in patients with SSc. Randomized clinical trials are required to establish the best treatment strategy for these patients.
Las manifestaciones osteoarticulares (MOA) están presentes de forma frecuente en pacientes con esclerosis sistémica (SSc). Generan gran discapacidad funcional con un impacto importante en la calidad de vida de los pacientes por lo que requieren especial atención por parte de los clínicos.
ObjetivosDeterminar la frecuencia de las MOA dentro de la SSc, su impacto en la condición general de los pacientes, y las diferentes herramientas disponibles de diagnóstico y tratamiento.
MetodologíaSe realizó una revisión sistemática de la literatura disponible desde enero de 1970 hasta diciembre de 2018, en las bases de datos de Medline, Embase, Lilacs, Scielo, Cochrane, y clinicaltrials.gov.
Resultados y conclusionesSe incluyó un total de 116 artículos en esta revisión. Se determinó el tipo y la prevalencia de las diferentes manifestaciones osteoarticulares reportadas en la literatura. Además de la evolución en los métodos diagnósticos de las mismas, cada vez existen más métodos validados para evaluar el impacto de las MOA en los pacientes con SSC. Se requieren ensayos clínicos aleatorizados que permitan establecer la mejor estrategia de tratamiento para estos pacientes.
Osteoarticular manifestations (OAM) are frequently present in patients with systemic sclerosis (SSc) with a reported prevalence between 40–80 % of cases.1,2 These OAM are related to important functional disability and a severe impact on patient’s life quality. Clements et al. previously alerted on the knowledge gap regarding OAM in SSc and the need to further investigate this issue.3
During the past few years the importance of OAM in SSc has grown, increasing the number of published literature regarding this topic. Given the amount of new information available, we decided to do a systematic review of the literature with the purpose of determining the frequency of reported OAM in SSc patients, the general impact of OAM in patient’s condition and the tools available for diagnosis and treatment
MethodologyA systematic review of the literature was performed for information published between January 1970 and December 2018. The search strategy was conducted in the medical research databases of: Medline, Embase, Lilacs, Scielo, Cochrane and clinicaltrials.gov. The terms used were: "Scleroderma, Systemic", “CREST syndrome”, “scleroderma”, “SSc”, “systemic sclerosis”, "Joint diseases", "Musculoskeletal Diseases", and “arthritis” (Supplementary material, Table S1) Search was limited by language to English and Spanish, and only articles regarding humans were included. The search included articles discussing research design, cohort, case control, and cross-sectional studies according to the objective of the review. Exclusion criteria considered were: pediatric population, individual case reports, genetic studies, narrative reviews, editor letters and opinion papers. Initially, 3613 articles were screened by title content, afterwards, two independent researchers reviewed the papers based on their abstracts. Duplicates were discarded (Fig. 1). In case of disagreement regarding the inclusion of an article, the article was reviewed by the two researchers. In case that no consensus was achieved the decision was made by the senior researcher. The first phase resulted in 130 articles to be selected by full text research (Fig. 1). After reading the studies related to musculoskeletal manifestations (21 articles), we considered that an independent systematic review focused on these manifestations should be performed. For this reason, we excluded these articles from the current revision. Fourteen additional articles were excluded based on their content (Table S2). Twenty-one papers were included after reviewing related articles and bibliography. Thus, a total of 116 articles were included (Table 1) in the present review. The information to be included was extracted independently by two independent researchers. In case of disagreement, the same methodology was used as in the selection of studies. The bias risk assessment was performed by two independent researchers (Table S3-S6).
Articles included in the systematic review.
| Author | Year | Study design | N | Mean duration of disease (years) | dcSSc (%) | lcSSc (%) | Main findings at osteoarticular level |
|---|---|---|---|---|---|---|---|
| Avouac J1 | 2012 | Review | – | – | – | – | Articular involvement is frequent in SSc. It contributes to disability and compromises patients’ quality of life. |
| Avouac J2 | 2010 | Cross-sectional based on data | 7286 | 10±9 | 42 | 58 | Synovitis, TFR, and JC present in 16 %, 11 %, 31 %, respectively. They are associated with a more severe disease and systemic inflammation. |
| Clements PJ3 | 2012 | Systematic review | – | – | – | – | Most of the tools used to assess arthritis in SSc patients have not been validated and additional work is needed to develop a “core set” of variables for assessment of arthritis in SSc and its response to treatment. |
| Ostojić P4 | 2006 | Cross-sectional | 105 | 5.3 | 47 | 53 | Musculoskeletal impairment is more common in dcSSc patients. |
| Schmeiser T5 | 2012 | Cross-sectional based on data | 58 | 12±9 | 24 | 76 | Arthritis was found in 31 % of patients, 19 % clinically and 26 % radiologically. |
| Tager RE6 | 1999 | Cross-sectional | 63 | 3.6 | 65 | 35 | Arthralgia/arthritis was found in 68 % of Black South African patients with SSc. |
| Wangkaew S7 | 2018 | Cross-sectional | 110 | 4.9 + 4.8 | 66 | 34 | dcSSC patients had more severe clinical hand complications than lcSSc. RF is associated with arthritis and high MRSS is associated with finger contractures and digital ulcers. |
| Arias-Nuñez MC8 | 2008 | Cross-sectional based on data | 78 | 8.3 | 30 | 70 | Arthralgia was found in 54 % of patients, and arthritis in 12 %. |
| La Montagna G9 | 2005 | Cross-sectional | 76 | 8 | 29 | 30 | Arthralgia was found in 38 % of patients, and arthritis in 13 %. TFR was found in 13 %. Joint involvement is a major determinant of disability. |
| Jaeger VK10 | 2018 | Cross-sectional | 944 | 14.8 | 37 | 57 | Arthritis was found in 11 % of patients, TFR in 4.6 %, and JC in 50 %. SSC patients perceive pain, digital ulcers and muscle weakness as some of the main factors driving their level of disability. |
| Misra R11 | 1995 | Cross-sectional | 34 | 7.5 | 53 | 47 | Synovitis was observed in 88 % of patients. RF was found in 33 % of SSc patients. |
| Allali F12 | 2007 | Cross-sectional based on data | 46 | 10.5 + 6.5 | 65 | 35 | SSc patients had radiological abnormalities in 80 % of the cases, including joint space narrowing (37 %) and erosions (43 %). |
| Lovell CR13 | 1979 | Cross-sectional | 24 | – | – | – | Radiological abnormalities in joints of SSc patients are common. |
| Armstrong RD14 | 1982 | Case series | 4 | – | – | – | Destructive arthritis may represent the coexistence of RA and SSc. |
| Horimoto AMC15 | 2016 | Cross-sectional | 61 | – | 33 | 48 | Arthritis was observed in 33 % of SSc patients. Overlap with RA was 6.6 %. Positive anti-CCP were found in 20 % of patients with arthritis versus no patients with SSc without arthritis. |
| Jinnin M16 | 2003 | Cross-sectional | 173 | 8 | 37 | 63 | Overlap with RA was present in 5.2 % of patients. Arthritis prior to Raynaud’s phenomenon, increased CRP and elevated RF were observed in SSc patients with RA with a significantly higher incidence than in those without. |
| Baron M17 | 1982 | Cross-sectional | 38 | 7.3 | – | – | The occurrence of arthropathy is common. Erosive disease did not correlate with disease duration, presence of RF, antinuclear antibodies, DTR, or the extent of the scleroderma skin changes. |
| AvouacJ 18 | 2016 | Prospective cohort | 1301 | 2±1 | 38 | 62 | Synovitis and TFR are independent predictive factors for disease progression in patients with early SSc. |
| Arslan Tas D19 | 2015 | Case-control | 175 | 7 | 67 | 33 | Sacroiliitis was found in 23 % of SSc patients, and was significantly different from the RA and healthy control groups. Male gender, diffuse subtype, inflammatory back pain and high C-reactive protein levels were found to be the significant risk factors for sacroiliitis. |
| Bálint Z20 | 2014 | Prospective cohort | 131 | 7.8 | 31 | 69 | Contractures predominantly develop during the early years of disease. Inflammation and skin-involvement are significant contributing factors for the development of contractures. A high number of joint-contractures might be an unfavorable prognostic factor in SSc. |
| Ashida R21 | 2007 | Cross-sectional | 350 | 7.8 | 43 | 57 | CP was found in 47 % of patients with SSc. Presence of CP may be a marker of esophageal involvement, pulmonary fibrosis and heart involvement. |
| Wiese AB22 | 2014 | Prospective cohort | 200 | 1.6 + 1.4 | 100 | 0 | Physician global health assessment had large correlations with MRSS (r=0.60) and physician-reported skin involvement visual analog scale in the last month (r=0.74). Skin variables were more responsive to change than musculoskeletal variables over a 1-year period. |
| Avouac J23 | 2011 | Prospective cohort | 103 | 12 | – | – | Multivariate Cox regression analysis did not identify any predictor of the progression of erosive arthritis. Digital ulcers were shown independently to predict the progression of acro-osteolysis and calcinosis (HR 12.43, and HR 3.16, respectively). dcSSc was shown to be an independent predictor of the progression of CP (HR 7.52). |
| Khanna PP24 | 2010 | Cross-sectional based on data | 134 | 0.8 | 100 | 0 | Forty-nine (37 %) patients had TFR at baseline, 50 % had resolution of their TFR, whereas 21 % developed new TFR. Change in TFR (P=0.04) and baseline MRSS (P=0.03) predicted change in MRSS over a 12-month period (Model R2=0.14). |
| Steen VD25 | 1997 | Prospective cohort | 1305 | 6 | 49 | 51 | TFR were detected most frequently in dcSSc. The presence of 1 or more TFR was a good predictor of both evolution to diffuse skin thickening and reduced survival. |
| Doré A26 | 2013 | Prospective cohort | 287 | 1 | 100 | 0 | Patients with TFRs had a >2-fold risk of developing renal crisis and cardiac and gastrointestinal disease complications. Patients with TFR had poorer 5- and 10-year survival rates. |
| Tas DA27 | 2012 | Cross-sectional | 28 | 1.8 | 61 | 36 | Arthritis was found to have correlation with heart involvement and FTP was correlated with lung involvement. |
| Arana-Ruiz JC28 | 2016 | Cross-sectional | 40 | 7 | 30 | 70 | Acro-osteolysis was more common in patients with higher MSC than in those with low MSC. |
| Johnstone EM29 | 2012 | Cross-sectional | 101 | 13 | 30 | 70 | Acro-osteolysis was strongly associated with severe digital ischemia. Patients with moderate/severe acro-osteolysis were more likely to have severe calcinosis, but this was not statistically significant after adjustment for potential confounders. |
| Koutaissoff S30 | 2011 | Cross-sectional | 335 | 9 | 24 | 64 | Although a rheumatoid arthritis-like or a collapse arthropathy can be observed in SSc patients, arthropathies are less common than previously reported. |
| Morardet L31 | 2016 | Cross-sectional | 155 | 9+8 | 42 | 58 | Acro-osteolysis and calcinosis are independently associated with the late NVC pattern and particularly with severe capillary loss. Severe acro-osteolysis was more likely to occur with neoangiogenesis |
| Valenzuela A32 | 2016 | Retrospective cohort | 5218 | 13.5 | 38 | 62 | In multivariate analysis, the strongest associations with calcinosis were digital ulcers (OR=3.9) and osteoporosis (OR=4.2). |
| Avouac J33 | 2006 | Cross-sectional | 120 | 6 | 43 | 57 | Flexion contracture was associated with disability and occurred in dcSSc patients. Calcinosis and acro-osteolysis were both associated with vascular complications. |
| La Montagna G34 | 2002 | Retrospective cohort | 100 | 17.4 + 10.5 | 26 | 74 | Foot involvement in SSc has a later onset compared with hand involvement, and is relatively less frequent but can be disabling. |
| Taccari E35 | 1989 | Case report | 2 | – | 100 | 0 | Avascular osteonecrosis could be related to the osteoarticular progression of the longstanding SSc. |
| Frerix M36 | 2016 | Case series | 9 | 10.7 | 44 | 56 | Osteonecrosis of the lunate bone is a frequent and so far under-recognized manifestation of SSc which might be linked to SSc-related vasculopathy. |
| Matsumoto AK37 | 1999 | Case report | 3 | – | – | – | Osteonecrosis may represent an underrecognized cause of wrist pain in scleroderma patients. |
| La Montagna G38 | 1991 | Cross-sectional | 90 | 9 | 38 | 38 | Early menopause can play a role in the induction of osteopenia in SSc. |
| Rios-Fernández R39 | 2012 | Cross-sectional | 200 | 15.4±10.9 | 18 | 74 | The prevalence of osteopenia/osteoporosis was 50 % in the control population and 77 % in SSc. The study could not demonstrate a relationship of vitamin D deficiency with low mineral density. |
| Omair MA40 | 2014 | Cross-sectional | 45 | 7 | 40 | 60 | Low BMD was observed in 62 % of patients, 36 % of those had OP. Low BMD was associated with non-Caucasian race, postmenopausal status, low BMI. |
| Caimmi C41 | 2016 | Cross-sectional | 106 | 10 + 5.8 | 35 | 65 | The study found a positive correlation between BMI and total femur and whole body Z score and between age and neck femur Z score. Lung involvement was shown to correlate with a lower whole body Z score in multivariate analysis. |
| Frediani B42 | 2004 | Cross-sectional | 55 | 10.9 | 36 | 34 | BMD and SI were lower in women with dcSSc. BMD and SI were lower in women in whom one or more internal organs were involved. |
| Kilic G43 | 2016 | Cross-sectional | 43 | 10.9 + 7.1 | 42 | 46 | Patients with SSc had low BMI and low vitamin D levels compared to patients with RA. Total hip and femoral neck BMD and T score at the femoral neck and total hip were significantly lower in patients with SSc versus RA. |
| Avouac J44 | 2012 | Cross-sectional | 71 | 10 + 9 | 28 | 72 | The prevalence of OP and fracture in SSc was increased compared to healthy women and reached the high prevalence associated with RA. Age and vitamin D deficiency were identified as risk factors of fracture in SSc. |
| Mok CC45 | 2013 | Cross-sectional | 84 | 7.8 + 6.4 | 21 | 79 | BMD of the spine and hip is significantly lower in patients with SSc than in healthy subjects, which is independent of age, sex, menopause, low BMI and altered body composition. |
| Souza RBC46 | 2006 | Cross-sectional | 43 | 13.2 + 8.0 | 91 | 9 | A higher frequency of OP in the lumbar spine and femoral neck was observed in SSc patients when compared to controls. Multiple linear regression analysis revealed an association between the presence of SSc and low BMD. |
| Atteritano M47 | 2013 | Cross-sectional | 54 | 5 + 2 | 37 | 63 | Vertebral fractures are frequent in subjects with SSc, and suggest that lower levels of 25(OH)D3 may play a role in the risk of OP and vertebral fractures. |
| Omair MA48 | 2013 | Systematic Review | – | – | – | – | The data suggest that patients with SSc are at risk of low BMD and fracture, especially when other risk factors for OP are present. |
| Yacoub Y49 | 2012 | Cross-sectional | 60 | 9.63±5.9 | 85 | 15 | Morrocan patients had high frequency of OP comparing to healthy controls. Bone loss seems to be associated with prolonged disease duration, severe joint involvement, malabsorption syndrome and immunological status. |
| Sampaio-Barros PD50 | 2005 | Cross-sectional | 61 | – | 34 | 66 | A low BMD in appendicular sites was observed in fertile and postmenopausal SSc patients when compared to a control healthy group, associated to a low BMI. Low BMD was independent of the SSc clinical variants, race and previous use of corticosteroids and cyclophosphamide. |
| Koumakis E51 | 2015 | Cross-sectional | 65 | 10.2±8.6 | 32 | 68 | TBS was significantly lower in SSc compared to controls and did not differ from RA, despite lower cumulative and daily GC dose. A low TBS was independently associated with daily GC dose (OR 5.6) and a T score ≤ –2.5 SD (OR 5.0) in SSc. |
| Di Munno O52 | 1995 | Cross-sectional | 43 | 5.9 | 21 | 58 | The patients showed a lower BMD than the controls at the radial, lumbar, and total body determinations. Patients with dcSSc had lower values than those with lcSSc. There was a negative correlation between BMD and the duration of the disease. |
| Fauny M53 | 2018 | Cross-sectional | 70 | 10 | 23 | 77 | The VF prevalence on CT-scan was low (4.3 %) while 50 % of the patients presented a SBAC-L1≤145 HU. The presence of calcinosis, periarticular calcifications or acro-osteolysis was linked with low SBAC-L1 and should lead to an osteoporosis screening, especially for patients under 63 years old. |
| Ruaro B54 | 2018 | Cross-sectional | 84 | 9.8 + 7.9 | – | – | The study showed significantly lower bone quality (lower TBS and BMD) in SSc and RA patients compared with healthy subjects. The bone quality seemed lower in SSc patients with more altered microvasculature (late NVC pattern). |
| Marot M55 | 2015 | Cross-sectional | 33 | 9.5 + 8.4 | 9 | 79 | Patients with SSc had an increased prevalence of OP and HR-pQCT showed impaired trabecular bone compartment. Low BMI, high age, digital ulcers and ACAs were identified as independent risk factors for bone damage. |
| Polimeni M56 | 2012 | Cross-sectional | 78 | 5 | 21 | 63 | The presence of anti-CCP is associated with the presence of arthralgia; however, it is not linked to arthritis, presence of RF or radiological erosions. |
| Morita Y57 | 2008 | Cross-sectional | 159 | – | 28 | 72 | In patients with SSc-RA, serum anti-CCP levels were significantly higher than those seen in SSc. Sensitivity and specificity of anti-CCP for diagnosis of SSc-RA were 86 % and 97 %, respectively. |
| Santiago M58 | 2008 | Cross-sectional | 242 | – | 39 | 54 | The presence of anti-CCP in SSc was only correlated with the presence of arthritis. |
| Stamenković B59 | 2012 | Cross-sectional | 82 | – | 28 | 72 | There was a statistically significant association between positive anti-CCP and arthritis and the presence of marginal bone erosions. |
| Ingegnoli F60 | 2007 | Cross-sectional | 75 | – | 41 | 59 | Statistically significant association was found between anti-CCP positivity and the presence of arthritis and marginal erosions. |
| Ueda-Hayakawa I61 | 2010 | Cross-sectional | 146 | – | 41 | 59 | The serum titer of anti-CCP is higher in SSc–RA overlap patients than in SSc patients with or without arthralgia. |
| Kamalaksha S62 | 2018 | Prospective cohort | 132 | – | 25 | 67 | Statistically significant associations of double antibody positivity (anti-CCp and RF) with arthralgia and erosions were demonstrated. Significant association between anti-CCP and erosions was also confirmed. |
| Laustriat G63 | 2018 | Meta-analysis | 1231 | – | – | – | The prevalence of anti-CCP in scleroderma is 9.2 %. The study found an increased risk for erosive arthritis, pulmonary fibrosis, esophageal involvement and diffuse skin involvement in SSc patients with positive anti-CCP. |
| Serup J64 | 1986 | Prospective cohort | 117 | – | – | – | The study suggested that increased ANA and anti-DNA antibodies in localized scleroderma are associated with joint manifestations. |
| Foocharoen C65 | 2016 | Retrospective cohort | 294 | – | 69 | 31 | ATA positivity was associated with a high frequency of hand deformity (OR 7.01). ACA negativity was associated with a short duration of pulmonary fibrosis in dcSSc and a lower frequency of RP in lcSSc. |
| Radić M66 | 2006 | Cross-sectional | 28 | 3.3 | – | – | The patients with MPC and PIP joints flexion contractures had a higher mean value of ATA titers than those with no contractures. The titers of ATA positively correlated with flexion contractures. |
| Gheita TA67 | 2012 | Cross-sectional | 40 | 6.1 | 48 | 52 | The level of COMP was higher in females and significantly higher in the SSc patients with arthritis compared to those without. |
| Yanaba K68 | 2012 | Cross-sectional | 57 | 3.6 | 51 | 49 | Serum PAF-AH levels were increased in patients with SSc and associated with a lower frequency of pitting scars/digital ulcers and arthritis/arthralgias. |
| La Montagna G69 | 2003 | Cross-sectional | 40 | 12.3±9.5 | 22 | 32 | YKL-40 serum levels are increased in SSc and they are correlated with joint involvement. |
| Bassett LW70 | 1981 | Cross-sectional | 55 | 11 | – | – | The presence of a nonrheumatoid erosive arthropathy was found in 22 % of SSc patients. |
| Blocka KL71 | 1981 | Prospective cohort | 65 | 14.5 | 85 | 15 | All PSS findings showed progression, although isolated reversibility was noted. The CREST group showed a similar frequency and distribution of findings but with less tendency to progression. |
| Izquierdo YE72 | 2016 | Systematic review with meta-analysis | – | – | – | – | The prevalence of DPTH resorption was 28.3 %, I2=80.4 %. The prevalence of calcinosis was 15.6 %, I2=0 %. No study reported proliferation or erosions and only one study described sclerosis of DPTH in 5 individuals. |
| Freire V73 | 2013 | Cross-sectional | 44 | – | – | – | Synovitis was found in 39 % of patients, tenosynovitis in 27 %, and it had a layered pattern in 41 % of cases. Calcinosis was found in 39 % of patients with US, with a sensitivity of 89 %. Acroosteolysis was found in 20 % of patients with US with 90 % sensitivity. |
| Chitale S74 | 2010 | Cross-sectional | 17 | – | 18 | 82 | The study found a persistent inflammatory, erosive, peripheral arthropathy, similar to that seen in RA, in SSc patients with arthralgia without overt inflammatory joint disease. MRI is more sensitive than US in this setting. |
| Abdel-Magied RA75 | 2013 | Prospective cohort | 16 | 5.4 | 31 | 69 | SSc patients with arthralgia who have no obvious clinical inflammatory arthritis were found to have persistent inflammatory erosive arthropathy in their hands and wrists using US and MRI. |
| Cuomo G76 | 2009 | Cross-sectional | 45 | 6 | 38 | 62 | The prevalence of synovitis as detected by US was found to be significantly higher than that found by clinical examination. US indicated a significantly higher number of joints with osteophytes than X-rays. |
| Elhai M77 | 2012 | Cross-sectional | 52 | 8.6 + 8.6 | 40 | 60 | Joint involvement in SSc is underestimated by a single clinical examination. It is characterized by mild inflammatory changes and the specific findings include sclerotic US aspects together with calcinosis. |
| Kilic E78 | 2015 | Cross-sectional | 52 | 9.34 + 8.27 | 48 | 42 | Patients with SSc had significantly higher MASEI scores than the healthy controls. Except for plantar aponeurosis, the tendons and ligaments were thicker in the SSc group. |
| Iagnocco A79 | 2013 | Cross-sectional | 46 | 11.3 | 39 | 61 | Study focused on hand and wrist joints PDUS assessment of inflammatory and structural abnormalities in SSc. The wrists were more frequently the site of inflammatory findings. The presence of synovial hypertrophy, joint effusion, cortical irregularities, and TS was significantly higher in SSc patients than in healthy controls. |
| Lescoat A80 | 2018 | Cross-sectional | 103 | 7.6 | 33 | 67 | Patients fulfilling the 2013 ACR/EULAR classification criteria for SSc. The two main PDUS features were Doppler-positive/inflammatory synovitis and sclerosing TS. Sclerosing TS was more frequent in men and was associated with anti-RNA polymerase III antibodies, dcSSc, and inflammatory arthralgia. Inflammatory TS or synovitis were associated with CRP levels >5mg/l, and inflammatory arthralgia. |
| Akbayrak E81 | 2013 | Cross-sectional | 26 | 7.9 + 5.3 | 23 | 77 | Erosions, bone marrow edema, synovitis, and joint effusions on low-field MRI are frequent in patients with pure SSc. All bones and joints could be affected, but synovitis and bone marrow edema occurred predominantly in the proximal row of carpal bones, most frequently affecting the lunate bone. |
| Low AHL82 | 2009 | Cross-sectional | 17 | – | 59 | 41 | Inflammatory MRI findings were found in 59 % of patients including synovitis, erosions, joint effusion and TS. |
| Tehranzadeh J83 | 2006 | Cross-sectional | 72 | – | – | – | Enhanced MR imaging of the hand and wrist is a superior technique for detection of TS. |
| Stoenoiu MS84 | 2013 | Cross-sectional | 15 | 7.5 | 100 | 0 | No clinically overt arthritis or tenosynovitis was detected. Synovitis, TS and tendon tear were identified in 8, 4 and 2 patients, respectively, by both US and MRI. Using MRI, deep connective tissue infiltrates surrounding tendons were present in all sites with TFRs but in only one patient without TFRs. |
| Stamm T85 | 2014 | Cross-sectional with qualitative analysis | 229 | 10 | – | – | A large number of similar problems are mentioned as ‘typical’ by patients with different rheumatic conditions. |
| Malcarne VL86 | 2007 | Cross-sectional | 114 | 4.3 + 2.9 | 64 | 36 | For disability, significant correlates were physician-determined joint tenderness and number of tender points, and patient-reported joint pain on motion, joint contracture, extremity ulcers other than digital, and dyspnea. |
| Willems LM87 | 2014 | Cross-sectional | 537 | 8 | 36 | 55 | Symptoms experienced by ≥70 % of patients in all countries were fatigue, RP, joint pain, and muscle pain. Thirty symptoms had an impact on daily activities in ≥50 % of patients who reported that the symptom was present in all countries. |
| Hudson M88 | 2011 | Cross-sectional | 464 | 10.9 + 9.4 | 13 | 36 | The highest rated symptoms in terms of frequency and moderate to severe impact on daily activities, respectively, were: fatigue (89 and 72 %), RP (86 and 67 %), hand stiffness (81 and 59 %), joint pain (81 and 64 %) and difficulty sleeping (76 and 59 %). |
| Kwakkenbos L89 | 2017 | Prospective cohort | 696 | 11.6 + 8.7 | 42 | 57 | Presence of joint contractures was associated with significant decrements in seven domains (small or small to moderate effect sizes). This study further establishes the validity of the PROMIS-29v2 in SSc. |
| Kwakkenbos L90 | 2018 | Prospective cohort | 1193 | 11.2 + 8.7 | 40 | 60 | Hand function was measured using the CHFS. The presence of moderate or severe small joint contractures, the presence of digital ulcers, and severity of RP had the largest associations with impaired hand function. |
| Hyphantis TN91 | 2007 | Cross-sectional | 56 | 15.5 + 12.2 | 30 | 70 | WHOQOL-BREF was used to assess HRQOL. HRQOL perceived by SSc patients was significantly impaired compared with healthy controls. SSc patients had impaired physical health quality of life in comparison with RA, SLE, and SS patients when age, pain, psychopathology, and coping strategies were taken into account. |
| Cole JC92 | 2006 | Validation study using confirmatory factor analysis | 387 | 2.2 | 84 | 16 | A single-factor HAQ-DI is appropriate for trials in SSc and, in addition, HAQ-DI scores among patients with SSc and early RA can be compared legitimately with one another. |
| Johnson SR93 | 2006 | Case-control | 350 | 7.1 + 5.9 | 49 | 51 | SSc patients with joint involvement had significantly poorer HAQ-DI scores than patients with PsA, and had higher visual analog scale pain scores than RA patients. The SF-36 Physical Component Summary and HAQ-DI score in SSc patients were adversely affected by joint involvement and ≥ 11 tender points. |
| Peytrignet S94 | 2018 | Prospective cohort | 326 | 1 | 100 | 0 | Patients with dcSSc displayed high levels of disability, with ‘grip’ and ‘activity’ being most affected. Of the activities assessed in the CHFS, those involving fine finger movements were most affected. HAQ-DI and CHFS were highly correlated. Worsening HAQ-DI over 12 months was strongly associated with increasing MRSS, decreasing hand function and increasing fatigue. |
| Sandqvist G95 | 2009 | Prospective cohort | 43 | 2 | 28 | 72 | Hand mobility and activities of daily life capacity were maintained during the first years of SSc. At the individual level 72 % of the patients showed a change in HAMIS score. Grip force and perceived hand function were moderately impaired at baseline and during the follow-up. |
| Cinar FI96 | 2014 | Cross-sectional with qualitative analysis | 19 | 12 | 47 | 53 | Impaired hand function affects the daily life activities of patients with SSc, and patients have developed some coping strategies to overcome these difficulties. |
| Torok KS97 | 2010 | Prospective cohort | 80* | 5.8±5.6 + | 81 | 19 | The delta FTP is a valid and reliable measure of finger motion in patients with SSc which outperforms the FTP. |
| Schouffoer AA98 | 2016 | Randomized, controlled trial | 53 | 9.2 | 57 | 43 | Significant correlations were seen between the MHQ total score and the HAQ, HAMIS, SODA, SODA Pain and MRSS. The effect size of the MHQ total score within the intervention was larger than that of all other outcome measures. Similar results were obtained for the standardized response mean. |
| Roberts-Thomson AJ99 | 2006 | Cross-sectional | 30 | 23.6 ¥ | 40 | 60 | The HAI was confirmed as a reliable measure which clearly distinguished patients with increasing hand deformity. The HAI correlated significantly with measures of global functional impairment, hand strength, and prehensile gripability. |
| Erol K100 | 2018 | Cross-sectional | 101 | 10.1 + 7.1 | 42 | 58 | DHI revealed similar functional loss and correlated with various measurements related to HRQOL in patients with SSc and RA. In SSc, hand span, grip strength and MRSS had major influences on hand functions. |
| Poole JL101 | 2013 | Retrospective cohort | 156 | 12.2 + 9.7 | – | – | The SSc and RA participants had weaker pinch, decreased joint motion and more activity limitations than the DMII and OA groups. There were no significant differences between the groups for right hand grip strength and pegboard dexterity, and applied dexterity. |
| Lóránd V102 | 2016 | Prospective cohort | 77 | 10.5 + 9.5 | 65 | 35 | DAS28-ESR, DAS28-CRP, SDAI and CDAI showed significant correlation with EScSG-AI, HAQ-DI, CHFS and the physical component of SF-36. With the exception of DAS28-CRP, the other three indices also discriminated between subgroups of SSc based on the value of EScSG-AI. DAS28-ESR showed the best performance regarding reliability and construct validity. |
| Choi HJ103 | 2017 | Cross-sectional | 835 | – | – | – | The prevalence of FM in patients with rheumatologic diseases was found to be between 1.4 % and 25 %. |
| Overman CL104 | 2016 | Cross-sectional | 6120 | – | – | – | The prevalence of severe fatigue in SSc patients was 48 %. Severe fatigue was associated with having fibromyalgia, having multiple rheumatic diseases without fibromyalgia, younger age, and lower education level. |
| Thombs BD105 | 2008 | Systematic review | 106 | 11.9 + 7.9 | 26 | 74 | GFI scores in SSc were significantly higher than in two large population samples and than in two samples of cancer patients in remission. SSc GFI scores were similar to scores from patients with RA, AS and SLE, and to scores from six studies of cancer patients in active treatment. |
| Bruni C106 | 2017 | Case-control | 20 | – | – | – | In SSc patients with joint involvement after mean 10.8±2.1 months of hydroxychloroquine treatment, global health was statistically significantly improved, with a trend for improvement in synovitis on US. |
| Nacci F107 | 2007 | Pilot study | 7 | 3.6 + 2.5 | 29 | 71 | After 6 months of intravenous immunoglobulins therapy, joint pain and tenderness decreased significantly, and hand function improved significantly, together with the quality of life. |
| Omair M108 | 2011 | Retrospective cohort | 10 | 24 | 60 | 40 | At 12 months, the median swollen joint count and tender joint count significantly decreased from 10 to 0, and 15 to 3, respectively. The median pain score decreased from 6 to 3.5. The median skin score remained unchanged at 6 months. One patient developed uncomplicated herpes zoster. After 30 months, 3 patients (30 %) developed malignancy. No death or other adverse events were observed. |
| Distler JHW109 | 2011 | Expert consensus | – | – | – | – | Most of the experts do not recommend the routine use of TNF-α antagonists in SSc. Arthritis might be a potential indication in SSc, although controlled clinical trials with TNF-α antagonists are needed before general recommendations can be given. |
| Elhai M110 | 2013 | Prospective cohort | 20 | 5 - 13¶ | 60 | 40 | After 5 months, tocilizumab induced a significant improvement in the DAS-28 and its components, with 10/15 patients achieving a EULAR good response. After 11 months’ treatment of patients with abatacept, joint parameters improved significantly, with 6/11 patients fulfilling EULAR good-response criteria. |
| Allanore Y111 | ND | Randomized, controlled trial | 22 | – | – | – | In progress. |
| DeLea SL112 | 2011 | Prospective cohort | 12 | – | – | – | SSc patients responded to injection in 83.3 % of cases. The therapeutic duration was 4.0±2.2 months. Reduced Raynaud’s attacks and healing of digital ulcers occurred in 83 % of subjects. |
| Seeger MW113 | 1987 | Prospective cohort | 19 | 2 | – | – | There was no evidence that the use of splints served to maintain PIP extension when compared with the control hand. |
| Norris RW114 | 1985 | Series of cases | 6 | 11 | – | – | There was 20 arthroplasties performed in this series. Hand function was assessed in 8 patients and in 6 of them there was improved qualitative function. The post-operative complication rate was low. |
| Bogoch E115 | 2005 | Systematic review | – | – | – | – | The goals of surgery for advanced SSc affecting the hand are limited and include pain relief through sympathectomy and increased perfusion, repositioning the digit, providing a functional position of fusion, and modest mobilization through resection arthroplasty |
| Gilbart MK116 | 2004 | Series of cases | 10 | – | – | – | The results of hand surgery for SSc are reliable, but goals must be limited and patient expectations should be modest. |
*Divided into three groups of 39 - 17 - 24 patients, according to the study phase. + In validation phase. ¥ Mean duration of lcSSc. ¶ Mean duration from patients treated with tocilizumab and abatacept respectively. (-) No data.
TFR (Tendon friction rubs), JC (Joint contractures), SSc (Systemic sclerosis), dcSSc (difusse SSc), lcSSc (Limited SSc), RF (rheumatoid factor), MRSS (modified Rodnan skin score), RA (rheumatoid arthritis), SLE (systemic lupus erythematosus), SS (Sjögren syndrome), PsA (psoriatic arthritis), DMII (Diabetes mellitus type 2), OA (osteoarthritis), FM (Fibromyalgia), AS (ankylosing spondylitis), DTR (distal tuft resorption), CP (Contracture of phalanges), FTP (finger-to-palm), MSC (Medsger severity scale), BMD (bone mineral density), BMI (body mass index), SI (Stiffness Index), OP (osteoporosis), TBS (Trabecular bone score), GC (Glucocorticoid), HU (Hounsfield Units), SBAC-L1 (scanographic bone attenuation coefficient of the first lumbar vertebra), NVC (nailfold videocapillaroscopy), HR-pQCT (High-Resolution peripheral Quantitative Computed Tomography), anti-CCP (cyclic citrullinated peptide antibodies), SSc-RA (Overlap SSc with RA), ANA (Antinuclear antibodies), ATA (Anti-topoisomerase I antibody), RP (Raynaud’s phenomenon), ACA (Anticentromere antibodies), MCP (metacarpophalangeal), PIP (proximal interphalangeal), COMP (Cartilage oligomeric matrix protein), PAF-AH (Platelet-activating factor acetylhydrolase), PPS (progressive systemic sclerosis), DPTH (distal phalanx tuft of the hand), US (ultrasonography), MRI (magnetic resonance imaging), MASEI (Madrid Sonography Enthesitis Index), PDUS (power Doppler ultrasound), TS (tenosynovitis), PROMIS (Patient-Reported Outcomes Measurement Information System), CHFS (Cochin Hand Function Scale), HRQOL (health-related quality of life), WHOQOL-BREF (World Health Organization Quality of Life Instrument, Short-Form), HAQ-DI (Health Assessment Questionnaire-Disability Index), SF-36 (Short Form-36 Health Survey), HAMIS (Hand Mobility in Scleroderma), MHQ (Michigan Hand Questionnaire), SODA (Sequential Occupational Dexterity Assessment), HAI (hand anatomic index), DHI (Duruöz Hand Index), DAS28-ESR (Disease Activity Score 28 using ESR), DAS28-CRP (Disease Activity Score 28 using CRP), SDAI (Simplified Disease Activity Index), CDAI (Clinical Disease Activity Index), EScSG-AI (European Scleroderma Study Group Activity Index), GFI (General Fatigue Index).
SSc can have different levels of OAM affecting directly articular units or their adjacent tissue (Table 2). OAM are more frequently seen in the diffuse variety of SSc and their development is related to severity biomarkers of vascular, muscular and pulmonary compromise.2,4
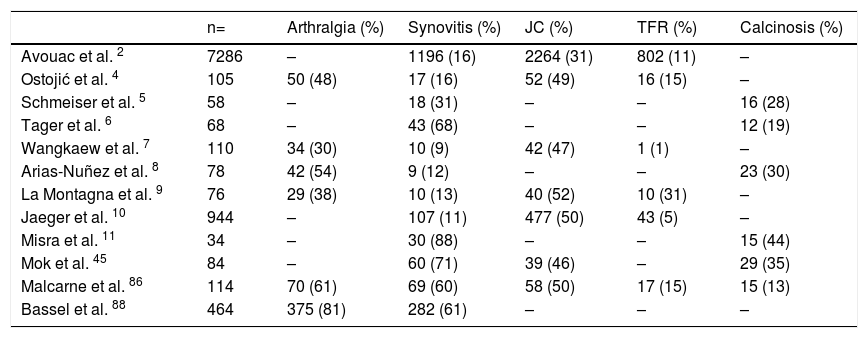
Prevalence of OAM in SSc.
| n= | Arthralgia (%) | Synovitis (%) | JC (%) | TFR (%) | Calcinosis (%) | |
|---|---|---|---|---|---|---|
| Avouac et al. 2 | 7286 | – | 1196 (16) | 2264 (31) | 802 (11) | – |
| Ostojić et al. 4 | 105 | 50 (48) | 17 (16) | 52 (49) | 16 (15) | – |
| Schmeiser et al. 5 | 58 | – | 18 (31) | – | – | 16 (28) |
| Tager et al. 6 | 68 | – | 43 (68) | – | – | 12 (19) |
| Wangkaew et al. 7 | 110 | 34 (30) | 10 (9) | 42 (47) | 1 (1) | – |
| Arias-Nuñez et al. 8 | 78 | 42 (54) | 9 (12) | – | – | 23 (30) |
| La Montagna et al. 9 | 76 | 29 (38) | 10 (13) | 40 (52) | 10 (31) | – |
| Jaeger et al. 10 | 944 | – | 107 (11) | 477 (50) | 43 (5) | – |
| Misra et al. 11 | 34 | – | 30 (88) | – | – | 15 (44) |
| Mok et al. 45 | 84 | – | 60 (71) | 39 (46) | – | 29 (35) |
| Malcarne et al. 86 | 114 | 70 (61) | 69 (60) | 58 (50) | 17 (15) | 15 (13) |
| Bassel et al. 88 | 464 | 375 (81) | 282 (61) | – | – | – |
(-) No data.
OAM Osteoarticular manifestations, JC Joint contracture, TFR Tendon friction rub, ND No data.
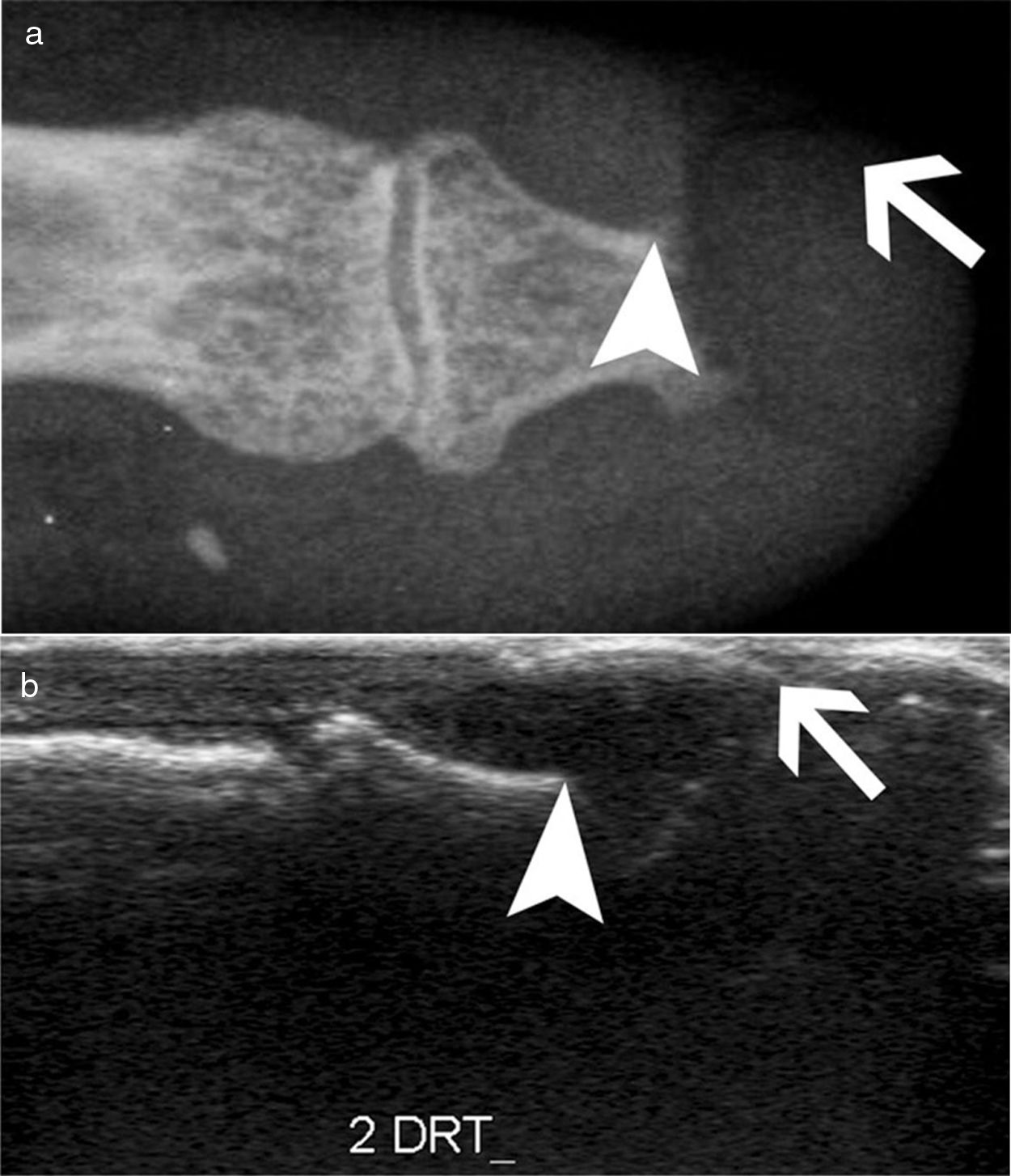
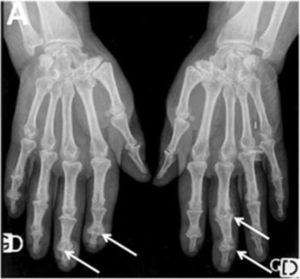
Arthritis and synovitis are frequent manifestations during the course of the disease. Literature reports 9–70 % of OAM among patients with similar early and late disease stages2,4–11 (Fig. 2). The most frequent manifestation is mild arthritis with small and discrete erosions.11–13 However, the presence of erosive arthritis similar to that seen in rheumatoid arthritis (RA) patients has been reported (Fig. 3); which opens the debate regarding the presence of a clinical overlap between RA and SSc or an isolated manifestation of the latter.11,14,15 Overlap is highly suspected in patients who develop articular manifestations and Raynaud's phenomenon.16 Monoarthritis, oligoarthritis or polyarticular involvement17 are more frequently seen in diffuse SSc compared to the limited variety,2 although some studies report that deforming arthritis manifestations are more prevalent in SSc limited variety.11 OAM are also related to acute phase reactants with an OR of 2.1 (95 % CI 1.67–2.64).2 Furthermore, the presence of arthritis is a predictive factor for: general disease progression, reduced left ventricular function, development of digital ulcers and worsening in Rodnan score18; thus making it a highly valuable severity feature to evaluate in all SSc patients.18 Sacroiliac joint manifestations are not unusual, and the presence of high C-reactive protein (CRP) level or back pain should alert the clinician for the possibility of sacroiliitis.19
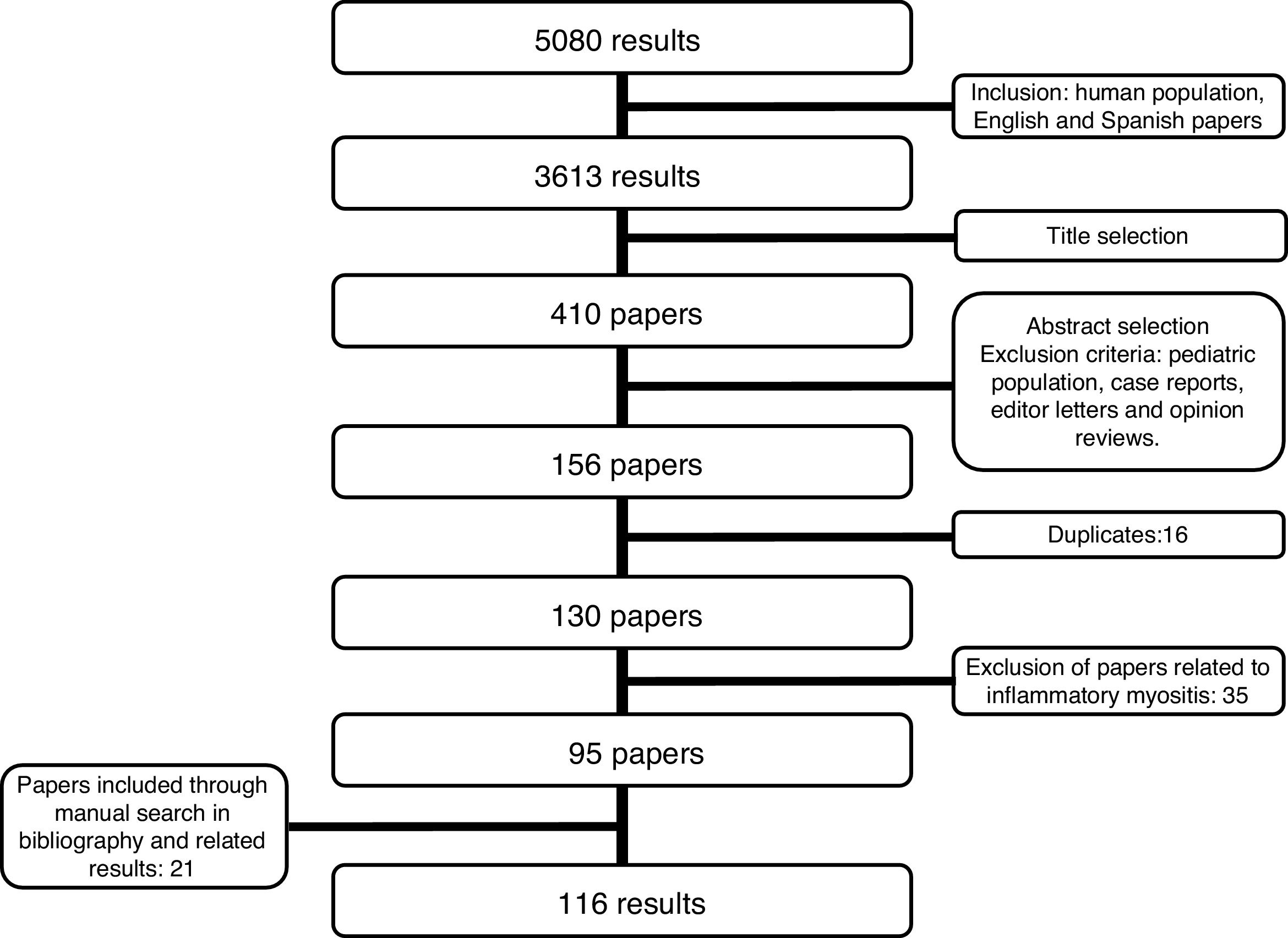
Detection of inflammatory joint and tendon involvement with MRI (arrows). Adapted (with permission) from Avouac et al.1
Erosive radiographic arthritis characterized by erosions and joint space narrowing involving the PIP and DIP joints (arrows). Adapted (with permission) from: Avouac et al.1
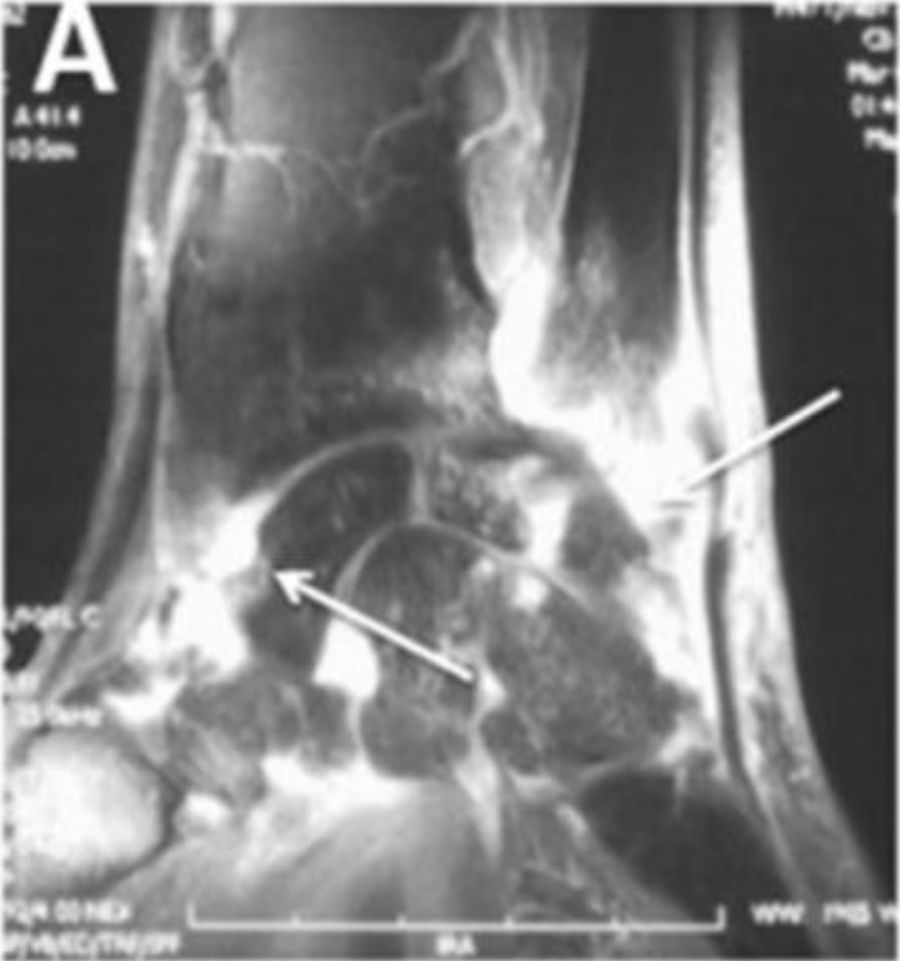
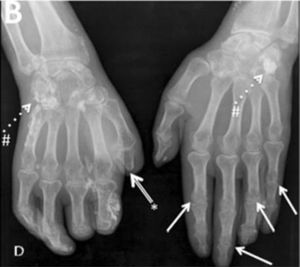
Joint contractures are very frequent during the course of the disease with a prevalence of 30–80 % in all patients throughout all disease stages.2,4,9,10,20–22 The articular units involved are mainly the first and second metacarpophalangeal (MCP) joints with damage described in up to 82 % of patients with joint contracture. The involvement of wrists, shoulders and second and third proximal interphalangeal (PIP) joints is usually described20 (Fig. 4), while the hips, knees and elbows are seldom involved. Patients with: diffuse SSc, positive titers for anti-topoisomerase I, and pulmonary, cardiac and esophageal involvement are more prone to joint contractures.2,7,20,21 Additionally, the diffuse SSc variety is an independent predictor for contracture progression.23 Also, it has been described that patients with more than four joints involved per hand have a lower survival rate, thus, articular contractures can be interpreted as a disease severity marker.20
Flexion contracture involving the hands. Adapted (with permission) from Tas et al.27
TFR also appear in patients with SSc with a reported prevalence of 5–37 %, ocurring more often in patients with diffuse SSc.2,4,10,22,24 TFR manifest mainly in hands, elbows and ankles.24,25 They are found more frequently in young patients and during early stages of the disease (OR 2.58, 95 % CI 1.87–3.56), and their presence is related to a progression of the disease, and other severe manifestations such as pulmonary, renal and vascular compromise.2,18,24,26 Additionally, patients with early diffuse SSc who develop TFR have a lower five to ten-year survival, and more severe compromise of their life quality.24
In general, synovitis, articular contractures and TFR manifest together, thus, the presence of any one of the OAM is a risk factor for the development of other osteoarticular involvement.2
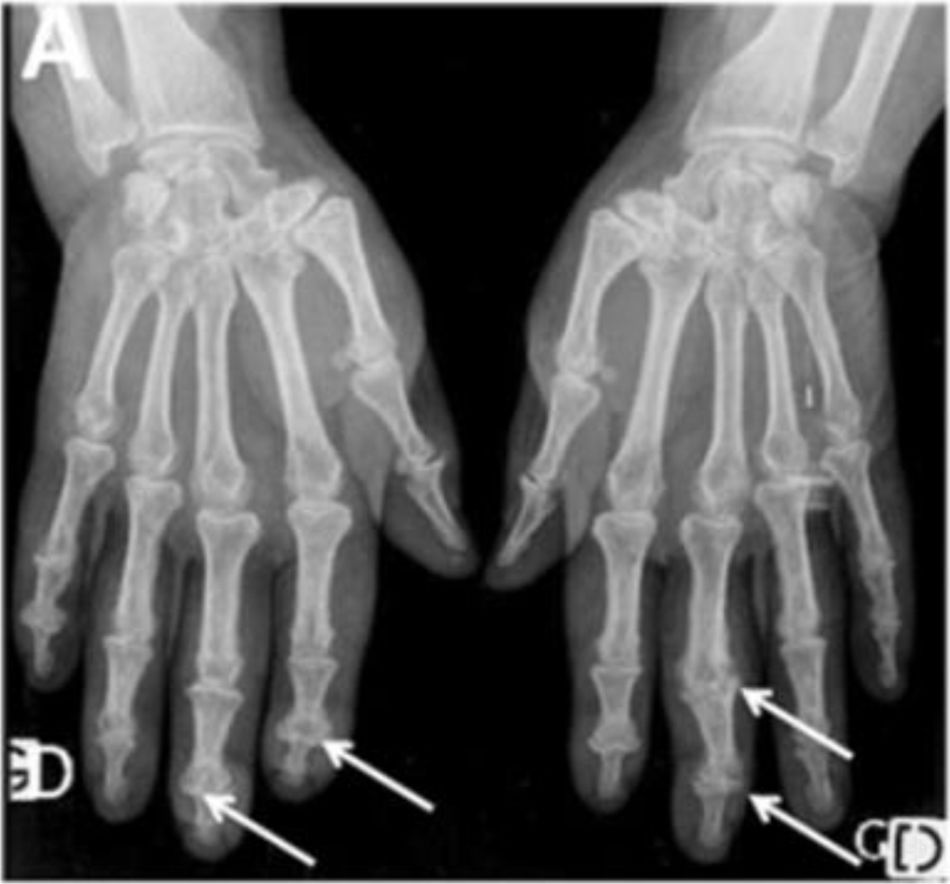
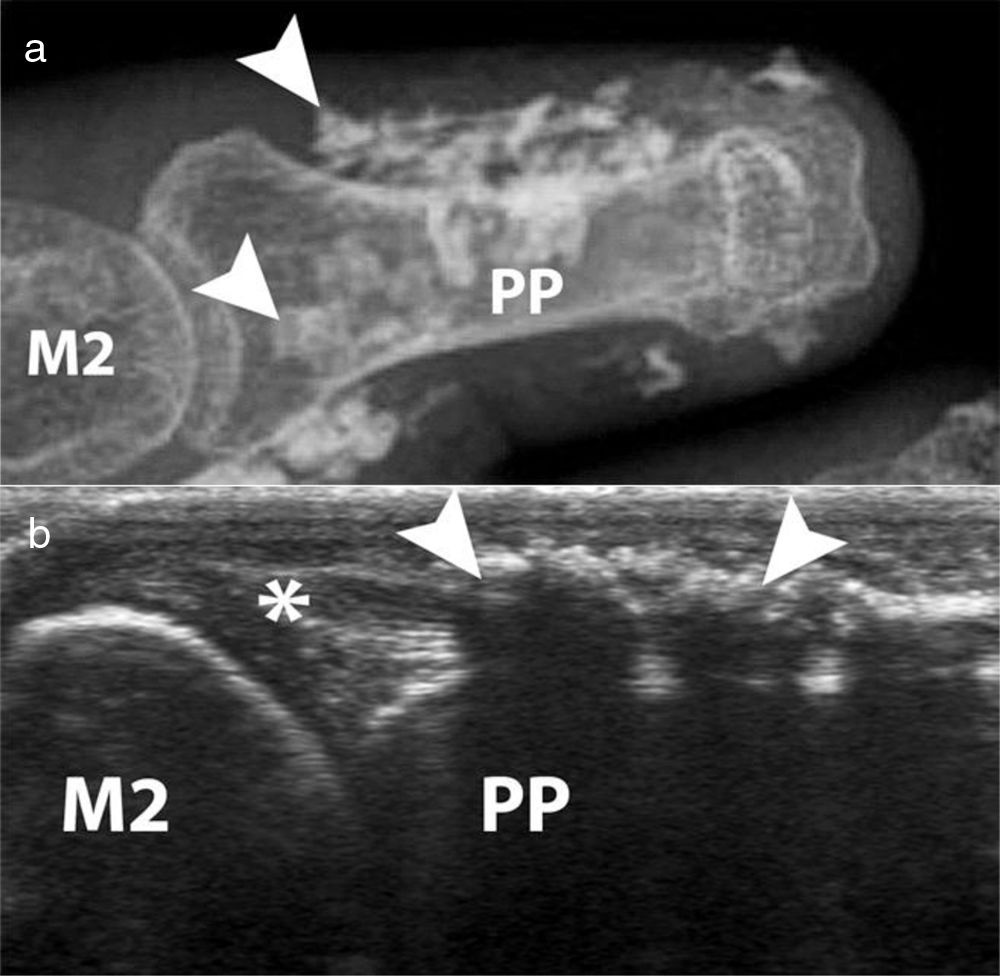
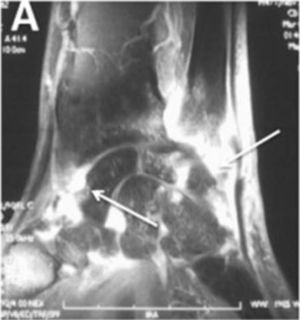
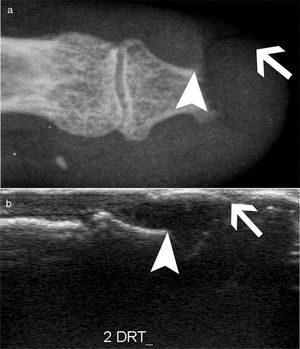
AcroosteolysisDistal phalanges bone resorption, or acroosteolysis, is another usual OAM, seen in up to 25 % of patients27 (Fig. 5). Some studies have described this manifestation related to more severe and systemic compromise of SSc.28 Furthermore, acroosteolysis is associated with digital ischemia severity,29,30 as well as with the presence of advanced videocapillaroscopy patterns, particularly capillary loss and angiogenesis.31 Similarly, the presence of digital ulcers is an independent predictor for acroosteolysis progression.23 Thus, distal bone resorption may correspond to an ischemic manifestation of SSc.
Acroosteolysis of the index finger in a SSc patient. a. plain radiograph imaging. b. ultrasonography imaging. Adapted (with permission) from: Freire et al.73
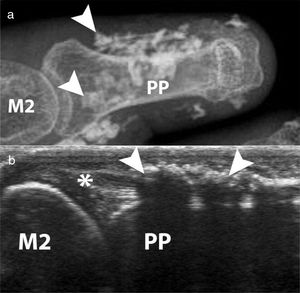
Calcinosis, which has been described in up to 25 % of patients, is more frequently described in older patients and is related to the presence of osteoporosis and digital ulcers32,33 (Fig. 6). Additionally, digital ulcers are an independent progression factor for calcinosis.23 Some studies suggest a possible role for vascular damage in the pathogenesis of calcinosis since it is associated to advanced videocapillaroscopy patterns and digital ulcers.31
Calcinosis of the index finger in a SSc patient. a. plain radiograph imaging. b. ultrasonography imaging. Adapted (with permission) from: Freire et al.73
Osteoarticular manifestations in the feet tend to appear later in the disease course and cause severe disability. At the time of SSc diagnosis, articular contractures, TFR and acroosteolysis involvement of the feet are far less common than these of the hands; nonetheless, feet arthralgia might be a common complaint.34 Moreover, feet involvement worsens during the course of the disease unlike hand involvement which tends to remain steady once it is manifested.34
Avascular necrosisAvascular necrosis in the femoral head has been reported as an infrequent OAM, although severe, in SSc patients. In early stages of the disease the patients may be asymptomatic. However, as it progresses the cardinal symptom is pain in the groin, thigh and buttocks.35 Some rare cases involving the carpal bones have been reported, yet necrosis of the lunate bone might be underestimated and frequently misinterpreted as carpitis (erosive arthritis in the wrist).36,37
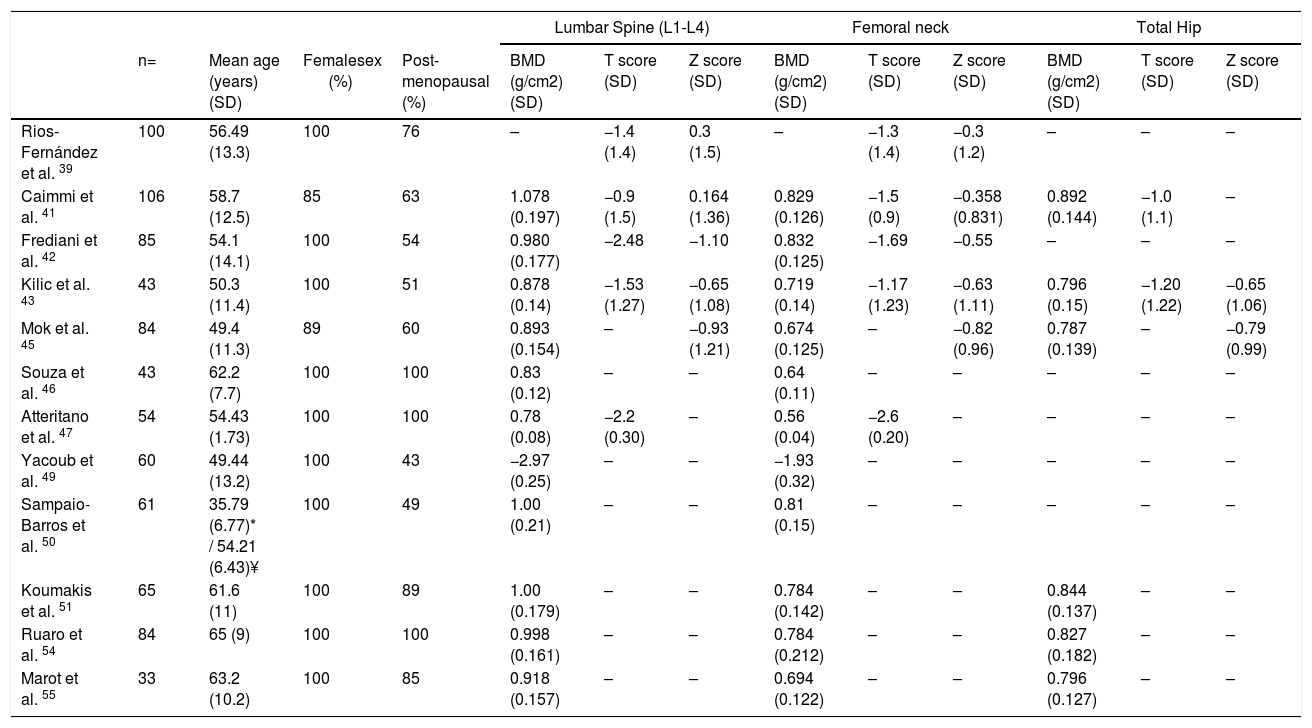
Bone mineral density in SScSSc patients have multiple known risk factors for the development of osteoporosis, i.e., chronic inflammation, steroids use, immobility and early menopause.38 Low bone mineral density (BMD) has been reported in around 27 %–62 % of SSc patients,38–45 while osteoporosis has been reported varying between 10 %–51 % of patients depending on the studied populations41–48 (Table 3). In SSc patients low BMD is more frequently seen in the femoral neck compared to the lumbar spine (0.829 + 0.126mg/cm2 vs. 1.078 + 0.197mg/cm2).41 The change in BMD seems to be influenced by the lower body mass index reported in this patients group compared to normal population.41,42,46
Values of BMD, T scores and Z scores of patients with SSc.
| Lumbar Spine (L1-L4) | Femoral neck | Total Hip | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n= | Mean age (years) (SD) | Femalesex (%) | Post-menopausal (%) | BMD (g/cm2) (SD) | T score (SD) | Z score (SD) | BMD (g/cm2) (SD) | T score (SD) | Z score (SD) | BMD (g/cm2) (SD) | T score (SD) | Z score (SD) | |
| Rios-Fernández et al. 39 | 100 | 56.49 (13.3) | 100 | 76 | – | −1.4 (1.4) | 0.3 (1.5) | – | −1.3 (1.4) | −0.3 (1.2) | – | – | – |
| Caimmi et al. 41 | 106 | 58.7 (12.5) | 85 | 63 | 1.078 (0.197) | −0.9 (1.5) | 0.164 (1.36) | 0.829 (0.126) | −1.5 (0.9) | −0.358 (0.831) | 0.892 (0.144) | −1.0 (1.1) | – |
| Frediani et al. 42 | 85 | 54.1 (14.1) | 100 | 54 | 0.980 (0.177) | −2.48 | −1.10 | 0.832 (0.125) | −1.69 | −0.55 | – | – | – |
| Kilic et al. 43 | 43 | 50.3 (11.4) | 100 | 51 | 0.878 (0.14) | −1.53 (1.27) | −0.65 (1.08) | 0.719 (0.14) | −1.17 (1.23) | −0.63 (1.11) | 0.796 (0.15) | −1.20 (1.22) | −0.65 (1.06) |
| Mok et al. 45 | 84 | 49.4 (11.3) | 89 | 60 | 0.893 (0.154) | – | −0.93 (1.21) | 0.674 (0.125) | – | −0.82 (0.96) | 0.787 (0.139) | – | −0.79 (0.99) |
| Souza et al. 46 | 43 | 62.2 (7.7) | 100 | 100 | 0.83 (0.12) | – | – | 0.64 (0.11) | – | – | – | – | – |
| Atteritano et al. 47 | 54 | 54.43 (1.73) | 100 | 100 | 0.78 (0.08) | −2.2 (0.30) | – | 0.56 (0.04) | −2.6 (0.20) | – | – | – | – |
| Yacoub et al. 49 | 60 | 49.44 (13.2) | 100 | 43 | −2.97 (0.25) | – | – | −1.93 (0.32) | – | – | – | – | – |
| Sampaio-Barros et al. 50 | 61 | 35.79 (6.77)* / 54.21 (6.43)¥ | 100 | 49 | 1.00 (0.21) | – | – | 0.81 (0.15) | – | – | – | – | – |
| Koumakis et al. 51 | 65 | 61.6 (11) | 100 | 89 | 1.00 (0.179) | – | – | 0.784 (0.142) | – | – | 0.844 (0.137) | – | – |
| Ruaro et al. 54 | 84 | 65 (9) | 100 | 100 | 0.998 (0.161) | – | – | 0.784 (0.212) | – | – | 0.827 (0.182) | – | – |
| Marot et al. 55 | 33 | 63.2 (10.2) | 100 | 85 | 0.918 (0.157) | – | – | 0.694 (0.122) | – | – | 0.796 (0.127) | – | – |
(-) No data.
*From fertile group.
¥ From postmenopausal group.
BMD Bone mineral density, SD Standard deviation.
In a study in Moroccan population by Yacoub et al. the SSc population had a lower BMD, as well as lower vitamin D levels compared to healthy controls. In addition, there was an association between a low BMD and low vitamin D levels with the presence of severe joint involvement.49 Similarly, in two Spanish populations the presence of SSc and the longer duration of the disease were associated with a lower BMD when compared with a group of healthy women. However, vitamin D deficiency was not associated with lower BMD.39
Similar results were found in the femoral neck bone density in a group of 43 patients with SSc when compared with matched controls with RA43; however, a previous study with a larger number of patients did not find this difference.44 The cumulative dose of steroids is not a risk factor for the development of osteoporosis or fractures in SSc when evaluated with bone densitometry44,50; although patients receiving doses of prednisone of 5mg or more per day have an altered bone microarchitecture when evaluated by tomography.51 In a prospective study of 50 Canadian SSc patients, those who were non-Caucasian had a lower BMD than Caucasians.40 The presence of calcinosis seems also to influence the appearance of osteoporosis32; nonetheless, this finding is not reproducible in all series.52 A low BMD in SSc patients is not associated with the severity of involvement of other organs.45
The presence of fragility fractures is not negligible. Omair and colleagues reported fragility fractures in up to 14 % of patients.40 In a previous study, up to 24 % of patients had a documented vertebral fracture, with a predominance of grade 1 fractures in the Genant classification.47 Avouac et al. found that 35 % had fragility fractures in a cohort of 71 patients with SSc, the number of fractures was similar to that found in patients with RA, and was higher than the reported in other SSc cohorts, probably due to the older age of patients in this cohort.44
Fauny et al. found that despite the increased risk of bone mineral density alteration, only 43 % of SSc patients are screened with Dual Energy X-ray Absorptiometry (DEXA).53 In addition, there are complementary strategies applied to tomography that allow to determine fracture risk in SSc patients, which seem to be more sensitive than DEXA in this specific population.53 The Trabecular Bone Score (TBS), a recent tool to evaluate bone microarchitecture, has been used in patients with SSc with good results.51,54 Ruaro et al. reported TBS as capable of discriminating patients with altered bone microstructure that increases the risk for fracture.54 In addition, TBS appears to be more altered in patients with early or active videocapillaroscopy patterns, suggesting a correlation between microvascular damage and bone involvement.54 In a previous study by Marot et al. an important compromise of trabecular bone was found, which seems to be related to the presence of digital ulcers.55 Bone microstructural characterization through TBS opens the possibility to its potential use as a complementary diagnostic tool for bone involvement secondary to corticosteroids, thus TBS could be used with DEXA to detect osteoporosis induced by corticosteroids.51
Serological markersAnti-cyclic citrullinated peptide antibodies (anti-CCP)Several studies have looked at the relationship of anti-CCP with the development of joint manifestations in SSc. Anti-CCP positivity is reported in between 3 %–14 % of SSc patients according to the test generation and the cut-off point used.56–59 In Latin American population anti-CCP positivity has been described in up to 12 % of cases with SSc, however, their association with arthritis in this demographic group is not completely clear.56 Due to the high specificity of these antibodies for RA diagnosis, there is controversy regarding the usefulness of anti-CCP to diagnose the overlap of SSc with RA (SSc-RA). Santiago et al. found a significant association between positive titers of anti-CCP and the onset of arthritis, although there was no statistically significant association between the presence of these antibodies and the diagnosis of SSc-RA58; unlike other studies where anti-CCP were considered useful for identifying SSc-RA patients.57,60,61 Stamenkovic et al. also reported a significant relationship between anti-CCP and clinically evident arthritis.59 Similarly, a recent study found that the presence of anti-CCP is related to the appearance of bone erosions, and when associated with the presence of positive rheumatoid factor (RF) is related to the development of arthralgia in SSc patients.62 A meta-analysis by Laustriat et al. reported that in addition to conferring high risk of erosive arthritis (OR=22.48 [95 % CI: 10.71–47.21]), anti-CCP positivity in SSc patients increases the risk for pulmonary fibrosis, as well as for esophageal and diffuse skin involvements.63
Other antibodiesThe presence of antinuclear and double-stranded DNA antibodies (ANA and anti-dsDNA, respectively) in limited SSc patients seems to be related to joint manifestations.11,64 Antibodies against SCL-70 (anti SCL-70) are higher among patients with low BMD.39,40,41 In addition, an association of anti SCL-70 with the development of articular contractures and deformity in hands has been reported (OR 7.01, 95 % CI 1.02–48.69), although it is not associated with the presence of arthralgia, or TFR.21,65 There is some debate about whether the presence of anti-topoisomerase I antibodies is associated with the presence of joint manifestations, especially joint contractures, since some of the studies that have established this association have selection biases.66
Cartilage oligomeric matrix protein (COMP)COMP, a protein found in cartilage, and believed to be an important regulator of collagen fibers type I and II maintenance, has been studied as a possible marker of arthritis in SSc. A study of 40 SSc patients found that COMP levels were related to disease activity, presence of arthritis, anti-CCP and anti SCL-70 antibodies.67 However, a significant number of patients show positive COMP, so it does not seem to be very specific to identify arthritis.67
Platelet-activating factor acetylhydrolase (PAF-AH)Normal levels of PAF-AH correspond to a risk factor for arthralgia or arthritis (OR 19.8, [95 % CI 2.4–164] and OR 1.86, [95 % CI 1.33–2.59] respectively), which suggests that the presence of elevated PAF-AH has a possible protective role for joint involvement.68
YKL-40 or human cartilage glycoprotein 39 (HC gp-39)YKL-40 was evaluated in a study of 40 SSc patients by La Montagna et al. reporting that YKL-40 levels are elevated in patients with arthralgia or arthritis, which suggests that it can be a possible serological marker of joint involvement in SSc.69
Radiological evaluationConventional radiographyConventional radiography is a useful tool to address joint involvement in SSc used in conjunction with RF and anti-CCP, especially in cases where doubt exists of a possible overlap with RA.27 The findings can be divided into three patterns: articular degenerative damage, periarticular fibrotic changes, or articular inflammatory involvement; although this is a classification that is not reproducible in all studies.9
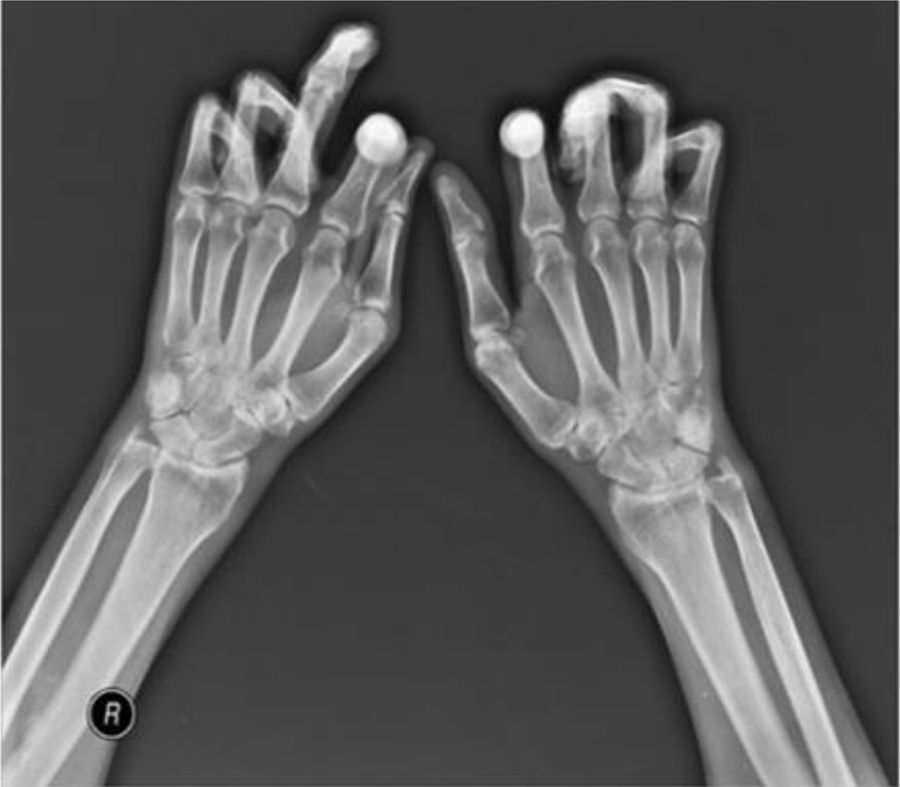
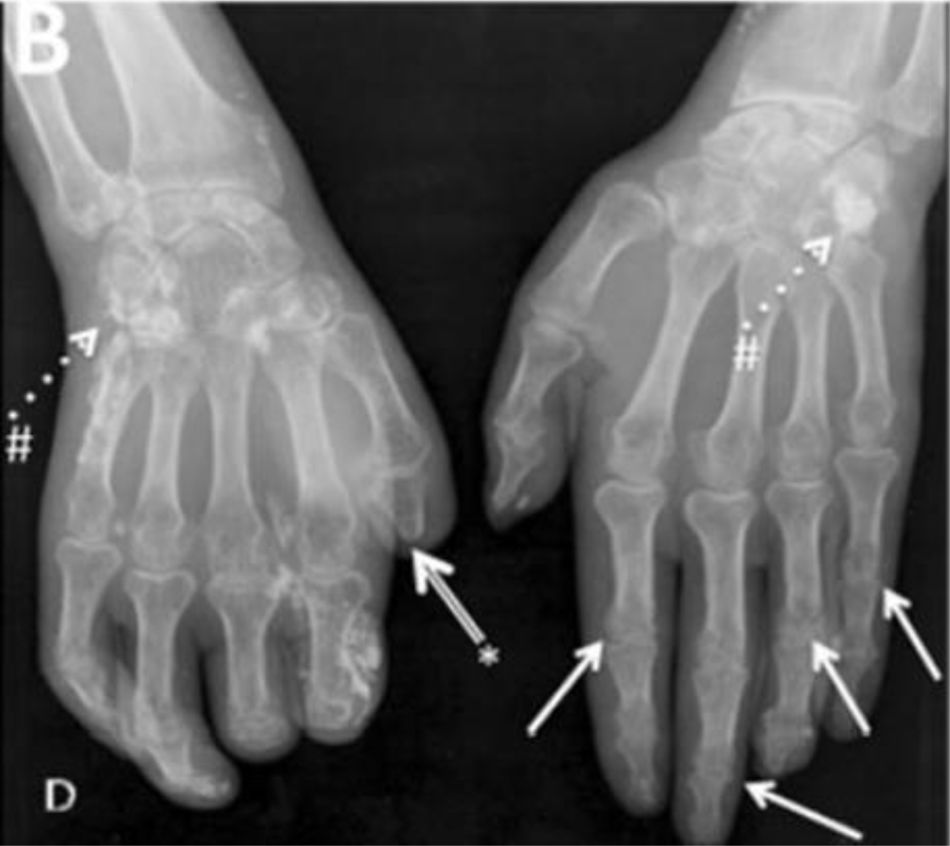
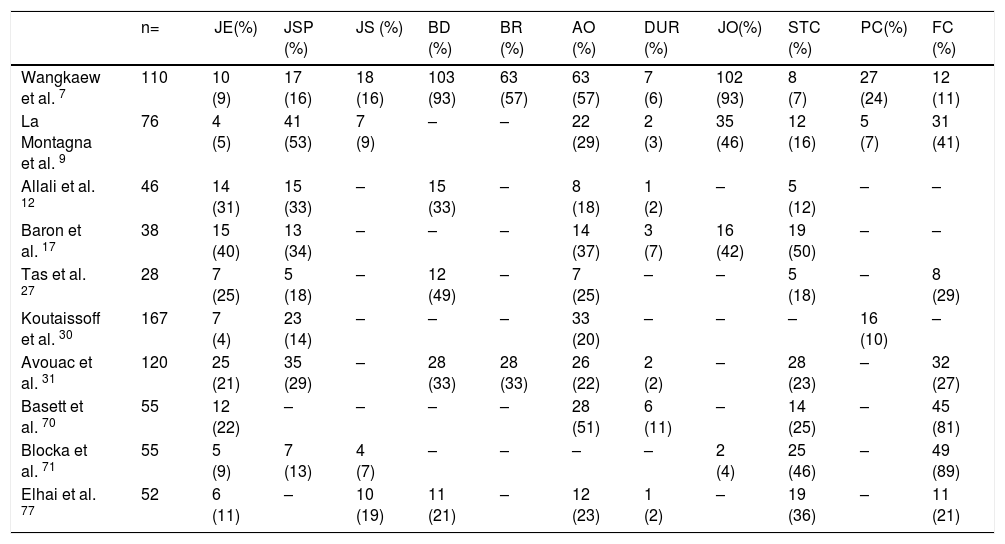
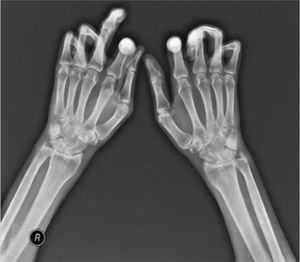
One of the most frequent findings described in simple X-rays is juxta-articular demineralization, reported in up to 42 % of cases with SSc. Decreased joint space and small and discrete joint erosions are common, up to 33 % and 31 % respectively12,27,33,70,71 (Fig. 7). Flexion deformities are present in a considerable number of patients.70 Extra-articular calcifications can appear in up to 18 % of cases12,27,30,33 (Table 4). Unlike RA, involvement of styloid process is uncommon, occurring only in 2 % of patients.12 In the distal phalangeal tuft (DPT) the most frequently described finding corresponds to DPT resorption reported in up to 28 % of the cases.12,30,70,72 Other findings reported are calcinosis in up to 12 %,30 as well as DPT proliferation or erosions, although with less frequency.72
Typical hand findings in simple radiography of a patient with SSc. (white arrow: yuxta articular erosions with joint space loss, asterix: acroosteolysis, hash: calcinosis). Adapted (with permission) from: Avouac et al.1
Radiographic changes in hands of patients with SSc.
| n= | JE(%) | JSP (%) | JS (%) | BD (%) | BR (%) | AO (%) | DUR (%) | JO(%) | STC (%) | PC(%) | FC (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wangkaew et al. 7 | 110 | 10 (9) | 17 (16) | 18 (16) | 103 (93) | 63 (57) | 63 (57) | 7 (6) | 102 (93) | 8 (7) | 27 (24) | 12 (11) |
| La Montagna et al. 9 | 76 | 4 (5) | 41 (53) | 7 (9) | – | – | 22 (29) | 2 (3) | 35 (46) | 12 (16) | 5 (7) | 31 (41) |
| Allali et al. 12 | 46 | 14 (31) | 15 (33) | – | 15 (33) | – | 8 (18) | 1 (2) | – | 5 (12) | – | – |
| Baron et al. 17 | 38 | 15 (40) | 13 (34) | – | – | – | 14 (37) | 3 (7) | 16 (42) | 19 (50) | – | – |
| Tas et al. 27 | 28 | 7 (25) | 5 (18) | – | 12 (49) | – | 7 (25) | – | – | 5 (18) | – | 8 (29) |
| Koutaissoff et al. 30 | 167 | 7 (4) | 23 (14) | – | – | – | 33 (20) | – | – | – | 16 (10) | – |
| Avouac et al. 31 | 120 | 25 (21) | 35 (29) | – | 28 (33) | 28 (33) | 26 (22) | 2 (2) | – | 28 (23) | – | 32 (27) |
| Basett et al. 70 | 55 | 12 (22) | – | – | – | – | 28 (51) | 6 (11) | – | 14 (25) | – | 45 (81) |
| Blocka et al. 71 | 55 | 5 (9) | 7 (13) | 4 (7) | – | – | – | – | 2 (4) | 25 (46) | – | 49 (89) |
| Elhai et al. 77 | 52 | 6 (11) | – | 10 (19) | 11 (21) | – | 12 (23) | 1 (2) | – | 19 (36) | – | 11 (21) |
(-) No data.
JE Joint erosion, JSP Joint space narrowing, JS Joint subluxation, BD Bone demineralization, BR Bone resorption, AO Acro-osteolysis, DUR Distal ulnar resorption, JO Juxta-articular osteopenia, STC Soft tissue calcification, PC Para-articular calcification, FC Flexion contracture.
When feet radiographs are evaluated, there is a lower frequency of acroosteolysis, erosions and calcinosis compared to hands. However, there are no significant differences in demineralization, decreased joint space, or joint subluxations.34
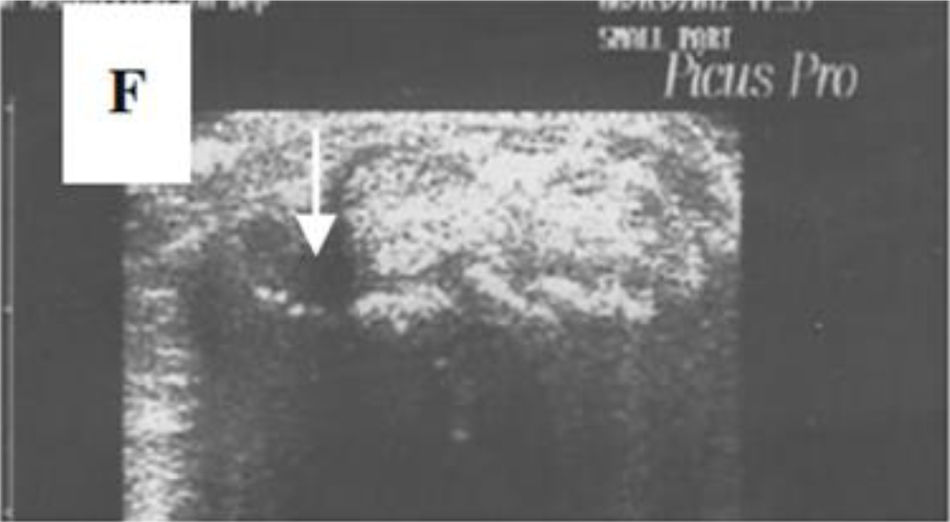
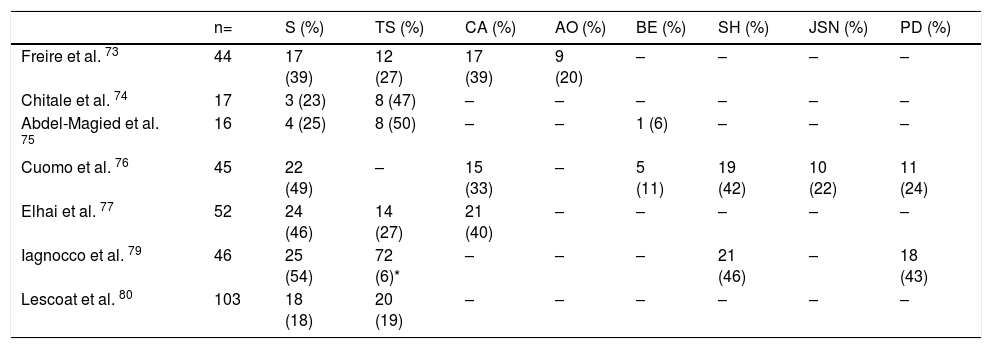
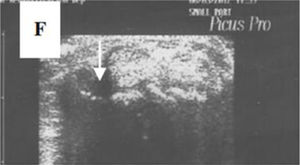
Ultrasonography (US)Due to the poor sensitivity of conventional radiography to detect early findings of joint involvement, the role of ultrasonography in this scenario has been evaluated (Table 5). Freire et al. evaluated the US findings at the joint level in SSc patients with recent diagnosis.73 Synovitis was found in 39 % of patients, mostly mild. In addition, 27 % of patients presented tenosynovitis with greater involvement in extensor tendon sheaths than in flexors73 (Fig. 8). US is able to identify arthritis and tenosynovitis during the follow-up of the disease in SSc patients with arthralgias without clinically evident inflammatory changes, although it is not equally useful to identify erosions.74–76 Sclerosing tenosynovitis and soft tissue calcifications are specific findings of SSc that allow for differentiation from other pathologies such as RA.77 Likewise, US has an acceptable sensitivity to detect calcinosis and acroosteolysis, with a value of 89 % and 90 %, respectively,73 and is able to detect the presence of enthesopathy in SSc patients.78
US changes in joints of patients with SSc.
| n= | S (%) | TS (%) | CA (%) | AO (%) | BE (%) | SH (%) | JSN (%) | PD (%) | |
|---|---|---|---|---|---|---|---|---|---|
| Freire et al. 73 | 44 | 17 (39) | 12 (27) | 17 (39) | 9 (20) | – | – | – | – |
| Chitale et al. 74 | 17 | 3 (23) | 8 (47) | – | – | – | – | – | – |
| Abdel-Magied et al. 75 | 16 | 4 (25) | 8 (50) | – | – | 1 (6) | – | – | – |
| Cuomo et al. 76 | 45 | 22 (49) | – | 15 (33) | – | 5 (11) | 19 (42) | 10 (22) | 11 (24) |
| Elhai et al. 77 | 52 | 24 (46) | 14 (27) | 21 (40) | – | – | – | – | – |
| Iagnocco et al. 79 | 46 | 25 (54) | 72 (6)* | – | – | – | 21 (46) | – | 18 (43) |
| Lescoat et al. 80 | 103 | 18 (18) | 20 (19) | – | – | – | – | – | – |
* From 1196 synovial tendon sites examined.
(-) No data.
S Synovitis, TS Tenosynovitis, CA Calcinosis, AO Acroosteolysis, BE Bone erosions, SH Synovial hypertrophy, JSN Joint space narrowing, PD Power Doppler.
A transverse ultrasonographic view of the common extensor tendon at the level of the wrist showing by grayscale tenosynovitis in the form of effusion (arrow). Adapted (with permission) from: Abdel-Magied et al.75
Iagnocco et al. evaluated the prevalence of abnormal US findings combined with Power Doppler in carpal and hand joints from SSc patients.79 Similar to previously published studies, a high prevalence of abnormal findings was reported when compared to healthy population. The most frequent findings described were: synovial hypertrophy, joint effusion and tenosynovitis. In addition, the use of Power Doppler US improves the ability to recognize local inflammatory signs, often in the absence of clinically evident synovitis.79 Lescoat et al. in a recent study confirmed these results, reporting inflammatory synovitis in 17.5 % of patients, and sclerosing tenosynovitis in up to 18.5 %.80 These findings were associated with elevated CRP, clinically evident synovitis and pericarditis. In turn, sclerosing tenosynovitis was more frequently seen in men and was associated with pulmonary compromise, antibodies against RNA polymerase III, and diffuse type SSc.80
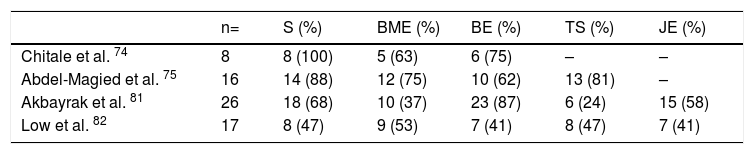
Magnetic resonance imaging (MRI)Interest in MRI usefulness to detect joint involvement in SSc patients has increased in recent years. Synovitis with mild erosions and joint effusion associated with the presence of bone marrow edema are predominantly described (Table 6).81,82 Articular MRI is more sensitive than ultrasound to identify joint involvement in patients with arthralgia without clinically evident synovitis.74,75 Unlike US and radiography, MRI is able to identify early changes, especially at the level of the carpus with predominant involvement of the lunate bone, which seems to be a distinctive feature of the disease.81
MRI changes in hands of patients with SSc.
| n= | S (%) | BME (%) | BE (%) | TS (%) | JE (%) | |
|---|---|---|---|---|---|---|
| Chitale et al. 74 | 8 | 8 (100) | 5 (63) | 6 (75) | – | – |
| Abdel-Magied et al. 75 | 16 | 14 (88) | 12 (75) | 10 (62) | 13 (81) | – |
| Akbayrak et al. 81 | 26 | 18 (68) | 10 (37) | 23 (87) | 6 (24) | 15 (58) |
| Low et al. 82 | 17 | 8 (47) | 9 (53) | 7 (41) | 8 (47) | 7 (41) |
(-) No data.
S Synovitis, BME Bone marrow edema, BE Bone erosions, TS Tenosynovitis, JE Joint effusion.
MRI has a high yield to identify tenosynovitis involving the hands in patients with arthritis,83 as well as changes in SSc patients, in whom tenosynovitis is described in up to 88 % of those with arthralgia.74,75 Similarly, in patients with evidence of TFR, MRI identifies tenosynovitis in up to 50 % of cases.84
Clinimetry and impact on general functionality and quality of lifeThe quality of life of patients with SSc is affected at different levels,85 largely due to skin involvement86 and osteoarticular commitment that affects the development of daily activities in a moderate to severe degree.10,87,89 Moreover, psychological stress and the perception of disability negatively affect the quality of life in these patients.90 There are multiple factors that are associated with the loss of hand function, especially the presence of joint contractures, digital ulcers, and severe Raynaud's phenomenon.91
The Health Assessment Questionnaire (HAQ) disability index (DI), the Short Form 36 (SF-36) health survey physical component score (PCS) and the mental component score (MCS) have been validated in SSc.92 Wiese et al. documented that there is a correlation between the HAQ DI and SF36 PCS (r=−0.79), as well as between HAQ DI and global health measurements given by physicians and patients with early diffuse SSc (r=0.43 and r=0.57, respectively).22 These measurements had an important association with the modified Rodnan score, although they did not have the same association with musculoskeletal manifestations or OAM.22 Additionally, using the same instruments, the disability generated by joint involvement is greater than that presented in PsA patients, with more intense pain than in RA patients.93 In a subsequent study of 326 patients with early diffuse SSc it was found that the loss of hand function, determined by the Cochin Hand Function Scale (CHFS), also contributes significantly to the disability generated by SSc.94
Kwakkenbos et al. validated in a cohort of 696 patients the PROMIS-29v2, a tool that evaluates seven domains related to quality of life in addition to the intensity of pain in SSc patients.89 They found that SSc negatively impacts quality of life largely due to joint contractures and gastrointestinal symptoms.89
The hand mobility test in SSc (HAMIS) was evaluated in a longitudinal study of 43 SSc patients with less than three years’ duration of the disease.95 A follow-up of at least four years was carried out, reporting that, although it varied in 72 % of the patients, there were no significant changes in the mobility of the hand during the first years of the disease.95 In turn, in patients with longer duration of the disease, there is an important limitation to perform movements that involve handgrip or twisting.96 The delta finger-to-palm test has also been validated to evaluate the mobility of the hands in SSc patients,97 as well as the Michigan Hand Questionnaire (MHQ).98 Similarly, the hand anatomic index (HAI) corresponds to a useful tool to objectively assess hand deformity and secondary functional impairment in SSc patients.99
Erol et al., used the health-related quality of life (HRQoL) with the Duruöz Hand Index (DHI) to assess hand function in 44 SSc patients compared to RA patients.100 They described that the loss of hand function in SSc is not only similar to that of RA, although joint changes in both diseases are different, but it has an important influence on the quality of life in these patients.100 A previous study that used HAMIS and Cochin Scale (CS) reported similar findings.101
Furthermore, Lorand et al., validated the usefulness of the Disease Activity Score 28 (DAS 28) using PCR and ESR, as well as of the Simplified Disease Activity Index (SDAI) and the Clinical Disease Activity Index (CDAI), to assess joint involvement in SSc.102 They reported that, although the four instruments are valid to determine severity of joint involvement in SSc patients, DAS28-ESR showed the best performance regarding reliability and construct validity.102
The levels of fatigue reported in SSc patients, measured by means of the general fatigue index (IGF), are similar to the ones reported by patients with neoplasms, RA or systemic lupus erythematosus (SLE).103 In addition, fatigue has a negative impact at many different levels in patients with autoimmune diseases, including SSc.104 Fibromyalgia, a disease frequently associated with high rates of fatigue, is very common in SSc patients, occurring in between 57 %–72 %.105
TreatmentThere are few studies on specific treatment of OAM in SSc. The first line treatments for the management of synovitis are adapted from the RA management schemes. A small retrospective study with patients from the EUSTAR database suggests a beneficial effect of hydroxychloroquine in the management of the inflammatory joint involvement in SSc.106 Patients who develop contractures in the first years of the disease could benefit from the use of cyclophosphamide, methotrexate or leflunomide.20 Immunoglobulin, used in a treatment scheme over six months, could be effective in reducing joint symptoms and secondary impact on quality of life in SSc patients with severe joint involvement refractory to first-line treatments.107
The possible role of anti TNF-α in the management of SSc has increased the interest of different groups worldwide. There are reports of studies that give a possible role for this group of treatments in the management of arthritis in the SSc population.108 In a study conducted by EUSTAR in 2011 in centers specialized in SSc management, it was evidenced that the majority of experts from this society recommended the use of anti TNF-α molecules only in randomized clinical trials since their potential adverse effects on the disease are unknown.109 However, due to the difficulty of conducting a randomized clinical trial with biological drugs, only observational studies have been published in this field.
Elhai et al. evaluated through an observational study the effect of tocilizumab, a humanized antibody against the IL-6 receptor, and abatacept, a recombinant fusion protein that modulates T-cell co-stimulation mediated by CTLA4, in the management of refractory polyarthritis or myopathy associated with SSc.110 DAS 28 was used to evaluate the response to treatment, and HAQ-DI to evaluate its impact on quality of life, suggesting that both drugs could improve polyarthritis secondary to SSc.110 A phase 3 study is currently underway to evaluate the response to rituximab compared with placebo in SSc patients with OAM who do not respond to first-line treatments.111
In SSc patients with painful hands of multicausal origin, including osteoarticular involvement, the carpal tunnel hydrodissection with lidocaine guided by US, associated to local corticoid injection reduces pain scores by up to 67 %, with a sustained duration of up to six months.112
Orthotic management for joint contractures can be ineffective in late stages of the disease, not only because it exacerbates symptoms such as Raynaud's phenomenon, but because it does not generate significant relief in the deformities generated by contractures.113 On the contrary, surgical management can be considered to reduce pain in patients with advanced disease refractory to other treatments.114 Procedures such as: sympathectomy, calcinosis resection, arthroplasties or capsulotomies have been practiced but the real benefits are few, so they have fall into disuse.115,116
In conclusion, OAM in SSc are frequent. They also have an important impact on patient's quality of life, especially due to the limitation in hand functionality. Treatment is difficult and requires a multidisciplinary approach. Due to the above, OAM in SSc require early identification and intervention. In addition, further research including good quality randomized clinical trials is required to establish the best treatment strategy for these patients.
Conflicts of interestNone.
Please cite this article as: Sebastian M–R, Eliana OC, Gerardo Q-L. Manifestaciones osteoarticulares de esclerosis sistémica: una sistemática. Rev Colomb Reumatol. 2020;27:85–110.