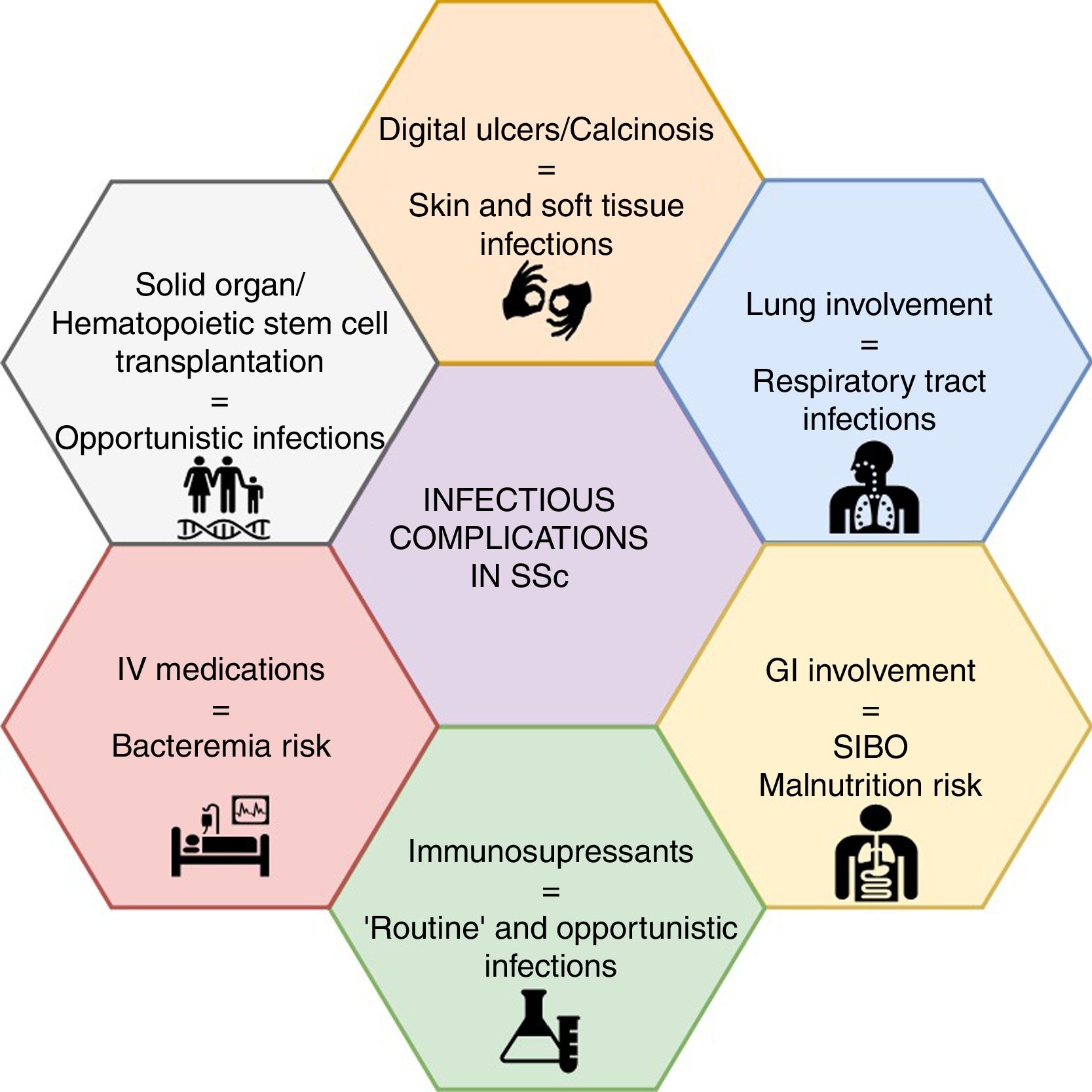
Mounting evidence has shown non-systemic sclerosis (SSc) related complications as a rising cause of hospital admission and mortality, out of which infections are among the top-five causes. Patients with SSc are at an increased risk of infection due to several features of the treatment options and to the disease itself. For instance, lung involvement is associated with a higher frequency of respiratory infections, whereas the presence of digital ulcers or calcinosis may result in skin and soft tissue infections, and even osteomyelitis. On the other hand, the growing trend towards immunomodulation and immunosuppression in patients with autoimmune diseases will place SSc patients at a higher risk of infectious complications, including opportunistic infections. A low suspicion threshold and an increasing awareness among treating specialists, particularly rheumatologists, are warranted for prevention, early diagnosis and management of infectious complications. Nonetheless, data on risk management strategies in SSc, such as vaccination and antimicrobial prophylaxis, are scarce. A narrative non-systematic review was performed to provide an update of infectious complications in patients with SSc.
La evidencia creciente muestra que las complicaciones no asociadas a la esclerosis sistémica (ES) son una causa cada vez más frecuente de hospitalización y mortalidad, dentro de las cuales las infecciones se encuentran entre las primeras cinco causas. Los pacientes con ES presentan un riesgo elevado de infección asociado a las opciones terapéuticas y por la enfermedad misma. Por ejemplo, el compromiso pulmonar se asocia a una mayor frecuencia de infecciones respiratorias, mientras que la presencia de úlceras digitales o calcinosis pueden resultar en infecciones de piel y tejidos blandos, e incluso osteomielitis. Por otro lado, la tendencia creciente hacia la inmunomodulación y la inmunosupresión como tratamiento de las enfermedades autoinmunes, pondrá a estos pacientes en un mayor riesgo de infecciones, incluidas las infecciones oportunistas. Son necesarios un umbral bajo de sospecha y un alto nivel de alerta entre las especialidades tratantes, particularmente los reumatólogos, para la prevención, el diagnóstico temprano y el manejo de las complicaciones infecciosas. Sin embargo, la información respecto a estrategias de gestión de riesgo en ES, como la vacunación o la profilaxis antibiótica, es escasa. Se realizó una revisión narrativa no sistemática que presenta una actualización sobre las complicaciones infecciosas en pacientes con ES.
Systemic sclerosis (SSc), also called scleroderma, is an immune-mediated chronic connective tissue disease characterized by a disproportionate deposition of collagen in the skin and internal organs; fibrosis and vasculopathy are its main features.1,2 Two main subsets, limited and diffuse, are described according to the extension of cutaneous involvement.3 SSc presents the highest standardized mortality ratio (SMR, i.e., risk of death as compared with the general population) among rheumatic diseases.4 As a systemic disease, a myriad of signs and symptoms may be present, particularly renal, cardiopulmonary, gastrointestinal, and musculoskeletal. Thus, its management is an increasing challenge for rheumatologists and related specialties.
With regard to infectious diseases, patients with SSc are at an increased risk of infection due to the disease itself, mainly respiratory infections due to interstitial lung disease (ILD)5 and skin/soft tissue infections due to the presence of digital ulcers (DU) or calcinosis, that can progress to osteomyelitis.6,7
As well, the growing trend towards immunomodulation and immunosuppression will place SSc patients at a higher risk of infections, as increasingly observed in other autoimmune diseases,8 including opportunistic infections (OIs). Medications such as cyclophosphamide (CYC), mycophenolate mofetil (MMF), methotrexate (MTX), azathioprine (AZA), glucocorticoids, and biological therapy (i.e., rituximab [RTX]) are recommended for the management of disease manifestations.9–12 Several novel immunologic targets are under research with promising results.13 In addition, the intravenous route of application of prostanoids and some immunosuppressants may generate an increased risk of infection. Further, the prolonged use of proton pump inhibitors (PPI) and the use of antibiotic courses for microbial overgrowth are interventions to be considered as risk factors for infection.
Hereby, we offer an updated review of infectious diseases in patients with SSc, including their epidemiology, clinical manifestations and risk-management strategies.
MethodsA narrative non-systematic review was performed. We completed a literature search in 4 databases (Cochrane Database, Medline, Embase and LILACS [which includes literature from Latin America]). Articles in English, Spanish, French, Italian, Portuguese and German were included. The search strategy included the following terms: “Systemic scleroderma”, “Infection”, “Infectious Disease Medicine”, “Communicable Diseases”. Gray literature was obtained using these terms in Google and a ‘snowball’ strategy was applied to retrieve useful literature from the revised articles’ references.
Infections and SSc: epidemiologyRecent advances in the treatment of SSc have influenced the causes of death and in-hospital admission. A shift from renal crisis to cardiovascular (e.g., congestive heart failure [CHF]), pulmonary (i.e., ILD and pulmonary artery hypertension [PAH]), neoplastic and infectious causes as main drivers of death and hospitalization has been observed.5,14–26 A meta-analysis that included data until 2006 found a SMR of 3.53, which has not changed significantly over the last 40 years.14 Rubio-Rivas et al. reported a lower SMR (2.72) using a similar approach; the authors estimated a cumulative survival from onset (first Raynaud’s symptom) at 87% and 74% at 5 and 10 years, respectively, whereas from diagnosis the survival was 75% and 62%, respectively. Further, they described a trend towards improvement in mortality between the studies performed prior and after 1990, although statically significant only at 5-year survival from diagnosis (71% vs. 84%).27 Previously, the main driver of death in patients with SSc was renal crisis. The use of angiotensin-converting enzyme inhibitors led to a profound reduction from 42% of cases to less than 6% in the early 2000s.22 However, mounting evidence has shown that non-SSc-related causes for both death and admission are approaching 50%,14,15,17,20,22,23 out of which infections are becoming an important cause.
Infection as a cause of deathTwo meta-analyses including data from 1960 to the first decade of the 2000s reported a 7% death frequency secondary to infection in SSc.14,19 Further, reports from the international European Scleroderma Trials and Research (EUSTAR) database, ranked infections among the top-five causes of death.15,24 A study using death certificates from France supported a similar frequency and reported infection as the cause of death in 11% of certificates. The authors reported a high mortality from lung infections and a five-fold higher rate of infectious deaths among patients with SSc compared with the general population24; similar evidence has been reported from several cohorts around the world.16,17,20,21,23,28
Some reports suggest a temporal shift in causes of death with infections being on the rise. Steen et al. reported that the proportion of infections as a cause of death among their cohort (1972–2001) ranged from 2% (1972–76) to 9% (1997–2001); although no statistical differences between periods were observed, a trend towards a higher frequency appears to be present.22 Further, two studies with a 10-year difference in the USA, based on the Healthcare Cost and Utilization Project-Nationwide Inpatient Sample (HCUP-NIS) databases and using a similar methodology, presented a significant rise in infections as a cause of death and admission26,29; Chung et al. (2002–2003) showed that infections were among the top-three causes of death, accounting for 13% of the events.26 Whereas Poudel et al., ten years later (2012–2013) reported that the most common diagnoses of deceased inpatients were infection/septicemia (32.7%), followed by pulmonary (20%) and cardiovascular (15.7%) involvement. Infection (other than opportunistic) had a high adjusted OR for mortality (3.36, CI 2.73–4.41).29
Infection as a cause of admissionSeveral cohorts have reported infections as a common cause of admission in patients with SSc, being ranked among the top three causes and as the most common non-SSc-related cause.17,18,21,26,29,30 Respiratory, skin/soft tissue, gastrointestinal, and urinary tract systems are the most common sites of infection causing hospital admission, although their frequencies differ by cohort. Infections have been associated with higher costs, longer length of stay and mortality.29
The occurrence of infections in SSc may be explained by an increasing use of immunosuppressive agents. Poudel et al. explored this hypothesis by comparing non-SSc-related hospitalizations against SSc-related hospitalizations with infection/septicemia, using a surrogate variable (a code for “chemotherapy” was available, which may represent the use of CYC or RTX, although it is not possible to ascertain). They found a significantly higher proportion of chemotherapy use in SSc hospitalizations with infection/septicemia, both in those who lived (9.2% vs. 4.1%, p < 0.0001) and those who died (17.4% vs. 6.3%, p = 0.0005). Those patients who died had higher proportions of PAH (43.3%), respiratory failure (72.4%), and CHF (38.4%), which may represent a more aggressive disease that required stronger immunosuppression and therefore, a higher risk of infection. Interestingly, the authors found that a higher income was associated with a higher risk of death. They proposed an access paradox, in which, among other causes, a higher income may represent a better access to immunosuppressive agents.29 Conversely, another study analyzing the immunosuppressive therapy hypothesis found no statistical association between respiratory infection and the use of immunosuppressants as a group.17 In this line, recent studies with Tocilizumab (TCZ)31,32 and Abatacept (ABA)33 do not support an increased risk of infections, as will be discussed later.
Infections and SSc: organ-specific involvementInfectious complications in SSc can be attributed to disease- or drug-associated factors (Fig. 1). The former appears to be more frequent, mainly represented by soft tissue and pulmonary infections.
Skin and soft tissue infectionsSkin involvement due to DU is one of the most frequent complications of SSc, and it can be associated with soft tissue infection in 19–54% of patients.6,7,34–36 The etiological mechanism of DU differs between ischemic ulcers, those related to articular deformities, and those associated with calcinosis.5 With regard to infectious complications, literature reports do not usually discriminate by mechanism, thus defining a clear risk difference is difficult.
Nonetheless, particularly in the presence of calcinosis, the development of cellulitis is a frequent complication.37,38 Progression to streptococcal pyomyositis,39 and cases of necrotizing fasciitis40 have also been reported.
Some studies have reported more infectious complications among the limited cutaneous SSc subset patients.41 DU infections can progress to osteomyelitis, with an incidence as high as 8%, which can increase to 17% in patients with active DU6,42–44 and 42% when those ulcers have any sign of infection.7 The most frequently isolated pathogens are Staphylococcus aureus, Pseudomonas aeruginosa and Escherichia coli, a pivotal information for the election of empiric antibiotic treatment.45 Furthermore, disease related disability is worsened by these infections, since 8–16% of patients require surgical amputation as part of their management.46 Patients who need surgical debridement, amputation or other acute intervention, will present an increased risk of wound infection, necrosis and slow wound healing, thus requiring a careful monitorization.47
Respiratory involvementIn some SSc series, the respiratory system is the most frequent site of infectious complications, accounting for up to 24% of the reported events.8 Diverse mechanisms have been proposed to explain this phenomenon, including ILD, which develops in almost 50–60 % of SSc patients, being the main cause of death in several series48,49; 40% of these deaths are attributed to lung infection.50 Due to pulmonary structural involvement, bacterial infections are frequent (e.g., pneumonia with or without necrosis, lung abscess, empyema, etc.).51 As patients with lung involvement present frequent flares, it is vital to rule out infectious complications as a cause of acute respiratory symptoms; early bronchoscopy may be necessary to identify infectious agents.52 Procalcitonin has showed some usefulness in this scenario, where a positive value can suggest an active bacterial infection, although the threshold is not well established.53,54
On the other side, esophageal dysmotility has been associated with aspiration pneumonia (OR 1.23, CI 1.05–10.55), major infection (OR 3.22, CI 1.01–10.51)30 and in-hospital death25; some authors have proposed that microaspiration may contribute to ILD.55,56 Pneumonia may be difficult to distinguish from ILD.52 Thus, Sehra et al.25 proposed that “strict aspiration precautions should be considered for SSc patients who are admitted to the hospital, including simple measures like elevation of the head of the bed, avoiding over-sedation, and swallowing evaluations to probably help decrease in-hospital mortality”.
Bacterial pneumonia and TB have also been reported in SSc. Two population-based studies in Taiwan (2000–2006 and 2001–2012) explored the risk of TB in SSc57 and rheumatic diseases,58 compared to age-matched healthy patients. SSc patients were found to have a significant 2.81-fold greater risk of overall TB infection (Incidence Rate Ratio [IRR] 2.81, CI 1.36–5.37) and 2.53-fold greater risk of pulmonary TB (IRR 2.53, CI 1.08–5.30); although non-significant, a trend towards a higher risk of extrapulmonary TB was reported (IRR 4.22, CI 0.27–17.57). SSc was found to be an independent risk factor for TB infection, with a hazard ratio (HR) of 2.99 (CI 1.58–5.63). Risk factors for TB in SSc were explored: age > 60 years and the presence of PAH were statistically significant in the multivariate analysis; interestingly, exposure to glucocorticoids or CYC were only significant in the univariate analysis.57 Lu et al. reported similar findings, although their methods differed, as the authors pooled together data from SSc and inflammatory myositis as a group.58 Moreover, among patients who received dexamethasone pulse therapy as part of their treatment, pulmonary TB was reported in 9% and extrapulmonary TB in 20%.59 Extrapulmonary TB has also been associated with the use of CYC, high doses of glucocorticoids and Tumor Necrosis Factor (TNF)-ɑ inhibitors.60,61 Some series have reported non-TB mycobacteria infections in 18% of patients with ILD.62
Lung involvement by other opportunistic microorganisms include infection by Pneumocystis jirovecii which, although seldom reported in SSc patients,63,64 has been associated with high dose glucocorticoids (i.e., ≥20 mg/day in this study) or lower glucocorticoids doses with concomitant use of CYC65 with a mortality close to 50%. Therefore, a low threshold of suspicion must be considered among rheumatologists and treating specialists to make the diagnosis in patients with highly immunosuppressive treatment who consult with progressive dyspnea and dry cough66; prophylaxis should be considered in high risk patients.67
Aspergillus fumigatus fungal ball has been found on large bullous spaces in patients with ILD, so it should be considered in patients with hemoptysis and lung masses.68 A case of pulmonary actinomycosis in a patient on infliximab was reported.69
Gastrointestinal involvementSmall intestinal bacterial overgrowth (SIBO) is a common gastrointestinal manifestation. Several antibiotics, such as amoxicillin, quinolones (e.g., ciprofloxacin), tetracyclines (e.g., doxycycline), metronidazole, minimally absorbable antibiotics (e.g., rifaximin) and trimethoprim/sulfamethoxazole (TMP/SMX) may be considered for SIBO treatment.70,71 Cyclical courses may be given monthly during 10–14 days. From an infectious diseases perspective, one should not forget that the regular use of antibiotics is linked with antibiotic associated diarrhea and a higher risk of Clostridioides difficile infection.72,73 Although clindamycin is often considered as the major driver of C. difficile infection, most of the mentioned antibiotics for the treatment of SIBO may be associated as well.73 A myriad of symptoms may be present and range from mild to severe systemic compromise. The main acute symptoms that should prompt active search are malaise, abdominal pain, diarrhea, nausea, vomiting, fever and leukocytosis.74 Moreover, the presence of gastrointestinal involvement has been related to a greater incidence of non-strongyloidiasis diarrhea (OR 2.28, CI 1.21–6.56).30
Interestingly, a high incidence of esophageal candidiasis was reported in the late 80 s in patients with SSc who needed PPIs and was linked by the authors to esophageal dysmotility, rather than to immunosuppressive treatment.75,76 Nonetheless, this phenomenon appears to be uncommon in recent literature. In fact, only one case was reported among the retrieved articles.30 Further, in our experience, esophageal candidiasis is seldom seen in clinical practice.
Helicobacter pylori prevalence in SSc patients is an interesting phenomenon, as it can be almost twice the one of immunocompetent population, although this prevalence is mainly driven by ELISA positivity.77 Its occurrence has been associated with the presence of disease activity and severity, and its detection and eradication have shown significant improvement in SSc symptoms and disease activity.78,79 Several eradication regimens are available and should be considered based on local epidemiology, patient preferences, risk factors and medical consideration.80
Another complication with the potential of infectious complications is the requirement of parenteral nutrition, mostly derived from gastrointestinal involvement, such as pseudo-obstruction, malabsorption, and malnutrition. It is considered as a safe intervention, although the incidence of bacteremia presents a variable rate (0.19–1 in 1000 catheter-days).81,82
Opportunistic infections and othersThe advent of biological therapy and the use of both glucocorticoids and other immunosuppressants in rheumatology practice render patients on these therapies prone to OI, for whom a specific definition has been proposed.83 Currently, OIs in SSc are considered to be less frequent than in other rheumatic diseases, given that most of the available information is limited to case reports. Thus, recommendations for a prophylactic or diagnostic approach are difficult, but they should be considered in highly immunosuppressed patients.
Extrapolated data of OIs from other diseases in which similar therapies are used (e.g., rheumatic, malignancies, transplants, etc.) might be considered. This kind of infections have been described in patients with high immunosuppression requirements, especially with high glucocorticoids dose (i.e., ≥30 mg/day), CYC or biological therapy.
Several different OIs have been reported in SSc, such as P. jirovecii pneumonia (as previously described)63–65; disseminated candidiasis on high glucocorticoids doses (i.e., prednisolone at a dose of 40 mg/day, followed by methylprednisolone pulse therapy)84; cryptococcosis, with pulmonary and extrapulmonary involvement85,86; cerebral toxoplasmosis87; tenosynovitis, septic arthritis and skin abscesses caused by atypical mycobacteria88–90; and infections by Nocardia farcinica with subcutaneous abscesses or pulmonary infections.91,92
Similarly, viral reactivations have been reported in patients on high immunosuppressive therapy. Cytomegalovirus (CMV) reactivations present complications similar to those described in other high risk groups, including splenic rupture, pneumonitis, pancreatitis, esophagitis or duodenal perforation, mainly related with high dose glucocorticoids and CYC.93–96 Human Herpes Virus 6 (HHV6) asymptomatic reactivation has been detected in SSc patients, although it may represent an epiphenomenon linked to immunosuppression, without clinical relevance.97 There are also reports of a Herpes Simplex Virus (HSV) -1 necrotizing pleuritis98 and parvovirus B19 alveolitis associated with ILD.99 Herpes Zoster Virus (HZV) reactivations can happen in 5% of patients, mainly with cutaneous involvement, but complications such as Guillain-Barré syndrome, have been reported.100 Hepatitis B Virus (HBV) reactivation prevalence in rheumatoid arthritis has been described in 3–3.5 %, but there are no data of its prevalence in SSc patients. Nevertheless, when high immunosuppressive doses are considered as part of SSc treatment, patients with a high risk profile (i.e., health care workers, high risk sexual activity, hemodialysis patients, injectable drug users, multiple sexual partners, and household contacts of HBV-infected individuals) must be screened for chronic HVB infection, and prophylaxis must be offered if indicated.101,102 HBV reactivation must be suspected when an increase in liver enzymes is observed, mainly alanine aminotransferase, particularly in patients with evidence of past HBV infection (i.e., positive HBV core antibody).102 If suspected, serum HBV DNA should be assessed and consultation with a specialist (e.g., gastroenterology, infectious diseases) is advisable. A low threshold of suspicion must be considered: although hepatotoxicity from medications is often seen, HBV reactivation may explain this finding and must be ruled out in at-risk patients.
Data on Human Papilloma Virus (HPV) infection incidence in SSc are contrasting, but up to a five-fold increased incidence when compared with healthy age matched controls has been reported.103,104 Routinely gynecological screening must be considered based on national guidelines.
Immunosupressants and infectionsCurrently, treatment strategies are directed towards management for specific organ involvement and symptoms relief, with immunomodulation as a pivotal strategy for the management of skin, lung and articular involvement.9,10 In selected patients, transplantation proceedings, such as hematopoietic stem cell transplantation (HSCT), are also available with a subsequent risk of infection.105 Solid organ transplantation is also feasible and is particularly offered for patients with organ-specific involvement, including lung (i.e., ILD or PAH), heart, and kidney; even heart-lung transplant is possible in selected cases. Clinical and survival outcomes are comparable to those observed in patients without SSc, including infectious events,106–109 for which recent guidelines have been issued.110 Further, other frequent interventions, particularly intravenous medications, such as prostanoids may turn SSc patients more prone to infections.5Table 1 summarizes potential infectious complications/etiologies by immunosuppressant in patients with SSc.
Potential infectious complications/etiologies by immunosuppressant in patients with Systemic sclerosis.
| Glucocorticoids (146,148) |
|
| Cyclophosphamide (5,112,114,118–127,184,185) |
|
| Methotrexate (102,125,126,144,145) |
|
| Azathioprine (102,112,115,125,126,135–138) |
|
| Mycophenolate mofetil (113,116,117,129–132) |
|
| Rituximab (102,125,153–155,157,158,160) |
|
| Tocilizumab (31,102,125,126,162–165,168) |
|
| Abatacept (102,125,126,169–171,174) |
|
| TNF α inhibitors (69,177–180) |
|
CMV, Cytomegalovirus; EBV, Epstein-Barr Virus; HBV, Hepatitis B Virus; HCV, Hepatitis C Virus; HSV, Herpes Simplex Virus; HZV, Herpes Zoster Virus; JC-virus, John Cunningham -virus; OI, Opportunistic Infection; PML, Progressive Multifocal Leukoencephalopathy; RTI, Respiratory Tract Infection; TB, Tuberculosis; TNF, Tumor Necrosis Factor; UTI, Urinary Tract Infection.
This table is based on references and author’s experience.
CYC is an alkylating agent that inhibits DNA replication, particularly in hematopoietic cells and lymphocytes due to their high proliferation rate, ultimately leading to their apoptosis.111 CYC appears as a first-line therapy for the treatment of ILD and is considered in severe skin involvement.9 This agent has been associated with respiratory infections, although the presence of lung structural changes that may account for an increased risk of infections should not be disregarded.5 Data on CYC and infections will be approached from two standpoints: regular- and high-dose, as infection risk is expected to differ. Although a recommended standard regular dose is lacking,10 a dose of 500−600 mg/m2 on a monthly basis is widely accepted, which will be discussed in this subheading. In contrast, high doses exceed this dosage and are mainly reported in Hematopoietic Stem Cell Transplant (HSCT) trials, a subject that will be addressed later.
Randomized controlled trials of CYC compared with a variety of interventions have shed light onto this subject. Ludici et al. reported UTIs in 6 patients (13.3%) and respiratory infections in 4 (8.8%).112 A recent metanalysis of CYC in connective tissue disease-associated ILD found no difference in pneumonia rates when compared either to placebo or MMF113; only two studies versus placebo were included: one study reported 6 out of 79 patients with pneumonia, as the only recorded infectious event,114 and, the other study reported no differences in infections between groups, although 13.6% (n = 3) of patients in the intervention group presented intercurrent upper respiratory infection115 with a more intense immunosuppressive therapy (i.e., glucocorticoids plus CYC followed by AZA). The comparison of CYC against MMF found similar results.113,116,117 In a meta-analysis of 18 studies until 2010 of CYC for lung involvement, Poormoghim et al reported only one discontinuation of CYC due to pneumonia and only one episode of HZV.118
Some other infectious complications have been reported. For instance, one of the frequent adverse events during CYC therapy is hematuria, which is usually considered secondary to CYC itself.118 However, BK-virus associated hemorrhagic cystitis in a patient with SSC treated with CYC has been reported, thus highlighting the need to rule out an infectious cause.119 Further, a case of hemorrhagic cystitis associated to adenovirus was described in a HSCT, probably secondary to CYC mobilization (considered as a high-dose).120
Although infections due to CYC appear to be rather infrequent, the experience in other rheumatic diseases should prompt awareness. Woytala et al reported infections in 37% of patients (n = 24) with different rheumatic diseases, including skin, upper and lower respiratory, and UTIs; few episodes of HZV (3%) were observed.121 Experience in systemic lupus erythematosus (SLE) has suggested an increased risk of infection from a variety of etiologies122–124; CYC has been considered as a low risk medication for progressive multifocal leukoencephalopathy (PML).125,126 An association with HZV appears to be present in rheumatoid arthritis (RA) on CYC.126 HBV reactivation has been reported in the literature and a shorter period to reactivation (8 weeks) compared to other immunosuppressants was identified.127
In conclusion, an increased risk of upper respiratory infections and probably UTIs appears to be associated with CYC. However, treatment interruption is not often needed.
Mycophenolate mofetilMMF is a potent inhibitor of inosine monophosphate dehydrogenase (IMPDH), which is pivotal for the proliferation of T and B lymphocytes.111,128 MMF is recommended among the first line therapies for ILD,9 although its safety in SSc treatment appears as an objective in the research agenda.10
A systematic review on MMF safety in SSc reported 34 infectious events in 487 included patients, including respiratory (n = 11), skin (n = 4), and UTIs (n = 2).129 Boulos et al. reported only one discontinuation due to recurrent infections after 5 years on therapy in patients treated with MMF (n = 74).130 Gordon et al.131 compared MMF alone vs. MMF plus belimumab in 20 patients, with a 1:1 randomization. No differences in infection rates were observed. Naidu et al. reported a similar infection rate compared to placebo in Indian patients.132
A recent meta-analysis of CYC in ILD in SSc that included two studies,113,116,117 found no difference in pneumonia rates when comparing MMF with CYC.
Data from renal transplant and SLE patients have reported a decreased production of IgG and IgM, thus increasing infection risk and suppressing response to some vaccines.133
These findings support an acceptable safety profile of MMF from an infectious risk standpoint, and may support its election over CYC, although evidence from lupus nephritis appears to show no difference between both.134
AzathioprineAzathioprine (AZA) is a cytotoxic agent that interferes with DNA and RNA cycles through the insertion of 6-tioguanine nucleotide.111 AZA is considered as a second line agent for maintenance therapy in ILD.9 Evidence of infections in SSc is scarce and its safety in these patients appears as an objective in the research agenda.10 Ludici et al. did not report infectious events in 24 patients on AZA,112 whereas Dheda et al. found one episode of TB in 8 patients assessed.115 Nonetheless, some evidence from the treatment of inflammatory bowel disease suggests a 3-fold increased risk of OIs with the use of AZA, particularly viral (i.e., HZV, HSV, CMV, Epstein-Barr Virus [EBV]),135 and a 7% frequency of infectious events.136–138 An association with HZV appears to be present in RA on AZA.126
MethotrexateMethotrexate (MTX) is a folic acid analogue that inhibits dihydrofolate reductase activity, thus undermining lymphocyte proliferation and promoting an anti-inflammatory milieu.139 MTX should be considered for the management of skin and articular involvement in SSc.9,10
The evidence from randomized controlled trials in the management of SSc has shown infections as a rare event in this population, with doses up to 25 mg/week.140–143 Evidence from IBD treatment appears to support its safety.138 MTX has been associated with a mild increased risk of infection, particularly skin/soft tissue and respiratory tract infections, in patients with rheumatoid arthritis (RA).144,145 Although an increased risk of HZV has been suggested, a large national database-based study in the USA found no association.126
GlucocorticoidsGlucocorticoids influence the inflammatory response through several pathways. Specifically, by a reduction in the innate response and in the production of inflammatory cytokines, an inhibition of both classic and alternative pathways of the complement system, and a reduced capacity for antigen presentation, lymphocyte proliferation and differentiation; a shift towards a Th2 phenotype is generated.146 Low dose glucocorticoids have been recommended, mainly based on expert opinion, for the management of articular symptoms9 and some evidence for myositis is available, although its use is debated; however, a wide share of patients with SSc receive them as comedication with other immunosuppressants.147 Immunological alterations explain the increased risk of infectious events due to both intra- or extra-cellular microorganisms, including bacteria, fungi, virus, mycobacteria and parasites146; OIs, such as P. jirovecii pneumonia, TB and HZV are increasing major concerns.148 High-dose glucocorticoids have been associated to an increased risk of fatal infections in patients with autoimmune diseases.149,150
Biological immunosupressants as potential novel interventions for SScRituximabRituximab (RTX) is a monoclonal antibody that targets CD20 and depletes most of B-cells virtually sparing plasmatic cells, thus altering several immunologic pathways.13,151 Although not readily approved by regulatory agencies for the treatment of SSc, RTX is recommended by experts for the treatment of refractory ILD and articular involvement.9 Mounting evidence supports its efficacy, either alone or in combination, for these systems and even in skin involvement.152–155 Infectious disease risk appears to depend on the underlying diagnosis, as it may be as high as 50% in patients with lymphoma or below 5% in patients with RA or systemic vasculitis. Hypogammaglobulinemia is the main driver of infection in these patients, in whom the highest risk occurs during the first 3 months of therapy, although substantial risk is present thereafter.156 Neutropenia can also occur and severe neutropenia is reported in up to 30% of patients. Early neutropenia is rare, nonetheless, the so-called late-onset neutropenia is observed in up to 15% of patients 1–5 months after the end of therapy. Its mechanism is still unknown as is considered to be immune-mediated; it can persist for months, may resolve spontaneously, and in persistent cases, its response to granulocyte colony-stimulating factor therapy is poor or transient. Its impact on infection risk remains to be elucidated.157 Different microorganisms have been reported as etiologies of infectious events on RTX for both neoplastic and autoimmune diseases, particularly by Enterobacteriaceae. Further, OIs have been reported including P. jirovecii pneumonia, A. fumigatus, Candida spp., HZV, CMV and PML,158 although for the latter, RTX has been considered as a low risk medication.125 However, evidence is conflicting as data derived from RA did not revealed an increased risk of OIs nor viral reactivations, although the lack of long-term follow-up hampers to ascertain this risk.159 Regarding specific data on SSc, Thibaut et al. reported in a literature review that infectious events are frequent, but usually not severe.153 Melsens et al. did not report infectious complications in 17 patients with SSc.154 Noteworthy, interim results from the EUSTAR Network reported infections in 76 out of 248 (30%) patients with SSc using RTX.155
One of the major concerns of RTX, is its increased risk of reactivation of latent HBV infection, that is considered the highest among all the available biologics: a rate of up to 16.9%, although this information comes from patients on concomitant antineoplastic agents.160 This risk appears to be lower among rheumatic diseases (<10%), thus, close monitoring is advised.102
TocilizumabTocilizumab is a humanized monoclonal antibody directed towards IL-6 receptor.161 TCZ is considered a promising molecule in the treatment of SSc and is recommended as an alternative in articular involvement.9,13 The FaSScinate phase 2 study explored its utility in SSc and reported that up to 56% of patients (n = 24) presented at least one infectious event. Noteworthy, this frequency did not differ from the placebo arm, although serious infectious events were more frequent in the TCZ arm (16% vs. 5%). One death due to lung infection was reported. The most frequent infections were nasopharyngitis (n = 5), UTI (n = 3) and HZV (n = 3).31 Data from the phase 3 trial did not report an increased risk of infection when compared to placebo.32 Noteworthy, Distler & Distler expressed a relevant concern on infectious disease risk: the authors of the study reported two events of proximal interphalangeal joint osteomyelitis in patients receiving TCZ, probably associated with digital ulcers. As TCZ inhibition of IL-6 has been shown to blunt PCR elevation and probably signs of infection, a low threshold of clinical suspicion is warranted162; from an infectious disease perspective, the follow-up of osteomyelitis under IL-6 inhibition may present a major challenge. Evidence from RA experience has suggested an increased risk of necrotizing skin infections, as well as S. aureus and non-TB mycobacteria disseminated infections.163–166 Neutropenia was observed in up to 30% of RA patients in pivotal studies, although less than 5% presented severe neutropenia. Its mechanism is still unknown, although a redistribution of the neutrophil compartment has been proposed. Its consequences on infection risk are unclear, although data from RA trials do not support an association with an increased risk; dose interruption is an effective risk mitigation strategy that yields a rapid recovery of neutrophil counts.167,168
AbataceptAbatacept is a soluble fusion molecule formed by the extra-cellular domain of CTLA-4, which inhibits the activation of T lymphocytes through its union with CD80 and CD86, thus blocking the interaction with CD28 161. The efficacy of ABA in the management of skin and articular involvement in SSc has been suggested 13. Chakravarty et al. (n = 10) found no statistical differences in infection rate between the intervention and the control group; two events of upper respiratory infection were reported in the ABA arm.169 Similarly, a retrospective study (n = 11) in patients with articular involvement reported 3 infectious events: two episodes of bronchitis and one HSV infection.170 Results from a phase 2 trial in SSc suggested a comparable infection risk profile between ABA and placebo.32 Pooled-evidence from RA studies does not support an increased risk for infection compared to other therapies,171–173 although the long-term extension (5 years) report of the ACQUIRE study showed that 70% of patients experienced an infectious event during follow up, mainly mild or moderate.174
BelimumabIn a pilot study, Gordon et al compared MMF alone vs. MMF plus belimumab (BEL) in 20 patients, with a 1:1 randomization. Although no differences between the interventions in the assessed outcomes were observed, basic science evidence supports its potential use13 and the authors suggest the performance of a larger clinical trial.131 No differences in infection rates were observed, with 18 in the MMF/BEL vs. 16 in the MMF alone. The most frequent infections in the MMF/BEL were upper respiratory involvement (n = 5), UTI (n = 3) and HSV stomatitis (n = 2).
Tumor necrosis factor α inhibitorsTumor Necrosis Factor (TNF) α inhibitors might play a role in the management of articular compromise, although they are not currently recommended by experts as a first line therapy.9,13,175 Data for etanercept, adalimumab and infliximab have been reported from small studies in patients with SSc.176,177 Regarding infectious disease risk, a low frequency of events has been reported: uncomplicated HZV (although this patient received concomitant prednisone and AZA),177 infected DU and one sepsis episode.178 Lam et al. reported no cases of OIs in 18 patients.179 Anecdotally, one case of pulmonary actinomycosis was reported on infliximab.69 Nonetheless, safety concerns based on data from other autoimmune/autoinflammatory diseases should be considered. Patients on TNF-α inhibitors may present a double risk of serious infections compared to placebo.180 Several infectious complications have been reported, namely respiratory infections, UTIs, gastrointestinal infections, skin/soft tissue infections, HZV, and HBV reactivation.180 OIs have been described, such as endemic mycoses, CMV, P. jirovecii pneumonia, Aspergillus spp., among others, in which atypical manifestations may be observed.180 As TB infection is a major concern on TNF-α inhibitors, active screening prior to biologic application must be performed. Non-TB-mycobacteria infections may be observed.180
Hematopoietic stem cell transplant and high dose CYCHematopoietic Stem Cell Transplant (HSCT) is an emerging therapeutic strategy in selected patients with SSC. The procedure is performed in three main phases: 1) mobilization of stem cells from the bone marrow to the peripheral blood using priming agents (i.e., high dose CYC), followed by the collection of the mobilized stem cells; 2) immunoablation or myeloablation through the administration of conditioning regimens (i.e., total body irradiation or alkylating agents); 3) infusion of autologous CD4+ stem cells, the so-called “transplantation”.181,182
Infectious complications are usual in the vast majority of transplantation procedures and three risk phases have been proposed. The first phase is known as ‘early pre-engraftment’, which occurs previous to the “transplantation” during the first 2–4 weeks, and is characterized by infections due to mucositis and neutropenia. The most frequent etiologies are bacterial (Gram-positive or Gram-negative), HSV and Candida spp. The second phase is known as ‘early post-engraftment’, and occurs from days 30–100 after the “transplantation”. The most frequent etiologies are bacterial, fungal (e.g., Aspergillus spp., P. jirovecii), and viral (e.g., CMV, EBV) infections. The last phase is known as ‘late post-engraftment’ and is characterized by infections due to encapsulated bacteria, Nocardia spp., Aspergillus spp., P. jirovecii, CMV, EBV, among others. Nonetheless, an increased incidence of respiratory virus infections and complications is present throughout all the phases.105 These periods are as applicable in patients with refractory systemic autoimmune diseases undergoing autologous stem cell transplantation. Szodoray et al. reported that immune reconstitution started with CD56 + NK cells around day 30, followed by CD8 + T cells around day 60 post-transplantation. The number of B cells and CD4 + T cells became normal within 150 days.183
The HSCT experience in SSc has illustrated the risk of infection. The SCOT trial compared CYC in a high dose regimen (i.e., initial intravenous dose of 500 mg/m2, followed by 11 monthly infusions of 750 mg/m2) with HSCT (which included a mobilization dose of CYC of 120 mg/Kg), and reported a similar infection rate among both groups. In the CYC arm, 84% of patients (n = 31) presented an infectious disease during follow-up, although only 9 patients presented a serious infection. The most frequent infections were UTIs (40%) and upper respiratory infections (21.6%); up to 10% of patients presented an infected DU. In contrast, in the HSCT group 97% of patients (n = 33) presented an infectious episode and serious infections appeared to be more frequent. The most frequent infections were HZV (38.2%), upper respiratory infection (26.5%) and pneumonia (26.5%); bacteremia was reported in 11.8% of patients. Varicella Zoster occurred in 12 patients in the HSCT compared to 1 in the CYC arm. Five cases of CMV reactivation were observed in the HSCT arm.184
The ASTIS trial compared CYC (i.e., 750 mg/m2 monthly for 12 months) with HSCT (i.e., mobilization dose of CYC of 200 mg/Kg for 4 days); viral infection or reactivation after randomization were recorded. Infections were infrequent among the CYC group (n = 77) with only one episode of HSV primary infection. In contrast, 27.8% of patients (n = 22) in the HSCT group presented an infectious disease. Reactivation of EBV (12.5%), HSV (22%) and recurrent CMV (18.7%) were the most frequent phenomena.185
The ASSIST trial compared CYC (i.e., 1000 mg/m2 monthly for 12 months) with HSCT (i.e., mobilization dose of CYC of 200 mg/Kg for 4 days). The authors reported only one episode of cellulitis on CYC and one episode of C. difficile positive stool sample in both arms, Micrococcus bacteremia and CMV reactivation were observed in the HSCT arm.186
Del Papa et al., in a retrospective study of HSCT in SSc, recorded eight cases of fever of unknown origin, five cases of fever with positive blood culture and three cases of pneumonia. All the observed infections resolved with adequate antibiotic treatment.182
Solid organ transplantationSolid organ transplant recipients also have an increased risk of infectious complications, in close relation to the dose and type of immunosuppressive drugs used. Other factors related include surgical complications, prolonged hospitalization, other invasive procedures, and viral reactivations, mainly CMV, with increased risk of opportunistic infections, allograft injury and rejection.187,188
In any post-transplant patient, the risk of infection is a function of the net state of immunosuppression. As described in bone marrow transplant recipients, the risk of infection has been divided into three periods, the first is referred as “early period”, which includes infections within the first month post transplantation and is characterized by health care-related infections, donor derived infections (e.g., HSV, rabies, HIV, Trypanosoma cruzi) and recipient derived infections, usually related to previous colonization. The second or “intermediate period” includes infections during the period from 1 to 6 months after transplantation. It is characterized by the greatest risk of opportunistic infections, mainly intracellular microorganisms, including P. jirovecii, TB, Cryptococcus neoformans, CMV, EBV, Toxoplasma gondii, among others. The third or “late period” occurs after the sixth month post transplantation and is characterized by an increased incidence of community acquired infections, mainly pneumonia and UTIs. Infections by Aspergillus spp., Zygomycota, CMV infections (colitis and retinitis), and post-transplantation lymphoproliferative disorder, associated in most cases to EBV have been described.188
In solid organ transplant recipients, the most common site of infection is the allograft. In SSc patients, the most frequently transplanted organ is the lung. Bacterial infection is the most frequent infectious complication (35–66%) for recipients with unilateral or bilateral lung transplant. Bacterial infections presenting in the immediate post transplantation period usually show relation with pre-transplantation colonization, the surgical procedure itself and accompanying technical complications, intubation or prolonged hospitalization. Late onset bacterial infections are related to increased immunosuppression due to rejection, invasive diagnostic procedures and the development of bronchiolitis obliterans.189
Other therapies and their potential risk of infectionThe intravenous route of some therapies for SSc management should raise awareness towards the potential for infectious complications. Prostanoids (e.g., epoprostenol, treprostinil) are recommended for the management of PAH, Raynaud’s phenomenon and DU.9,10 Cellulitis and sepsis have been described with their application. Among the most frequent sepsis etiologies are Staphylococcus epidermidis, Micrococcus spp., S. aureus, Pseudomonas spp., and Enterobacter spp.190,191 A higher infection rate associated with treprostinil compared to epoprostenol has been suggested.5,192,193
In patients with gastroesophageal reflux disease or gastrointestinal dysmotility, the use of high dose PPIs is recommended.9 Recently, the prolonged use of PPIs has been associated with a higher risk of C.difficile infection73,194,195 and community-acquired pneumonia, although evidence for the latter is conflicting.196–199
With regard to ILD management with anti-fibrotic therapy, current evidence supports its efficacy in SSc. Nintedanib is not associated with an increased risk of infection in SSc or other patient population.200–203 As observed with most tyrosine kinase inhibitors, a common side effect is diarrhea, which has not been associated with an infectious etiology. Although its cause is still unknown, receptor inhabitation and direct mucosal damage have been proposed.204,205 On the other hand, although direct evidence of pirfenidone in SSc is lacking, trials are ongoing; to date, pirfenidone has not been associated with an increased risk of infection in other patient population.206,207
Other therapeutic strategies addressing several domains of the disease have not been associated with a higher risk of infection in SSc. These include phosphodiesterase-5 inhibitors, calcium channel blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor antagonist, endothelin receptor antagonists, and riociguat.10,208–213
Infection preventionRisk management strategies, including prophylaxis and vaccination must be considered within the bundle of care. Tables 2 and 3 summarize screening recommendations, potential prophylaxis and available prophylactic therapies to be considered in patients with SSc. Several recommendations guidelines for the prevention of infection in patients receiving immunosuppressive treatments are available.157,168,214–223 However, there is a lack of information in the literature on infectious diseases prevention in patients with SSc, probably due to the low incidence of the disease. Thus, extrapolated data from other diseases, in which similar therapies are used (e.g., rheumatic, malignancies, transplants, etc.), might be considered. When considering vaccination and chemoprophylaxis for patients with SSc, the first thought a rheumatologist should have is: how immunosuppressed is my patient and what is the mechanism of immunosuppression? One shall not forget an increased risk of infections due to the disease itself. Vaccination, screening for latent or active infections, and several other measures for infection prevention should be performed prior to immunosuppressive agent application (215,223,224)
Screening recommendations and available prophylactic therapies proposed for autoimmune diseases.
| Recommendation by infectious disease | Available therapies |
|---|---|
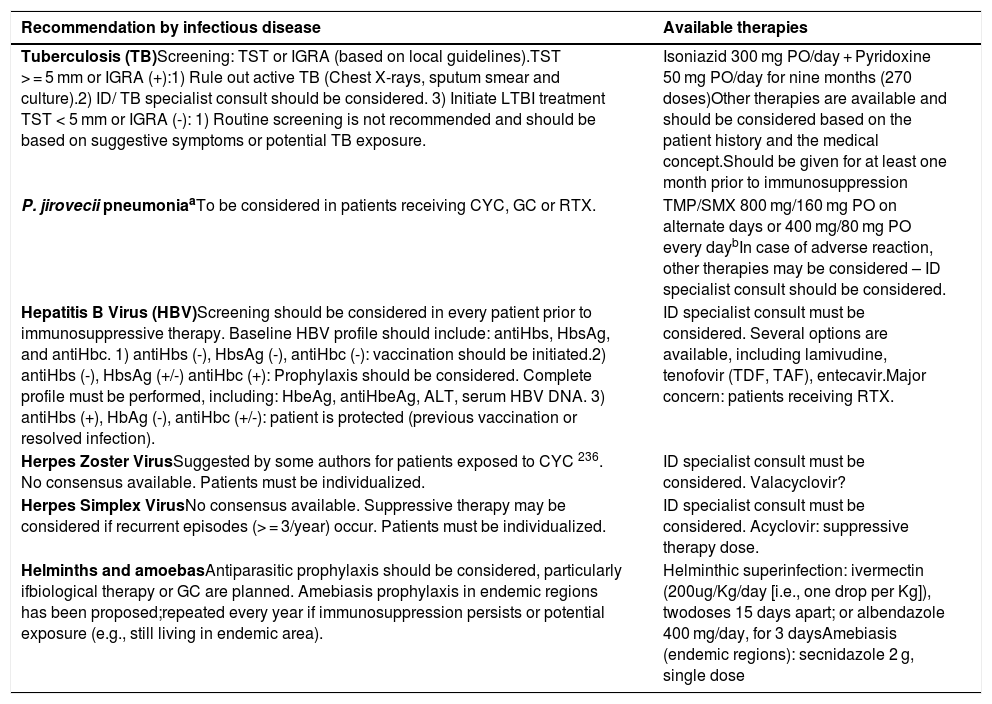
| Tuberculosis (TB)Screening: TST or IGRA (based on local guidelines).TST > = 5 mm or IGRA (+):1) Rule out active TB (Chest X-rays, sputum smear and culture).2) ID/ TB specialist consult should be considered. 3) Initiate LTBI treatment TST < 5 mm or IGRA (-): 1) Routine screening is not recommended and should be based on suggestive symptoms or potential TB exposure. | Isoniazid 300 mg PO/day + Pyridoxine 50 mg PO/day for nine months (270 doses)Other therapies are available and should be considered based on the patient history and the medical concept.Should be given for at least one month prior to immunosuppression |
| P. jirovecii pneumoniaaTo be considered in patients receiving CYC, GC or RTX. | TMP/SMX 800 mg/160 mg PO on alternate days or 400 mg/80 mg PO every daybIn case of adverse reaction, other therapies may be considered – ID specialist consult should be considered. |
| Hepatitis B Virus (HBV)Screening should be considered in every patient prior to immunosuppressive therapy. Baseline HBV profile should include: antiHbs, HbsAg, and antiHbc. 1) antiHbs (-), HbsAg (-), antiHbc (-): vaccination should be initiated.2) antiHbs (-), HbsAg (+/-) antiHbc (+): Prophylaxis should be considered. Complete profile must be performed, including: HbeAg, antiHbeAg, ALT, serum HBV DNA. 3) antiHbs (+), HbAg (-), antiHbc (+/-): patient is protected (previous vaccination or resolved infection). | ID specialist consult must be considered. Several options are available, including lamivudine, tenofovir (TDF, TAF), entecavir.Major concern: patients receiving RTX. |
| Herpes Zoster VirusSuggested by some authors for patients exposed to CYC 236. No consensus available. Patients must be individualized. | ID specialist consult must be considered. Valacyclovir? |
| Herpes Simplex VirusNo consensus available. Suppressive therapy may be considered if recurrent episodes (> = 3/year) occur. Patients must be individualized. | ID specialist consult must be considered. Acyclovir: suppressive therapy dose. |
| Helminths and amoebasAntiparasitic prophylaxis should be considered, particularly ifbiological therapy or GC are planned. Amebiasis prophylaxis in endemic regions has been proposed;repeated every year if immunosuppression persists or potential exposure (e.g., still living in endemic area). | Helminthic superinfection: ivermectin (200ug/Kg/day [i.e., one drop per Kg]), twodoses 15 days apart; or albendazole 400 mg/day, for 3 daysAmebiasis (endemic regions): secnidazole 2 g, single dose |
antiHbc, Hepatitis B virus total core antibody; antiHbeAg, Hepatitis B virus E antigen antibody; antiHbs, Hepatitis B virus surface antibody; CYC, Cyclophosphamide; GC, Glucocorticoids; HbeAg, Hepatitis B virus E antigen; HbsAG, Hepatitis B virus surface antigen; ID, Infectious diseases; IGRA, Interferon Gamma Release Assay; LTBI, Latent TB Infection; RTX, Rituximab; TAF, Tenofovir Alafenamide Fumarate; TDF, Tenofovir Disoproxil Fumarate; TMP/SMX, Trimethoprim/Sulfamethoxazole; TNF, Tumor Necrosis Factor; TST, Tuberculin Skin Test.
This table is based on references and author’s experience.
Potential prophylaxis to be considered in patients with SSC.
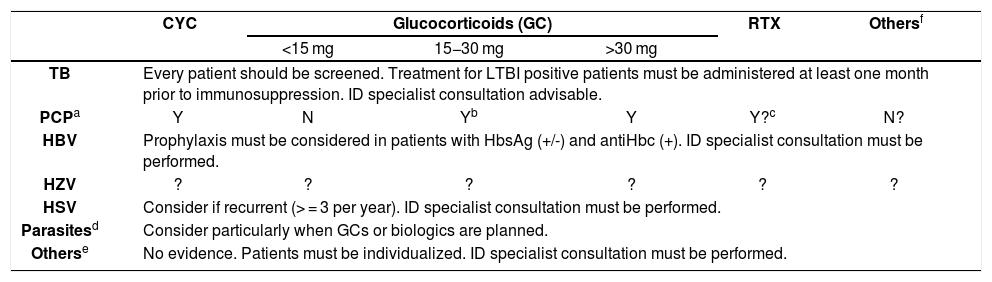
| CYC | Glucocorticoids (GC) | RTX | Othersf | |||
| <15 mg | 15−30 mg | >30 mg | ||||
| TB | Every patient should be screened. Treatment for LTBI positive patients must be administered at least one month prior to immunosuppression. ID specialist consultation advisable. | |||||
| PCPa | Y | N | Yb | Y | Y?c | N? |
| HBV | Prophylaxis must be considered in patients with HbsAg (+/-) and antiHbc (+). ID specialist consultation must be performed. | |||||
| HZV | ? | ? | ? | ? | ? | ? |
| HSV | Consider if recurrent (> = 3 per year). ID specialist consultation must be performed. | |||||
| Parasitesd | Consider particularly when GCs or biologics are planned. | |||||
| Otherse | No evidence. Patients must be individualized. ID specialist consultation must be performed. | |||||
antiHbc, HBV total core antibody; CYC, Cyclophosphamide; GC, Glucocorticoids; HbsAg, HBV surface antigen; HBV, Hepatitis B virus; HSV, Herpes Simplex Virus; HZV, Herpes Zoster Virus; ID, Infectious diseases; LTBI, Latent TB Infection; PCP, Pneumocystis jirovecii pneumonia; RTX, Rituximab; TB, Tuberculosis.
This table is based on references and author’s experience.
As shown, the most frequently used immunosuppressants in SSc may increase the risk of infectious complications.5 Experience from other autoimmune diseases has suggested the protective effect of chemoprophylaxis whenever immunosuppression is necessary.67
A particular interest and evidence for P. jirovecii pneumonia are available, in which contrasting incidences are identified depending on the underlying disease, from as low as 0.1% in RA to as high as 12% in granulomatosis with polyangiitis.225 One shall consider that P. jirovecii pneumonia has a low rate among patients with rheumatic diseases, although its course tends to be severe and potentially fatal.226 For instance, recent guidelines for the management of ANCA-associated vasculitis “encourage the use of prophylaxis against P. jirovecii in all patients treated with CYC, where not contraindicated”.227,228 Mounting evidence supports the utility of prophylaxis in patients on glucocorticoids, particularly in those with high doses (≥ 30 mg/day), since a dose-dependent risk is observed.67,229 In addition, the use of RTX also appears to be associated with a higher risk of P. jirovecii pneumonia and a protective effect of prophylaxis has been reported.230 Winthrop & Baddley recently proposed a tailored prophylaxis schema for different autoimmune rheumatic diseases on glucocorticoids, in which SSc patients with ≥ 30 mg/day dose would benefit and those with doses below 15 mg/day would not; those patients who receive between 15 mg and 30 mg/day should be assessed on an individual risk basis, based on the presence of at least one of these factors: baseline lymphopenia, low CD4 count, CYC use, anti-TNF or RTX use, initial glucocorticoids dose ≥ 60 mg/day (Table 3). Nonetheless, these recommendations are based on expert opinion and the authors advocate for further studies67; some other authors believe that “more personalized risk assessments are needed to inform P. jirovecii pneumonia prophylaxis”, as data are contrasting.226 There are no studies in SSc showing these outcomes, probably due to the difficulty in gathering large cohorts of patients with this disease. However, there are reports of SSc patients on these therapies who develop opportunistic infections by Mycobacterium avium intracellulare and P. jirovecii.231
As the optimal TMP/SMX prophylactic dose in rheumatic diseases is unclear, an open RCT in Japan explored this subject. Patients were mainly exposed to glucocorticoids (>0.6 mg/Kg/day), and received a daily low dose TMP/SMX (80 mg/400 mg, or 40 mg/200 mg, or escalating dose from 8 mg/40 mg until 40 mg/200 mg was reached); no events of P. jirovecii pneumonia were observed in any group. The authors suggested a better tolerability and safety of 40 mg/200 mg doses.232 Recommended regimens for ANCA-associated vasculitis prophylaxis on CYC are: TMP/SMX 160 mg/800 mg on alternate days or 80 mg/400 mg every day. In case of adverse reaction or contraindication, pentamidine, atovaquone or dapsone are alternatives,227,228 however, only the latter is available in Colombia. In this scenario, an infectious diseases specialist consultation is advisable. As quality evidence is unavailable regarding doses, one might extrapolate recommended doses for human immunodeficiency virus (HIV), namely dapsone 100 mg/day or, in situations in which the recommended agents cannot be administered or are not tolerated, clindamycin plus primaquine.233 British guidelines on ANCA-associated vasculitis suggest the use of similar prophylaxis regimen in any patient with immunosuppression, including RTX.234 Some authors have suggested that one shall not rely solely on CD4-cell count for the guidance of P. jirovecii prophylaxis in autoimmune/inflammatory diseases.235
The incidence of HZV in patients under immunosuppression is an increasing challenge, however, the utility of prophylaxis among patients with autoimmune rheumatic diseases is unclear and safety data for vaccination are lacking. A recent study in patients receiving CYC for SLE or vasculitis explored the effect of anti-viral prophylaxis with valacyclovir. The authors reported an increased risk of HZV in patients with lymphopenia <500/μL at CYC initiation (HR 4.36, CI 0.51-37-31) and suggested that valacyclovir should be considered in these scenarios; none of the 19 patients receiving valacyclovir presented HZV. Interestingly, a higher risk of HZV has been found in SLE, which may suggest a different risk based on the underlying disease.236 Data are lacking for SSc.
As aforementioned, SSc patients are at an increased risk of TB (and probably non-TB-mycobacteria) infection due to the disease itself (particularly in the presence of pulmonary involvement and PAH) and to pharmacological immunosuppression.57,58 Glucocorticoids and TNF-α inhibitors are well-known risk factors for TB.237,238 Therefore, screening for TB infection using either a tuberculin skin test or an interferon gamma release assay is the basis of active disease prevention, and should be implemented in patients who will undergo immunosuppression.215,224 Whenever latent TB infection is detected, it must be treated according to guidelines.224,239 Expert recommendations for RA suggest to screen for pulmonary non-TB-mycobacteria prior to biologic therapy in patients with lung structural abnormalities (particularly bronchiectasis) or those with unexplained chronic cough. Although there is a higher risk of non-TB-mycobacteria in patients with ILD, such as SSc,62 data to support the need of prophylaxis are not available.238
VaccinesSome preventable infectious diseases, such as influenza and pneumococcal pneumonia, may manifest more severely in patients with SSc. For instance, patients with ILD who become infected by influenza are at greater risk of respiratory failure and morbidity. Therefore, vaccination becomes an important strategy of care.240 However, safety and efficacy of vaccination in patients on immunosuppressive therapy and patients with autoimmunity has always concerned clinicians, as some studies have shown immunosuppressive therapy may hamper immunologic response to vaccination.241,242 These believes may explain a low vaccination rate against influenza in these patients, as described in a French cohort of patients with systemic autoimmune diseases on immunosuppressive therapy.243
InfluenzaRecent research on influenza vaccination in patients with SSc has proven that different presentations of the influenza vaccine are not only safe in this population, but effective. A study by Sampaio-Barros et al. evaluating the outcomes of vaccination with H1N1 vaccine, found that there were no statistically significant differences in seroprotection (83.7 vs. 76.1%; P = 0.20) or seroconversion (76.1 vs.72.8%; P = 0.61) rates between SSc patients and healthy controls, despite receiving different immunosuppressive medications. A similar incidence of systemic side effects after vaccination was also observed (25 vs. 31.5%; P = 0.33).244 Another study by Setti et al., assessed the effectiveness of influenza AH3N2/AH1N1/B vaccine in individuals with SSc compared to a healthy control group. Although a higher effectiveness was observed in the control group, a good humoral response was achieved by SSc patients with no higher incidence of adverse effects or alterations in the course of the disease, proving its safety and efficacy.240
Pneumococcal pneumoniaPublic health authorities recommend vaccination for Streptococcus pneumoniae (PCV13 or PPVP23) in immunocompromised adults245 and different studies have described a fair immune response to pneumococcal vaccine in patients with SSc. A study by Hesselstrand et al., comparing the immune response of patients with SSc with or without DMARDs (disease-modifying antirheumatic drugs) against a control group, receiving the PPVP23 or the PCV13 pneumococcal vaccine, found that the production of antibodies against pneumococcal serotypes in SSc patients without DMARDs was similar to that of the healthy control group. No differences were described between the PPVP23 and the PCV13 vaccine. However, patients on DMARDS had a lower antibody production.246 Other study by Mercado et al. described the immunogenicity of different pneumococcal antigens after vaccination with the PPVP23 in a group of 18 patients with SSC. They found that after vaccination, all patients had a two-fold rise in antibodies against the different antigens despite being treated with CYC. No differences were observed between limited SSc and diffuse SSc.247
Additional recommendations for immunization in patients with autoimmune inflammatory rheumatic diseases from the CDC,222 the Infectious Diseases Society of Colombia (ACIN),222 the Infectious Diseases Society of America (IDSA),222 the Colombian Association of Rheumatology (ASOREUMA)216 and the European League Against Rheumatism (EULAR),221 are summarized in Table 4. Novel interventions adapted to the South American context should be assessed for future recommendations on vaccination in patients with autoimmune diseases, such as vaccination for dengue virus, meningococcal disease and vaccination with the recently developed inactivated vaccine for HZV (Shingrix), which has shown to be more effective in preventing HZV and postherpetic neuralgia.248
Major recommendations for vaccination in patients with autoimmune inflammatory rheumatic diseases and patients with altered immunocompetence.
| Recommendation | EULAR (2011)(221) | IDSA (2013)(222) | ACIN (2016)(223) | ASOREUMA (2016)(216) | Pérez-Díaz et al. (2016)(215) | CDC (2018)(222) |
|---|---|---|---|---|---|---|
| X | X | X | X | X | X |
| X | X | X | |||
| X | X | X | X | X | |
| X | X | ||||
| X | X | X | X | X | X |
| X | |||||
| X | X | X | |||
| ||||||
| ||||||
| ||||||
| X | X | X | |||
| X | X | X | |||
| X | X | X | X | ||
| X | X | X | |||
| X | X | X | X | X | X |
| X | X | X | X | X | X |
| X | X | X | X | X | |
| X | X | X | X | ||
| X | X | X | X | ||
| X | X | X | X | ||
| X | X | X | X | ||
| X | X | ||||
| X | X | X | X | X | |
High level immunosuppression:
| Low level immunosuppression:
| |||||
ACIN, Asociación Colombiana de Infectología (Colombian Association of Infectious Diseases); ASOREUMA, Asociación Colombiana de Reumatología (Colombian Association of Rheumatology); BCG, Bacillus Calmette-Guerin; CDC, Centers for Disease Control; DMARDS, Disease-modifying anti-rheumatic drugs; EULAR, European League Against Rheumatism; GVHD, Graft-vs-Host disease; Hib, Haemophilus influenza b; IDSA, Infectious Diseases Society of America; HSCT, Hematopoietic Stem Cell Transplantation; PCV, Pneumococcal conjugate vaccine; PPSV, Pneumococcal polysaccharide vaccine; SLE, Systemic lupus erythematosus; TNF-α, Tumor necrosis factor α.
A baseline screening for different infectious diseases should be considered, as strong immunosuppression in patients with SSc is expected. Interpretation of screening tests has been discussed elegantly elsewhere.102,215,224 This screening should be based on local epidemiology, which should always include: TB, HIV, Hepatitis A Virus, HBV, Hepatitis C Virus, Toxoplasma, EBV, CMV, Varicella Zoster Virus, syphilis and a stool test. Some other entities to be considered are: Chagas (Trypanosoma cruzi) and Human T Lymphocyte Virus 1–2.215 Screening for histoplasmosis it not routinely recommended and should only be considered in endemic areas.215 Antiparasitic prophylaxis should be considered, particularly if biological therapy or glucocorticoids are planned. To prevent helminthic superinfection, the use of ivermectin (200ug/Kg/day [i.e., one drop per Kg]) in two doses 15 days apart, or albendazole 400 mg/day for 3 days is recommended.223 Amebiasis prophylaxis in endemic regions has been proposed using secnidazole 2 grams in a single dose, and repeating it every year if immunosuppression persists or if the patient is still living in an endemic area.215
ConclusionInfections are a growing concern in the management of SSc, as evidence suggests them as a rising cause of admission and mortality. Most of the studies on this issue come from the USA and Europe, with few studies from Asia, which may not represent Latin-American populations, thus, local studies are warranted. Patients with SSc present a higher risk of infectious complications due to the structural damage of the disease and immunosuppressive treatment. Novel therapeutic agents have shown promising results; however, an increasing incidence of infections may be expected as has been observed in other rheumatic diseases. Data on the effects of antimicrobial prophylaxis and risk management strategies in SSc are lacking, although experience from other autoimmune diseases may shed light on this issue. An increasing awareness among treating specialties, particularly rheumatologists is warranted. The wide adoption of risk management strategies, including underused measures such as vaccination, is necessary.
FundingThe present work did not receive any funding.
Conflicts of interestThe authors declare there are no conflicts of interest relevant for the present manuscript.
We thank Dr. Diana Romero-Alvernia for her valuable comments on the manuscript.
Please cite this article as: Barahona-Correa JE, De la Hoz A, López MJ, Garzón J, Allanore Y, Quintana-López G. Infecciones y esclerosis sistémica: un desafío emergente.Rev Colomb Reumatol. 2020;27:62–84.