To evaluate the association between autoantibodies with clinical manifestations (extraglandular and glandular) and histopathological findings of minor salivary gland biopsy in primary Sjögren’s syndrome.
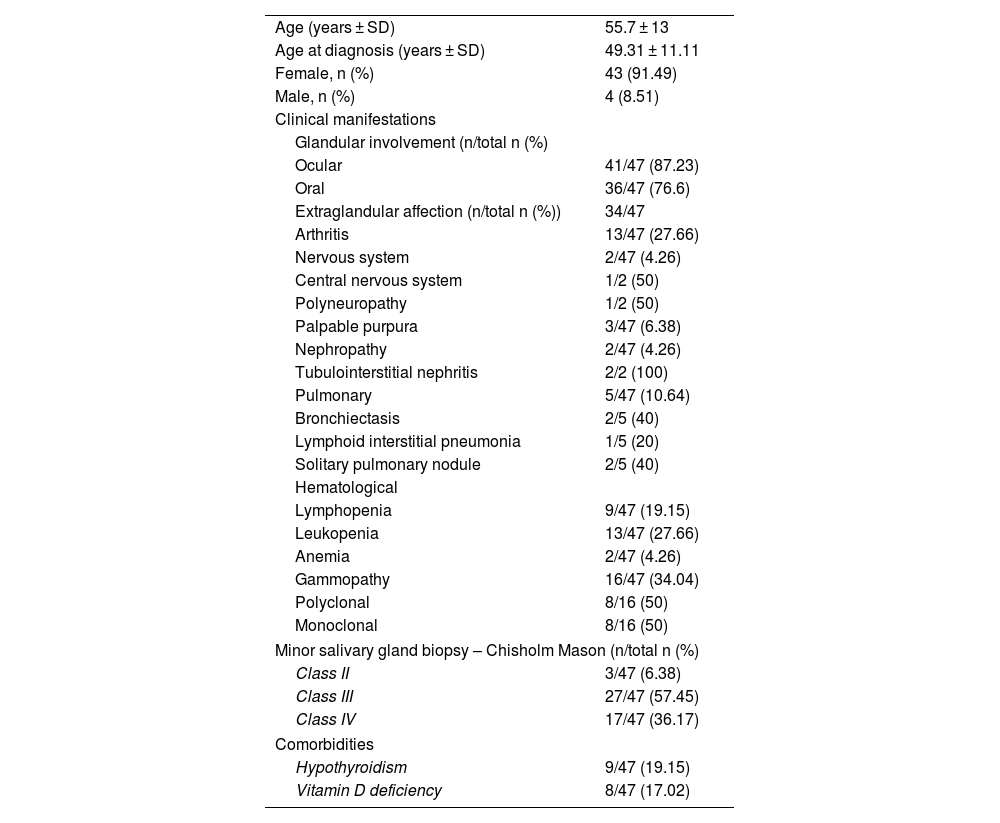
Materials and methodsObservational, descriptive, and cross-sectional study. Forty-seven patients with pSS according to the ACR/EULAR 2016 criteria were included. A face-to-face survey, a review of medical records, and the measurement of autoantibodies (Ab) anti-Ro 52, anti-Ro 60, anti-La, antinuclear antibodies (ANA), rheumatoid factor (RF) IgA, IgG and IgM, and anti-alpha fodrin IgA and IgG were done. Characterization of the population and analysis of the association between clinical characteristics, autoantibodies, and histopathology were performed.
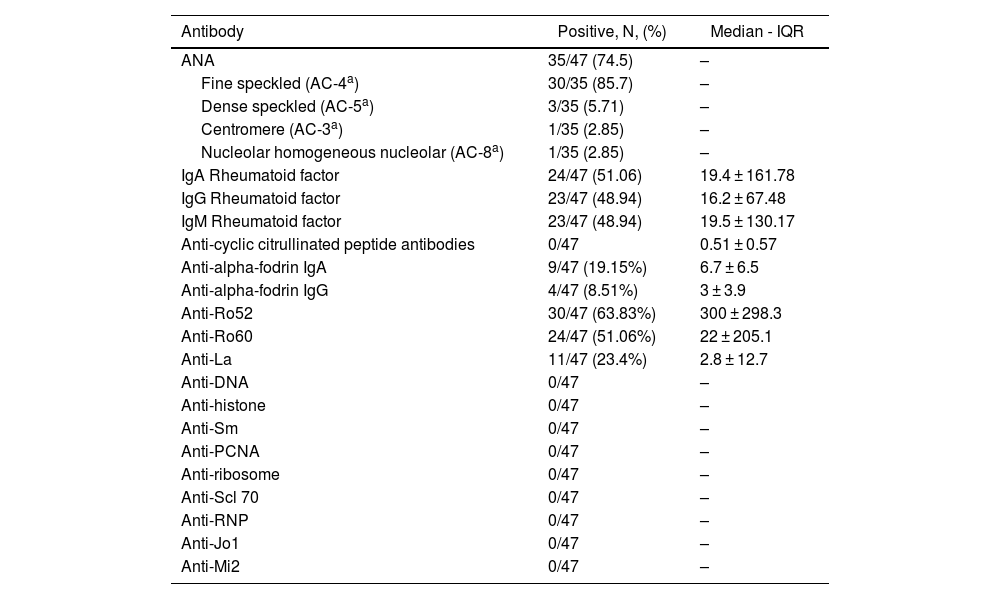
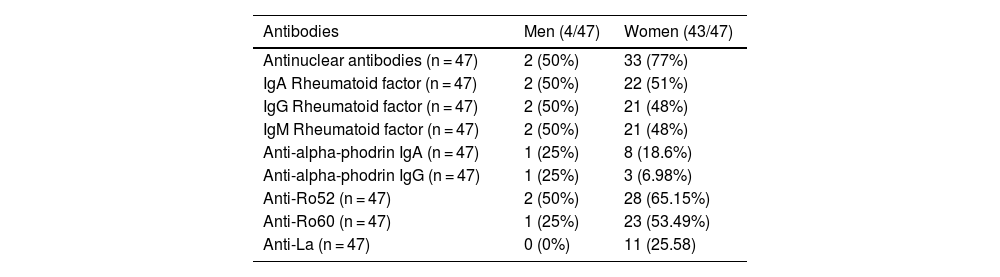
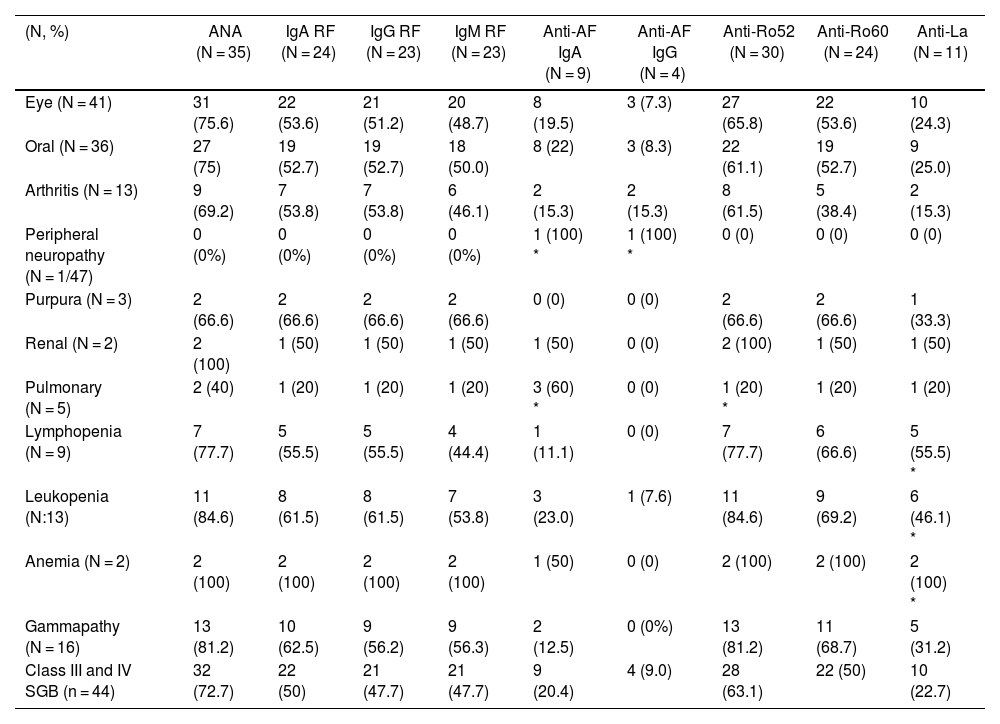
ResultsAssociation of anti-alpha fodrin IgA and anti-Ro 52 Ab was found with pulmonary involvement (P = .014 and P = .031 respectively) and anti-La antibodies with haematological manifestations, specifically leukopenia (P = .011), lymphopenia (P = .023), and anaemia (P = .09). We found no association between the histopathological findings of the minor salivary gland biopsy and extraglandular manifestations.
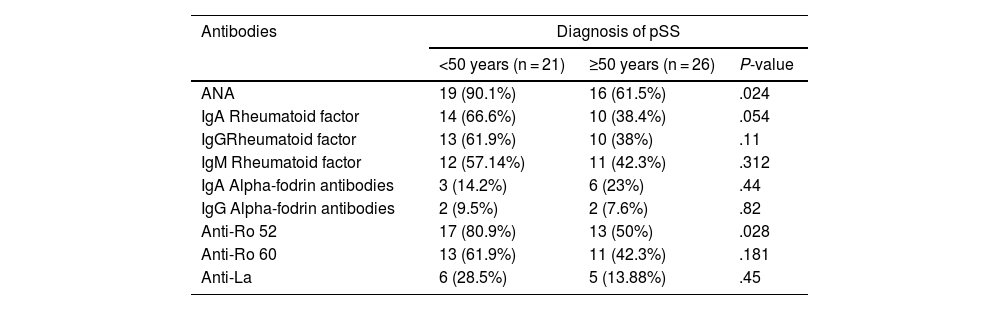
ConclusionsThe activation of B cells, reflected in the increased production of autoantibodies, is related to extraglandular manifestations in pSS, which is observed more frequently in patients with earlier diagnosis.
Evaluar la asociación entre los autoanticuerpos, las manifestaciones clínicas (extraglandulares y glandulares) y los hallazgos histopatológicos de la biopsia de glándula salival menor en el síndrome de Sjögren primario (SSp).
Materiales y métodosEstudio observacional, descriptivo, de corte transversal. Se incluyeron 47 pacientes con diagnóstico de SSp por criterios ACR/Eular 2016. Se hizo una encuesta presencial, así como una revisión de la historia clínica y la medición de los autoanticuerpos (Ac) anti-Ro 52, anti-Ro 60, anti-La, anticuerpos antinucleares (AAN), factor reumatoide (FR) IgA, IgG e IgM y anti-alfa fodrina IgA e IgG. Se hizo la caracterización de la población y el análisis de la asociación entre las características clínicas, los autoanticuerpos y la histopatología.
ResultadosSe encontró asociación de Ac anti-alfa fodrina IgA y anti-Ro 52 con el compromiso pulmonar (P = ,014 y P = ,031, respectivamente) y de los anticuerpos Anti-La con manifestaciones hematológicas, específicamente leucopenia (P = ,011), linfopenia (P = ,023) y anemia (P = ,09). No se encontró asociación entre los hallazgos histopatológicos de la biopsia de glándula salival menor y las manifestaciones extraglandulares.
ConclusionesLa activación de las células B, que se refleja en una mayor producción de autoanticuerpos, se relaciona con manifestaciones extraglandulares en el SSp, las cuales se observan con mayor frecuencia en pacientes con diagnóstico más temprano.