Systemic sclerosis (SSc) is an immune-mediated disease characterized by small vessel vasculopathy and fibroblast dysfunction that leads to increased production of extracellular matrix. Interstitial lung disease represents one of the most common complications, by affecting almost 70% of patients with SSc.
ObjectiveTo evaluate the effectiveness of the pharmacological treatments available for systemic sclerosis-associated interstitial lung disease (SSc-ILD) based on pulmonary function tests and radiologic findings.
Materials and methodsA systematic literature review and meta-analysis were conducted. A thorough literature search was made in EMBASE, PUBMED, and Cochrane CENTRAL to collect studies published between January 2015 and July 31 of 2019. The primary outcomes were forced vital capacity (FVC), diffusion capacity of carbon monoxide (DLCO), and high-resolution computed tomography findings (HR-CT). Studies using medications for the treatment of SSc-ILD including cyclophosphamide (CYC), mycophenolate mofetil (MMF), nintedanib, pirfenidone, or rituximab (RTX) were included. Effect measures were calculated based on available data, and a meta-analysis was made with these results.
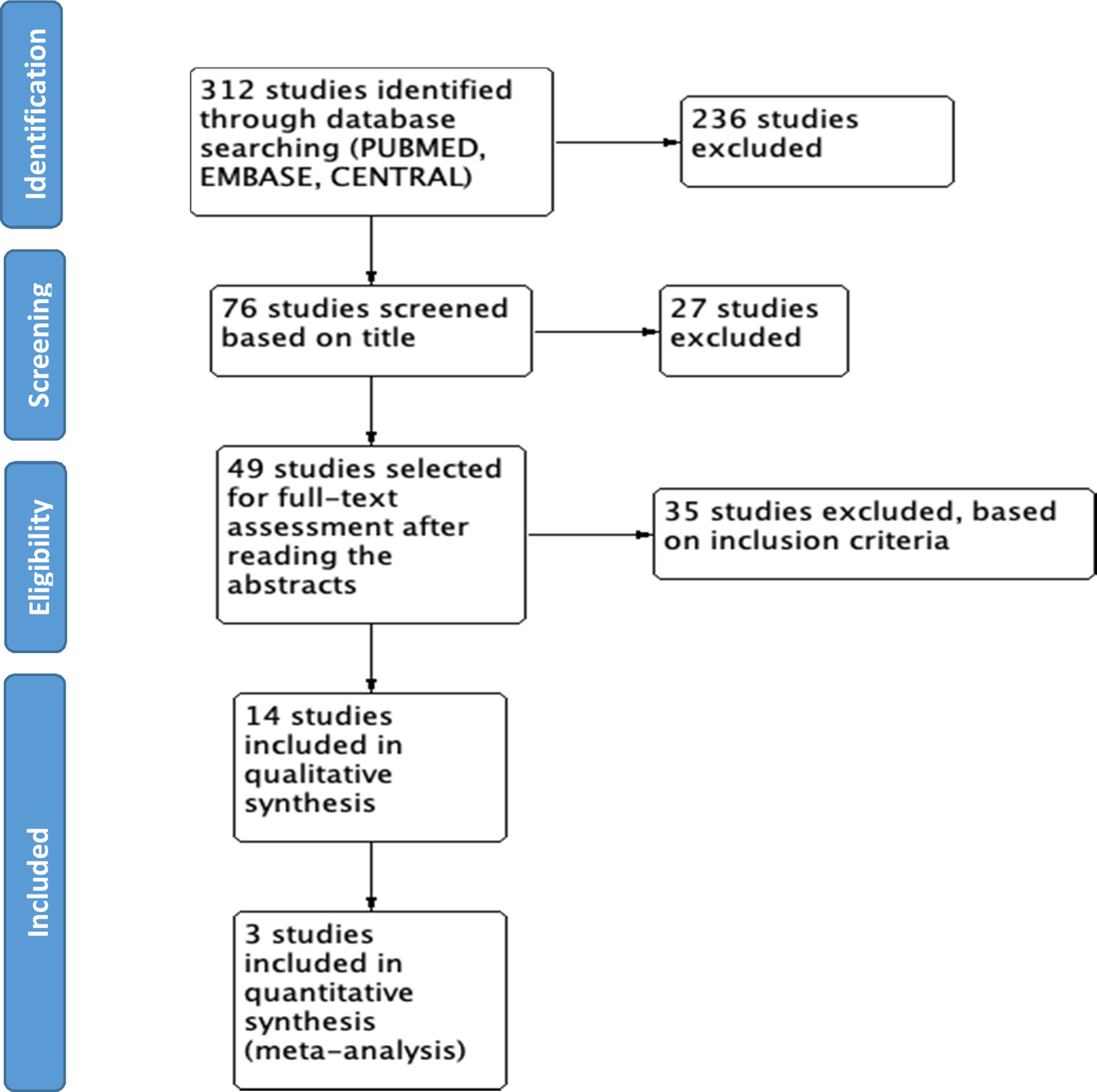
ResultsWe found a total of 312 studies. 49 studies were selected for full-text assessment after reading the abstracts. Finally, 14 studies were selected to be included in the final review. 2 meta-analyses, 8 clinical trials, 3 retrospective cohorts, and one nested case–control study were identified. The available evidence supports the use of CYC and MMF as the best options for the treatment of SSc-ILD, with MMF being the preferred option based on a better safety profile. Other medications such as RTX, pirfenidone, and nintedanib show potential as alternatives to CYC. The overall quality of evidence available is adequate based on generally well-designed studies. Meta-analysis was done by assessing >5% or >10% decrease of FVC when comparing pharmacological agents vs. placebo. Results show that the use of pharmacological agents is negatively associated with the worsening of FVC. However, high heterogeneity limits the number of studies used during quantitative analysis, affecting the overall results.
ConclusionsImmunosuppressive therapies remain as the cornerstone of treatment of SSc-ILD, as most evidence show improvement or slow progression of pulmonary function tests by using them, especially CYC and MMF. However, more evidence is required regarding the use of alternative pharmacological agents, in search of an improvement in the quality of life of these patients.
La esclerosis sistémica (ES) es una enfermedad mediada por el sistema inmunitario, caracterizada por vasculopatía de pequeños vasos y disfunción de los fibroblastos, que da lugar a una mayor producción de matriz extracelular. La enfermedad pulmonar intersticial representa una de las complicaciones más comunes, ya que afecta a cerca del 70% de los pacientes con ES.
ObjetivoEvaluar la efectividad de los tratamientos farmacológicos disponibles para la enfermedad pulmonar intersticial asociada a la esclerosis sistémica (EPI-ES) basándonos en las pruebas de función pulmonar y los hallazgos radiológicos.
Materiales y métodosSe llevó a cabo una revisión sistemática de la literatura y un meta-análisis. Se hizo una búsqueda exhaustiva de literatura en EMBASE, PUBMED y Cochrane CENTRAL con el fin de recopilar los estudios publicados entre enero de 2015 y el 31 de julio de 2019. Los resultados primarios fueron la capacidad vital forzada (CVF), la capacidad de difusión del monóxido de carbono (DLCO, por sus siglas en inglés), y los hallazgos de la tomografía computarizada de alta resolución (TCAR). Se incluyeron los estudios que utilizaron medicamentos para el tratamiento de la EPI-ES, incluyendo ciclofosfamida (CYC), micofenolato de mofetilo (MMF), nintedanib, pirfenidona o rituximab (RTX). Las medidas de efecto se calcularon en base a los datos disponibles, y se hizo un meta-análisis con estos resultados.
ResultadosEncontramos un total de 312 estudios. Después de leer los resúmenes, se seleccionaron 49 estudios para la evaluación del texto completo. Finalmente, se seleccionaron 14 estudios para incluirlos en la revisión final. Se identificaron 2 meta-análisis, 8 ensayos clínicos, 3 cohortes retrospectivas y un estudio de casos y controles anidado. La evidencia disponible respalda el uso de CYC y MMF como las mejores opciones para el tratamiento de la EPI-ES, siendo el MMF la opción preferida basada en un mejor perfil de seguridad. Otros medicamentos como RTX, pirfenidona y nintedanib muestran un potencial como alternativas a la CYC. La calidad global de la evidencia disponible es adecuada sobre la base de estudios por lo general bien diseñados. El meta-análisis se realizó evaluando una disminución >5% o >10% de la CVF al comparar los agentes farmacológicos vs. placebo. Los resultados muestran que el uso de agentes farmacológicos se asocia negativamente con el empeoramiento de la CVF. Sin embargo, la alta heterogeneidad limita el número de estudios utilizados durante el análisis cuantitativo, afectando los resultados generales.
ConclusionesLas terapias inmunosupresoras, especialmente la CYC y el MMF, siguen siendo la piedra angular del tratamiento de la EPI-ES, ya que la mayoría de la evidencia muestra una mejoría o una progresión lenta de las pruebas de función pulmonar al usarlas. Sin embargo, se requiere más evidencia con respecto al uso de agentes farmacológicos alternativos, en busca de una mejora de la calidad de vida de estos pacientes.
Systemic sclerosis (SSc) is a rheumatic, immune-mediated disease, in which the triggering event is still unknown.1 The pathophysiology of the disease is characterized by the development of small vessel vasculopathy, auto-antibodies production and humoral immune response, as well as fibroblast dysfunction that leads to increased production of extracellular matrix.2 Early SSc is characterized by microvascular dysfunction and autoimmune response,3,4 that associated with an aberrant repair mechanism of connective tissue, lead to the development of fibrosis, which could compromise several organ systems such as the skin, gastrointestinal tract, renal and respiratory systems.1,5
This is a rare disease with a prevalence of 3–24 cases per 100,000 people, being more frequent in North America and Australia, compared to Europe.6 The data found in the Colombian population is quite comparable, thus finding estimates that report a prevalence of 0.02% in the general population and 23.7 cases per 100,000 inhabitants.7,8 It is more common in women (3–8:1 ratio).9 Multi-systemic compromise is associated with increased morbidity and mortality,10 being mortality especially associated with the development of systemic sclerosis-associated interstitial lung disease (SSc-ILD) and pulmonary hypertension. These conditions are associated with 60% of the deaths of patients with SSc.11,12
Based on the clinical characteristics, SSc can be classified as 1: limited, 2: diffuse, and 3: sine scleroderma. The limited SSc is associated with cutaneous compromise below the elbows and knees, sparing the trunk. The diffuse SSc is associated with a cutaneous compromise that includes proximal limbs and the trunk. Finally, sine scleroderma is not associated with skin compromise and is the most difficult to be diagnosed,1 fortunately, it only represents 3% of the cases.13
Characterizing the subtypes of the disease is important to assess the risk of lung involvement. For example, diffuse SSc has the highest risk of developing SSc-ILD.12 Also, serological predictors have been described for the development of SSc-ILD: the presence of anti-SCL-70 antibodies, and high titers of ANAs with a nucleolar pattern.14
In 2013, the American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) developed a joint statement with the updated classification criteria for SSc,15 aiming to unify the clinical and laboratory aspects. These criteria include skin thickening, fingertip lesions, Raynaud phenomenon, abnormal nail-fold capillaries, telangiectasias, lung compromise (pulmonary hypertension and/or interstitial lung disease), and expression of anti-centromere, anti-SCL-70, and anti RNA polymerase III antibodies15; each criterion represents a score, and if the overall score is 9 or more, the patient is classified as having definitive SSc.
Once the disease has been diagnosed, considering the specific organ compromise that could be present in its early stages, the procedure is focused on the identification of organ compromise and giving an early treatment to improve the morbidity and mortality.10,16 This screening is based on the search for pulmonary hypertension, lung fibrosis, cardiac, renal and gastrointestinal alterations. To assess the pulmonary compromise, the following procedures should be performed: echocardiogram and pulmonary function tests (including spirometry, carbon monoxide diffusion (DLCO), and 6minutes walking test). Nevertheless, using only the pulmonary function tests provides a low sensitivity to detect SSc-ILD.16 The use of high-resolution computerized tomography (HRCT) has shown a better performance when used as a screening test.16,17 As reported by the most recent evidence-based European consensus on the identification and management of SSc-ILD, it is stated, among other agreed observations, that all patients with SSc (especially if they present with one or more risk factors) should be screened for SSc-ILD using HRCT, considering it as the most reliable tool for its diagnosis. Furthermore, it is asserted that pulmonary function tests (such as spirometry and DLCO) support both screening and diagnostic processes and should be done to guarantee a baseline parameter.18
Systemic sclerosis interstitial lung diseasePulmonary fibrosis represents a frequent complication associated with SSc, as it is present in approximately 75% of patients19 and is part of the minor classification criteria for the disease.15 Clinical manifestations include dry cough and/or dyspnea, but it could also be asymptomatic. Physical examination is characterized by lung auscultation of velcro-like crackles, but in advanced stages of the disease, it could present cyanosis and right heart failure signs.19,20
In the pathophysiology of the disease, the presence of major histocompatibility complex DR3 and DR52, and the presence of anti-topoisomerase I19 gained significance as it reflects the development of an inflammatory response in the lung, in which T-cells, specifically Th2 release cytokines such as IL-4 and IL-13, stimulating the proliferation of fibroblasts and collagen synthesis.21 Also in this process, B-cells are involved as they are hyperactive and overexpress CD-19, which is implicated in the production of autoantibodies and fibrosis through the production of IL-6 and TGF-β, that induce the synthesis of the extracellular matrix, and also inhibit collagen degradation.21,22 There is also the presence of autoantibodies against the platelet-derived growth factor (PDGF), which increases the production of reactive oxygen species, the expression of collagen I gene, and the conversion of fibroblasts into myofibroblasts.19
In 2002, the American Thoracic Society and the European Respiratory Society (ATS/ERS) classified the interstitial lung disease according to radiological and histological findings into usual interstitial pneumonia (UIP), non-specific interstitial pneumonia (NSIP), acute interstitial pneumonia (AIP), cryptogenic organizing pneumonia (COP), lymphoid interstitial pneumonia (LIP), respiratory bronchiolitis-associated interstitial lung disease (RB-ILD), and desquamative interstitial pneumonia (DIP)23,24; however, when interstitial lung disease is in advanced stage, it is difficult to classify.19 The most frequent type of SSc-ILD is NSIP, corresponding to 76% of the cases, followed by UIP in 11%.20 Despite the above, there are yet cases that utterly fall outside any of the presented categories and are identified as unclassifiable interstitial lung diseases (U-ILDs). For those patients which present with an U-ILD and have features of autoimmunity, but who do not fit into the specific criteria of any characterizable connective tissue disease (CTD), it has been proposed since 2015 by the European Respiratory Society/American Thoracic Society a distinct division recognized as “Interstitial pneumonia with autoimmune features” (IPAF) and delimited by three central domains: a clinical domain (extra-thoracic symptoms that suggest a CTD but on their own are not enough to assert a diagnosis), a serological domain (autoantibodies associated with CTD, high titers of antinuclear antibody (ANA) and rheumatoid factor (RF), or ANA of any titer with nucleolar or centromere-staining pattern) and a morphological domain (radiological patterns on HRCT and histopathological features from lung biopsy). Hence, the U-ILDs, together with the unclassifiable CTDs (U-CTDs) are found within a spectrum of undifferentiated variants of both ILDs, CTDs, and other systemic autoimmune diseases.25,26
In the general population, identifying the type of pulmonary fibrosis acquires importance as it defines the prognosis of the disease. UIP has a 5-year mortality of 60–80%,27 on the contrary, NSIP has a good prognosis with a 5-year mortality of 18%.28 However, Park and colleagues29 found better survival in UIP associated with collagen diseases, compared with idiopathic UIP. On the contrary, there were no differences between idiopathic and collagen disease associated NSIP, confirming that pulmonary fibrosis associated with rheumatic diseases has a better prognosis.
Regarding patients with SSc-ILD, studies such as the one conducted by Okamoto et al.30 found an 8-year global mortality of 34% identifying a higher risk of mortality in patients aged 58 years or older (RR: 10.6; 95% CI 2.0–194.9; P=0.005), acute exacerbations (RR: 6.1; 95% CI 1.6–20.6; P=0.0013), and as a cause of death it was observed: infection 33%, acute exacerbation 33%, and progressive worsening of ILD 15%. While investigating the pulmonary fibrosis pattern influence on survival, they found higher mortality in UIP vs. any other pattern.30
Severe disease predictors in SSc-ILD include African American ethnicity, being male, and a forced vital capacity (FVC) ≤50% below the predicted value.31 Besides, bad prognosis predictors include FVC, DLCO (as low the real value is regarding the predicted value, it represents a higher pulmonary compromise), and the compromise perceived in the HRCT.24,30
This study aimed to evaluate the effectiveness and level of evidence regarding the pharmacological treatments available for SSc-ILD based on the measurement of carbon monoxide diffusion (DLCO) test, forced vital capacity (FVC) and/or high-resolution computerized tomography (HRCT) findings.
Materials and methodsInclusion criteriaTypes of studiesA systematic literature search was developed in order to identify published studies in English, that include meta-analyses, systematic literature reviews, clinical trials, and observational studies including cohorts and case–control studies.
Types of participantsStudies needed to include patients aged 18 years or older, with a diagnosis of SSc based on the 2013 ACR/EULAR Classification Criteria, and diagnosis of interstitial lung disease secondary to SSc.
Types of interventionsStudies using approved medications for the treatment of SSc-ILD including cyclophosphamide, mycophenolate mofetil, tocilizumab, nintedanib, pirfenidone, rituximab, or fresolimumab.
Types of outcomes measuredThe outcomes established for this review were based on pulmonary function tests and radiologic findings using HRCT, which are the main methods for follow-up of patients with interstitial lung disease.
Primary outcomes assessed in this review include:
- •
Forced vital capacity (FVC): is the maximum capacity of air that a patient can exhale after a maximum inspiration. It measures the volume of air exhaled in a spirometer, after a maximal inspiration. It is reported as the percentage of the predicted value for the patient.
- •
Carbon monoxide diffusion (DLCO) test: it represents the passive diffusion of carbon monoxide from a high partial pressure environment, to another with lower partial pressure. It is measured with the velocity of carbon monoxide diffusion from an alveolus to a capillary for pressure gradient unit (mL/min/mmHg). It is reported as the percentage of the predicted value for the patient.
- •
High-resolution computed tomography (HRCT): is a method that allows the development of computerized body images based on transversal cuts between 1 and 1.5mm using X-rays. This diagnostic image is taken using a tomography and reconstructing it digitally.
Studies with the following characteristics were not selected for the systematic review:
- •
Patients <18 years of age
- •
Presence of other auto-immune comorbidities in patients
- •
Case reports or case series
- •
ILD from other etiologies
A systematic literature search was conducted to collect studies published between January 2015 and July 31 of 2019. The search was made in different databases that include: MEDLINE (PubMed), EMBASE, and Cochrane Central Register of Randomized Controlled Trials (CENTRAL).
Boolean operators AND, OR, NOT were used to build the search strategy. The terms that were defined for the search included: “interstitial lung disease”, “systemic sclerosis”, “scleroderma”, “antifibrotic agents”, “Pirfenidone”, “Nintedanib”, “Mycophenolate mofetil, “Cyclophosphamide” “Rituximab”, “Tocilizumab”, “Fresolizumab”, “High Resolution Computer Tomography”, “Lung Diffusion Capacity” and “Forced Vital Capacity”.
Data collection and selection of studiesAn independent literature search in electronic medical databases was performed by two different researchers, who applied the same search terms and strategy. Each one executed the process of selecting the studies that best fit the previously defined inclusion criteria. Initial screening of studies was conceived based on the title. Then, a new filter was applied by assessing the abstracts of the firstly identified studies. Finally, the ones that remained as useful for the review were thoroughly reviewed, in order to choose the ultimate studies to be included. The results from each researcher were compared. In the case of disparities regarding the inclusion of a study, a consensus was made between both researchers. If an agreement was not reached, a third senior researcher came to settle.
Data analysisQuality of evidence and risk of biasIn order to assess the methodological quality of the studies and the risk of bias, several tools and checklists were applied based on the study design. For meta-analyses and systematic literature reviews, the Assessment of Multiple Systematic Reviews (AMSTAR) tool was applied.32 For clinical trials, it was assessed following the recommendations from the Cochrane Handbook for Systematic Reviews of Interventions.33 For the cohort and case–control studies, the Scottish Intercollegiate Guidelines Network (SIGN) checklist was applied.34
Measures of treatment effectBased on the reported outcomes and the available data, a descriptive analysis was made using central tendency, dispersion, and frequency measurements. Effect measures (relative risk, odds ratio) were calculated when it was possible with the information given by the included studies.
Meta-analysisBased on the quantitative results available and the homogeneity of outcomes from the studies included, a meta-analysis was developed. Forest plots were built to calculate the overall effect measurement of the outcomes assessed, using a mixed-effect or a random effect model, depending on the nature of the data reported by the studies selected for quantitative analysis. I2 test was used to assess the heterogeneity in the meta-analysis. Funnel plots were built to assess the risk of publication bias. This process was made using the Cochrane Review Manager (RevMan) software version 5.3 (Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration, 2014).
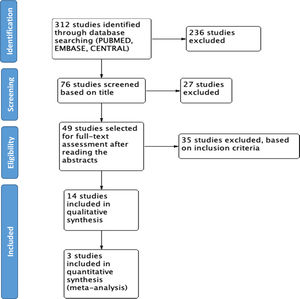
ResultsWe found 312 studies based on the search in the different databases. 58 studies were retrieved from MEDLINE PUBMED, 134 from ELSEVIER EMBASE, and 120 studies from Cochrane CENTRAL. With the initial screening based on the title, 76 studies were selected, and 236 were excluded for not being related; 49 studies were selected for full-text assessment after reading the abstracts. Finally, 14 studies were included in the final review (see Fig. 1).
Description of studies and quality assessmentThe description of the main characteristics of the final studies included in this review is summarized in Table 1.
Description of studies included in the systematic literature review.
| Study | Publication year | Country | Study design | Objective | Participants | Interventions | Outcomes |
|---|---|---|---|---|---|---|---|
| Distler O, et al.30 | 2019 | Europe (Austria, Czech Republic, Denmark, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Netherlands, Norway, Poland, Portugal, Spain, Sweden, United Kingdom) USA, Canada, South America (Argentina, Brazil, Chile), Asia (China, India, Malaysia, Japan, Thailand), Australia. | Randomized, placebo-controlled, double-blind clinical trial | To evaluate the safety and effectiveness of nintedanib as treatment for SSc-ILD. | Age: >18 years. Diagnosis of SSc based on the 2013 ACR/EULAR classification criteria. <7 years of disease duration. ILD diagnosed with HR-CT at least 12 months before the screening, with fibrosis in 10% of the lungs. FVC at least 40% of the predicted value. DLCO 30–89% of the predicted value. | Nintedanib (150mg twice daily) or placebo. | Annual rate of decline in FVC, absolute change in FVC, absolute change in DLCO, change in mRSS, SGRQ score. |
| Tashkin DP, et al.31 | 2006 | USA | Double-blind randomized clinical trial | To determine the effects of oral CYC on pulmonary function and health-related symptoms in patients with evidence of active alveolitis and SSc-ILD. | Diagnosis of limited or diffuse SSc. Evidence of active alveolitis on BAL fluid or HR-CT. Presence of ground-glass opacities. <7 years of disease duration. FVC 45–80% of predicted. Mahler baseline dyspnea index grade 2. | Oral CYC (initial 1mg/kg/day, increased monthly up to 2mg/kg/day) or placebo. | All outcomes were measured after 12 months of treatment: FVC% predicted, TLC, Mahler baseline dyspnea index, HAQ-score, QoL, SF-36. |
| Hoyles RK, et al.32 | 2006 | United Kingdom | Double-blind randomized clinical trial | To investigate the effects of IV CYC followed by AZA for the treatment of SSc-ILD. | Age 18–75 years. Diagnosis of SSc by fulfilling 1980 ACR preliminary classification criteria. Presence of ILD by HR-CT findings or thoracoscopy lung biopsy. Adequate compliance to therapy and attendance. | Oral prednisolone 20mg/day on alternate days and 6 IV infusions of CYC at a dose of 600mg/m2 every 4 weeks, followed by AZA 2.5mg/kg/day as maintenance, or placebo. | Change in FVC% predicted, change in DLCO% predicted. Change in dyspnea score. Change in HR-CT extent and pattern after 1 year of treatment. |
| Shenoy PD, et al.33 | 2016 | India | Single-center retrospective cohort study | To compare the efficacy of MMF and CYC in patients with SSc-ILD for the improvement of pulmonary function. | Age >18 years. Diagnosis of SSc by fulfilling the 2013 ACR/EULAR classification criteria. Evidence of ILD given by FVC <80%, and HR-CT findings. Treatment with CYC or MMF in the last 3 years. | MMF (initial 500mg/day increased to a maximum tolerated dose or 3g/day) or IV CYC (initial 600mg/m2 monthly for 6 months, increased to 1.2g as tolerated). | FVC% predicted measured at baseline, 3 and 6 months. |
| Jordan S, et al.34 | 2014 | 42 EUSTAR centers | Multicenter nested case–control study | To analyze the effects of RTX on the skin and lung fibrosis, and safety in a real-life clinical setting using the EUSTAR cohort. | Cases: patients receiving RTX in routine clinical practice. Controls: matched controls not receiving RTX as treatment. Inclusion criteria: fulfillment of the 1980 ACR classification criteria for SSc. Evidence of ILD given by FVC 70% predicted and HR-CT findings. Having at least one follow-up | RTX | Change in FVC% predicted from baseline to follow-up. Change in mRSS from baseline to follow-up. |
| van den Hombergh WMT, et al.35 | 2018 | Netherlands | Retrospective open-label cohort | To analyze whether the extent of inflammation, SSc disease duration, extent of ILD, or baseline DLCO <60%, modify the effect of IV CYC pulse therapy on pulmonary function after 12, 24 and 36 months, in patients with SSc-ILD. | All patients with SSc-ILD who started IV CYC pulse therapy from 2003. ILD was defined as the presence of ground-glass opacities, honeycombing, or pulmonary fibrosis on HR-CT. | IV CYC (750mg/m2 monthly). Some patients also received MMF following CYC infusions. Before 2013, some patients received AZA instead of MMF after CYC. | Pulmonary function tests at 0, 6, 12, 24 and 36 months of treatment were performed. FVC and DLCO values after 12, 24, and 36 months. |
| Sircar G, et al.36 | 2018 | India | Prospective randomized open-label clinical trial. | To evaluate the safety and efficacy of RTX compared to CYC in the treatment of SSc with pulmonary and skin manifestations. | Age: 18–60 years. Diagnosis of diffuse SSc by fulfilling 2013 ACR/EULAR classification criteria for SSc. Anti-Scl-70 positive. Presence of ILD given by HR-CT findings. FVC 45–80% of predicted. Maximum 3 years since onset of symptoms. NYHA class II-III. | RTX (2 pulses of 1000mg at 0 and 15 days, every 6 months) or IV CYC (500mg/m2 monthly for 24 weeks, followed by MMF or AZA). Patients also received prednisolone 10mg/day, calcium and vitamin D. | FVC% predicted at 24 months of follow-up. Absolute change in FVC after 6 months, mRSS at 6 months, Medsger's score, new onset or worsening of existing pulmonary hypertension. |
| Volkmann ER, et al.37 | 2017 | USA | Post hoc study from previous double-blind randomized clinical trials (SLS I and SLS II) | To compare MMF vs. placebo for the treatment of symptomatic SSc-ILD. | Age: >18 years. <7 years of disease duration. FVC 40 to 80–85% of predicted. DLCO >40% or 30–39% if no PHT. Ground glass opacities on HR-CT. | MMF (3g/day in divided doses) or placebo | % predicted of FVC, % predicted of DLCO, TDI, mRSS, safety |
| Tashkin DP, et al.38 | 2016 | USA | Randomized controlled double-blind clinical trial | To compare the efficacy and safety of MMF vs. Oral CYC followed by placebo in patients with symptomatic SSc-ILD | Diagnosis of SSc. Age 18–75 years. <7 years of disease duration. Predicted FCV <80%, but >45% exertional dyspnea grade 2 or higher based on Mahler baseline dyspnea index. Ground glass opacities on HR-CT | MMF (dose: 500–1.5g BID) for 24 months. Oral CYC (dose: initial 50–150mg, titrated to maximum dose of 1.8–2.3mg/kg daily) for 12 months followed by another 12 months receiving placebo. | Change in FVC as a % of the predicted normal value over 24 months of follow-up. Secondary outcomes include DLCO % predicted, and development of adverse events. |
| Zheng JN, et al.39 | 2019 | China | Network meta-analysis | To summarize and assess the relative efficacy and safety profile of 6 immunosuppressive agents for the treatment of SSc-ILD. | Studies investigating adults with diagnosis of SSc or SSc-ILD. Use of immunosuppressive therapies with or without glucocorticoids. At least 12 months receiving treatment and at least 12 months of follow-up. | Oral or IV CYC, MMF, AZA, MTX or combinations between them. | Change in FVC as a % of the predicted normal value. DLCO % predicted. Adverse events. |
| Barnes H, et al.40 | 2018 | Australia | Meta-analysis | To assess the efficacy and adverse effects of CYC in the treatment of SSc-ILD | Randomized controlled clinical trials investigating adults with a diagnosis of ILD associated with connective tissue diseases (SSc, RA, SLE, SJ, PM/DM, MCTD). At least 6 months receiving treatment and at least 12 months of follow-up. | Oral or IV CYC monotherapy or concomitantly with other immunomodulatory therapies, compared to placebo or MMF. | Change in FVC as a % of the predicted normal value. Change in DLCO % predicted. Adverse events. Health-related quality of life. |
| Adler S, et al.41 | 2018 | Europe (EUSTAR database) | Retrospective observational study | To analyze the current use of immunosuppressive drugs, test correlation between drug use and lung function tests, and define specific treatments for defined disease characteristics | Age >18 years. Diagnosis of SSc fulfilling 1980 ACR or 2013 ACR/EULAR classification criteria. Presence of ILD based on HR-CT findings and/or chest X-ray. | Immunosuppressive drugs including glucocorticoids, CYC, MMF, AZA, MTX, etc. | Baseline clinical characteristics including FVC and DLCO. Evolution of pulmonary function tests FVC and DLCO (no final values were given. Data was presented in a graphic). |
| Khanna D, et al.42 | 2016 | Canada, USA, Italy | Open-label, randomized phase 2 clinical trial | To assess the safety and tolerability of pirfenidone in patients with SSc-ILD. | Age: 18–75 years. Diagnosis of SSc fulfilling 1980 ACR classification criteria. Evidence of ILD based on HR-CT findings within 2 years since the beginning of the study. <7 years since the onset of SSc symptoms. Initial FVC >50%, DLCO >40%. | Pirfenidone: starting dose 801mg/day titrated to 2403mg/day, in 2-weeks vs. 4-weeks titration. Concomitant use of MMF vs. no use. | Adverse events, gastrointestinal symptoms using the UCLA SCTC GIT 2.0 questionnaire. FVC % predicted, DLCO % predicted, patient-reported outcomes, and mRSS. |
| Volkmann ER, et al.43 | 2016 | USA | Randomized double-blind clinical trial | To determine whether changes in CXCL4 levels can predict improvements in lung function in patients receiving MMF or CYC for the treatment of SSc-ILD. | Age: >18–75 years. Limited or diffuse SSc. Active ILD demonstrated by restrictive ventilatory pattern (FVC 40–80% of predicted). Exertional dyspnea (Mahler baseline dyspnea index grade 2). Ground glass opacities on HRCT. | CYC for 12 months followed by 12 months of placebo vs. MMF for 24 months. | Baseline clinical characteristics including FVC, DLCO, CXCL4 levels. Changes in FVC, DLCO and CXCL4 levels for 12–24 months. |
SSc-ILD: systemic sclerosis-associated interstitial lung disease; ILD: interstitial lung disease; HR-CT: high resolution computed tomography; FVC: forced vital capacity; DLCO: diffusion capacity for carbon monoxide; TLC: total lung capacity; mRSS: modified Rodnan skin score; SGRQ score: Saint George's respiratory capacity; CYC: cyclophosphamide; MMF: mycophenolate mofetil; RTX: rituximab; AZA: azathioprine; MTX: methotrexate; BAL: bronchoalveolar lavage; NYHA: New York Heart Association functional score; TDI: transitional dyspnea index; PHT: pulmonary hypertension; BID: twice per day; UCLA-SCTC GIT 2.0: University of California Los Angeles Scleroderma Clinical Trial Consortium Gastrointestinal Tract Instrument 2.0; EUSTAR: European Scleroderma Essay and Research Group.
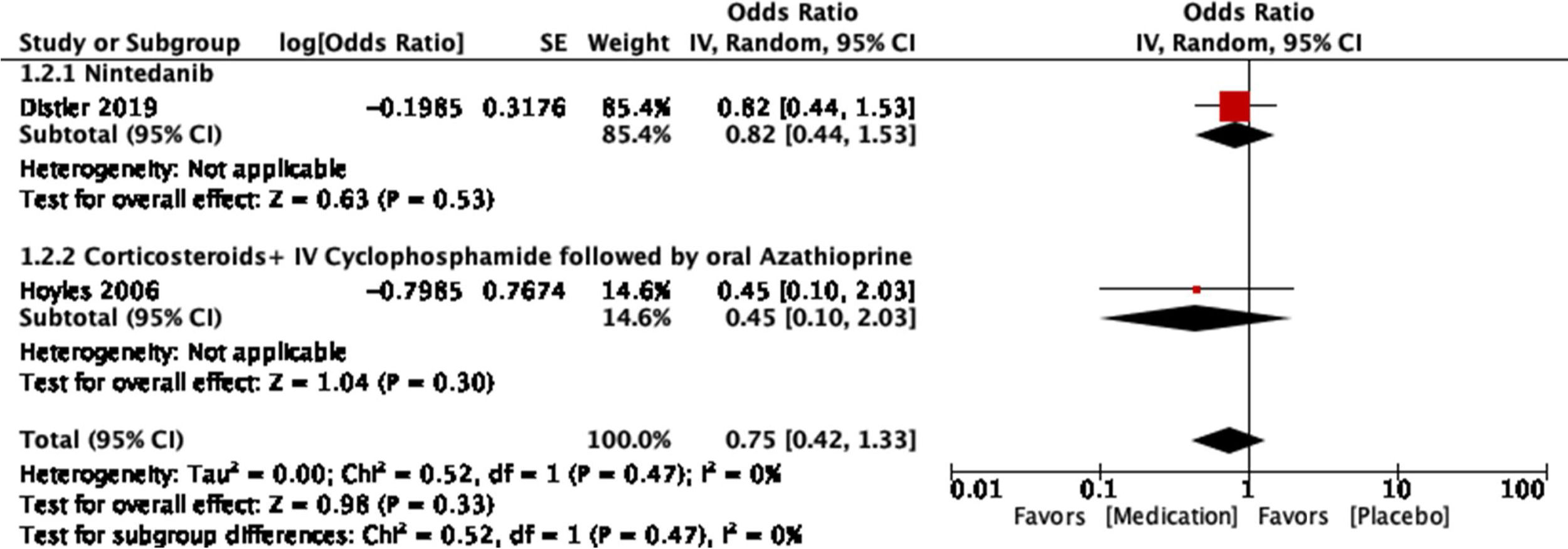
The study conducted by Distler and colleagues35 is a double-blind, randomized, placebo-controlled clinical trial. It seeks to evaluate the safety and effectiveness of nintedanib as a treatment for SSc-ILD. Initially, adequate randomization was identified, achieving a 1:1 ratio between placebo (288 patients) and nintedanib (288 patients). The clinical and demographic characteristics of both groups were statistically similar to each other. All data were reported even if they were not statistically significant. Funding bias can be seen as it was sponsored by the pharmaceutical company Boehringer Ingelheim. Concomitant treatment with mycophenolate mofetil (MMF) and its influence on outcomes was identified as a limitation, considering that this was not taken into account for the randomization process. Regarding the results, it can be said that at the end of a 52-week periodical follow-up, a statistically significant difference was identified for the main outcome: annual decrease in FVC with a difference of 41.0mL per year (95% CI: 2.9–79). No statistically significant differences are described for the key secondary endpoints: absolute change for the modified Rodnan skin score (mRSS) at week 52 and absolute change in the quality of life for total score through the SGRQ (St. George's Respiratory Questionnaire). Therefore, the researchers do not consider that there is enough evidence to support nintedanib as a disease-modifying agent for SSc-ILD, as it does not influence other complications of the disease.
Donald Tashkin and colleagues36 published a double-blind, randomized, placebo-controlled clinical trial that seeks to describe the effectiveness and safety of oral cyclophosphamide (CYC) on pulmonary function and health-related symptoms in patients with active alveolitis and SSc-ILD. 145 completed at least 6 months of treatment and were included in the analysis. The main limitation is the high rate of losses: only 54 and 55 patients completed the study in the CYC group and the placebo group, respectively. However, the follow-up losses are explained. The results after 12 months of follow-up show a discreet but statistically significant effect on the changes in FVC with a mean absolute difference of 2.53% (95% CI: 0.28–4.79%; P<0.03) favoring CYC in the primary analysis including only the baseline FVC, and of 2.97% (95% CI: 0.75–5.19%; P=0.009) in the secondary analysis when the baseline FVC and the worst score for fibrosis on high-resolution CT were included. Additional data favoring CYC was presented: a notable (but not statistically significant due to the CI) difference in total lung capacity (TLC) with an absolute difference in the percent of the predicted value of 4.09% (95% CI: 0.49–7.65%; P=0.026), a clinically significant improvement in both the Mahler Dyspnea Index (+1.4±0.23), in contrast to the worsening in the placebo group (−1.5±0.43) (P<0.001 with the use of a covariance model) as well as in the HAQ disability score with a −0.16% (95% CI: −0.28 to −0.04; P=0.009) difference with respect to the placebo group and in the vitality (7.99; 95% CI: 2.18–13.8; P=0.007) and transitional health (−0.66; 95% CI −1.02 to −0.30; P=0.003) domains of the SF-36 (quality of life). Regarding the scores for skin thickness, they also described a difference favoring CYC of −3.06 (95% CI: −3.54 to −0.52; P=0.008) in 85 patients with diffuse disease. No significant effects were identified in the measurement of gas exchange in either the DLCO or the DL: AE. The number of patients with serious adverse events during the follow-up year was higher in the CYC group than in the placebo group (20 vs. 16) although data is not considered statistically significant. Regarding non-serious adverse events, the only statistically significant were leukopenia and neutropenia in the CYC group (P<0.05). On this basis, CYC is presented as a potential treatment for SSc-ILD by significantly improving dyspnea, functionality, quality of life and skin thickness with a favorable safety profile in a risk–benefit context. Finally, it should be taken into account that at the time of publication no prospective randomized studies were assessing the effectiveness and safety of CYC for patients with this disease.
The study conducted by Hoyles and colleagues37 is a multicenter, prospective, randomized, double-blind trial. It seeks to evaluate the effects of intravenous corticosteroids and CYC therapy followed by oral azathioprine (AZA) for SSc-ILD. A total of 152 patients were initially screened, of whom 45 met the inclusion criteria and were randomized. Only 62% of patients (15 of the active treatment group and 13 of the placebo group) completed one year under the conditions of the study, nevertheless, the losses were explained. The baseline clinical and demographic features were similar, except for the interval between the time of diagnosis and the randomization: a mean of 33 months in the active treatment group and 66 months in the placebo group. The economic association between the authors and the pharmaceutical company Actelion is mentioned. The funding is not explicitly clarified, and it is not clear whether there are related economic conflicts. The main limitation was the small sample size as well as the subset of selected patients who were in a relatively stable phase of the disease. In the same way, the criteria used to categorize an adverse event as transient or as serious were not specified. Besides, the frequency of transient adverse events in the placebo group is not shown. The response to the primary outcome (change in FVC in the active treatment group vs. the placebo group) showed a trend toward statistical significance (P=0.08), without reaching it. An improvement of 4.19% in the predicted FVC is shown in the active treatment group (95% CI: −0.57 to 8.95). As for the remaining evaluated outcomes (DLCO, TLC, FEV1, dyspnea score, and improvement on serial HR-CT), no statistically significant differences were identified. Serious adverse events are said to have occurred at similar rates in the two groups although the appropriate operational definitions to verify this statement are not presented. Once the limitations and the results have been pointed out, it is proposed as a discussion that the tendency of the studied outcomes toward improvement is intuitively better than a tendency toward worsening. In the same way, they indicate that their results are consistent with other studies in the same context, although they differ in their statistical significance due to the small size of the sample. While they do not provide strong evidence, they postulate what is described as a treatment option.
Shenoy and colleagues38 developed a single center retrospective cohort study. It aimed to describe the efficacy and comparability between the use of CYC and MMF for the treatment of SSc-ILD; retrospectively collecting data from the last 3 years of SSc-ILD patients being treated at a clinical center in India by protocol-based administration of intravenous CYC and MMF. The CYC group had 23 patients and 34 patients for the MMF group. Being a retrospective study, the possible losses of patients are not taken into account. Both statistically significant and non-statistically significant data are presented, although confidence intervals (CIs) are not provided. The identified limitations are the lack of a control arm for those who were not treated and a longer follow-up period. After a 6-month follow-up, the average FVC increased to 55.99±13.47% from the baseline value of 53.44±13.69% in the MMF group (P=0.003) and to 53.09±14.93% from the baseline value of 48.74±15.67% in the group of CYC (P=0.003). The mean percentage increase in FVC was 10.84±13.81% in the CYC group and 6.07±11.92% in the MMF group. Similarly, the change in FVC at 6 months between the CYC group and the MMF group was not statistically significant (P=0.232). The percentages of the increase, decrease and non-change of the FVC are presented, respectively being 68.57%, 28.57% and 2.85% in the CYC group and 78.26%, 17.39% and 4.35% in the MMF group. In both groups, the treatment was well tolerated, with only 9 cases of lower respiratory tract infection in the CYC group and 4 cases in the MMF group. In summary, it is proposed that both CYC and MMF are equally effective in preserving the lung function of patients with SSc-ILD since they not only stabilize it but also increase it in most patients, with a very good safety profile. Finally, the authors support the use of these two medications and point out that MMF may be a better option on behalf of the long-term toxicity of CYC.
The study conducted by Suzana Jordan and colleagues,39 is a multicenter, case–control nested in a cohort (Cohort of the European Scleroderma Essay and Research Group (EUSTAR)) study. The objective was to analyze the effects of rituximab (RTX) on the skin and lung fibrosis and its safety in a real-life clinical setting. Matching and selection of control patients from the cohort was made considering the following criteria: mRSS, FVC, duration of follow-up, SSc subtype, disease duration, immunosuppressive co-treatment and those not treated with RTX. All patients agreed to be part of the EUSTAR cohort. 63 patients were included. The necessary measures to prevent the knowledge of the primary exposure from influencing the determination of cases were not taken, since it was based on the decisions of their physicians. The confusion proneness regarding the indication of receiving treatment (in this case RTX) according to the severity of the disease was identified as a limitation and as potential selection bias. It did not exclude as well the clinical effect by the application of methylprednisolone during the application of RTX, nevertheless, it is clarified that there were no differences with the patients who did not receive it (although the data is not presented). The CIs are not presented, and it is clarified that some of the data were collected retrospectively. Regarding patients with SSc-ILD, 9 cases were identified. The median disease duration was 6 years. FVC% predicted was stable (60.6±2.4 vs. 61.3±4.1%; P=0.5) after a 6-month follow-up in patients with RTX. Matched controls showed a decrease in FVC% predicted at follow-up. When comparing the absolute change in FVC% predicted, significant differences were observed between patients receiving RTX and matched controls (0.8±2.2 vs. −4.8±1.7; P=0.01). The DLCO moderately improved (41.1±2.8 vs. 44.8±2.7%; P=0.03) in patients with RTX, although the difference with the control group was not statistically significant (3.7±1.4 vs. 6.2±6.2; P=0.9). Regarding the safety profile, adverse effects were described only for the RTX group, with nausea and infections being the most frequent in 25% and 21% of patients, respectively. Despite the limitations of the study design, RTX, according to its pharmacodynamics and the disease pathophysiology is a potent anti-fibrinolytic agent with an acceptable safety profile in patients with SSc. The authors invite to expand the evidence in this regard with future randomized controlled trials.
Van den Hombergh and colleagues40 published a long-term observational open-label retrospective cohort study that includes prospective data collection and intention-to-treat analysis. It seeks to assess whether CYC pulse therapy can be more effective in the inflammatory phase of the disease and takes into account the following effect modifiers: disease duration, more ground-glass opacity than fibrosis, the extent of ILD, and DLCO >60% at baseline. Patients were retrospectively selected from a Dutch clinical center where, since 2003, the protocol stated the first-line treatment with CYC. The features of the included patients are presented, but it is not entirely clear if there are any additional exclusion criteria besides not having a baseline HR-CT or pulmonary function test and patients not treated with CYC. There is not an established comparator group, although the results are analyzed according to the differences between the effect modifiers. A total of 44 (58.6%) from 75 initially selected patients completed 12 cycles of CYC, however, the reasons for the losses are explained. Concomitant treatment with other drugs such as MMF (51 patients (68%)) is identified as a limitation (since 2013 it was included as a follow-up treatment standard in the clinical center). It is concluded that treatment with CYC involves stabilization of lung function in patients with SSc-ILD for 3 years (follow-up time), without this response to treatment having much to do with receiving it or not during the inflammatory phase of the disease. 54.7% of the patients remained stable (change in FVC<10% and DLCO<15%), 26.7% worsened (decrease>10% in FVC or>15% in DLCO) and 18.7% improved (increase in FVC>10% or an increase in DLCO>15% compared to baseline) (P=0.008). It also pretended to identify a subgroup of patients whose characteristics made them good responders; however, it was not possible. The only effect modifier that influenced the outcome was the short duration of the disease, although it may be secondary to the lower loss of FVC in the group of poor responders. The researchers explain their failure due to the lack of a more objective method (or a Gold Standard) to measure the amount of inflammation of the ILD.
Sircar and colleagues41 published a prospective, randomized, open-label, parallel-group, controlled trial that seeks to evaluate the safety and efficacy of RTX compared to CYC in the treatment of patients with early SSc. Once the inclusion and exclusion criteria were determined, 64 patients were recruited. Only 4 losses occurred, and they were clearly explained. Among the limitations and the validity of the study, the inclusion of patients belonging to a single ethnic group is identified, as it was done in India. In the same way, being open-labeled favors detection bias and observer bias. Compared to other previous trials, the present study differs by including younger patients (mean age of 36 years) with a shorter disease time (early-stage patients given for a mean disease duration of 22 months), with diffuse cutaneous SSc and positivity for anti-Scl70. Regarding the outcomes, an improvement in the FVC% predicted at 6 months is reported in patients who received RTX (from 61.30 at baseline to 67.52 at the end of the study; P=0.002) compared to those who received CYC (fall from 59.25 at baseline to 58.06 at the end of 6 months; P=0.496), thus establishing a mean statistically significant difference in favor of RTX of 9.46 (95% CI: 3.01, 15.90; P=0.003). A similar behavior can be seen in the secondary outcomes analyzed at 6 months: mRSS improved significantly in patients with RTX (from 21.77 at baseline to 12.10 at the end) in contrast to patients with CYC (from 23.893 at baseline to 18.33 at the end of the study), the 6-min walking test (+49.97m in the RTX group vs. +13.24m in the CYC group) and the Medsger severity scale (−3.66 in the RTX group vs. −3.64 in the CYC group) also improved (all P<0.001). Regarding the safety profile, the total number of adverse events was lower in the RTX group (30%, 9/30) than in the CYC group (70%, 21/30) (P=0.02) and major adverse events occurred in the CYC group only. Once the limitations and biases were mentioned, statistically significant data that position RTX as a safe and effective alternative for the treatment of patients with diffuse cutaneous SSc and positivity for anti-Scl-70 were presented.
Elizabeth Volkmann and colleagues42 conducted a post hoc analysis derived from the Scleroderma Lung Study (SLS) I and II trials. The aim of this study was to compare outcomes of patients assigned to MMF (from SLS II study) and of patients assigned to placebo (from SLS I study). The SLS I trial was designed to compare the use of CYC (titrated to 2mg/kg) vs. placebo for the treatment of SSc-ILD. In it, 162 patients were randomly assigned to receive either of both treatment alternatives during a year. An additional year of follow-up without treatment was included. The SLS II trial was designed to compare the use of CYC vs. MMF for the treatment of SSc-ILD. An overall of 142 patients were randomized to receive either MMF during 2 years of follow-up or oral CYC during 1 year followed by an additional year receiving placebo. A double-dummy design was applied in order to maintain the double-blinding. For the actual study, 69 patients were enrolled in the MMF group, and 79 patients in the placebo group. Baseline characteristics between the groups were similar. Results through a joint model analysis show that treatment with MMF was associated with an improvement of FVC% predicted after 24 months of follow-up. The improvements were more significant during the first 3–12 months of treatment, compared to placebo which presented a decline in FVC during the same period. Based on the intent-to-treat strategy, it was calculated that 64.4% of patients had any improvement after 12 months of follow-up, and 71% of patients presented it after 24 months of follow-up. The absolute improvement presented by patients after 24 months of follow-up was >5% of predicted FVC. The data from patients that completed the study show a greater percentage of patients presenting any improvement after 24 months of follow-up (75.5%). Another finding was that patients with higher initial predicted FVC presented improvements after 24 months of follow-up. Considering improvements in the measurement of DLCO, they found that treatment with MMF was associated with improved DLCO percentage predicted over 24 months of follow-up (P<0.001). During the first 3–12 months, patients from the MMF arm presented improvements, while those in the placebo arm showed significant declines. In conclusion, the evidence supports the use of MMF for the treatment of symptomatic SSc-ILD, in which it is associated with pulmonary function improvements compared to placebo. This study presents certain limitations, since it was made based on data from previous trials that were conducted in different periods. There is an unclear risk of selection bias, considering that the original trials randomized the participants, but the actual one was not able to do it in order to adequately control for external non-measured variables that may affect the results.
The study by Tashkin and colleagues,43 is a multicenter randomized controlled, double-blind parallel-group clinical trial (SLS II), aiming to compare the efficacy and safety of MMF vs. CYC in patients with symptomatic SSc-ILD. Patients were randomly assigned using a double-blind double-dummy design to receive MMF for 24 months, or oral CYC for 12 months followed by another 12 months receiving placebo. A post hoc frequency distribution for participants in whom a measurement was made at 24 months of follow-up and presented changes in FVC was applied to have a better understanding of the frequency and magnitude of individual changes in pulmonary function. An intention-to-treat analysis was applied to handle missing data. An overall of 142 patients was initially eligible; however, only 126 patients were finally included in the primary analysis as not all patients achieved the acceptable baseline HR-CT results. From them, 63 patients received MMF and 63 CYC. The course of the FVC percentage predicted did not differ between the treatment groups during the 24 months of follow-up. Each group showed improvements in FVC % predicted, with greater improvements reported at 21 months. At 24 months of follow-up, the improvement was 2.19% for MMF, and 2.88% for the CYC group. 71.7% of patients receiving MMF, and 64.7% of patients receiving CYC had improving values, and no statistical differences were identified. Regarding adverse events, more patients from the CYC group developed leucopenia. Thrombocytopenia was only registered in the CYC group. No differences were identified regarding the development of anemia or pneumonia. They concluded that treatment of SSc-ILD with MMF for 2 years or CYC for 1 year was associated with significant improvements in lung function. No significant differences were present regarding efficacy; however, the use of MMF presented less toxicity and better tolerance. Limitations that could be identified were loss of follow-up due to premature withdrawals, as well as the exclusion of patients at the beginning of the study for the assessment of the primary analysis. Also, the use of oral CYC only may be considered a limitation.
Ji-Na Zheng and colleagues44 developed a network meta-analysis in which the aim was to summarize and assess the relative efficacy and safety profile of six immunosuppressive therapies including CYC, MMF, AZA, Methotrexate (MTX), CYC+AZA, and CYC+MMF, in patients with SSc-ILD. They identified randomized clinical trials, cohorts, and case–control studies published up to July 31 of 2018 in PUBMED, EMBASE, and Cochrane CENTRAL. Furthermore, studies with concomitant glucocorticoid use were included. The immunosuppressive therapies should be given for at least 12 months, and 12 months was the minimum time of follow-up. They developed a traditional pairwise meta-analysis to directly compare the different therapies, and a network meta-analysis with a random-effect model to calculate CIs, mean differences and odds ratio for the outcomes. An overall of 1369 studies was identified through database searching and in additional records. 10 studies were finally included in the meta-analysis, with information from 939 patients. Regarding the change in FVC% predicted, they identified that the use of CYC (1.6; 95% CI=1.3–1.9) and CYC+AZA (2.7; 95% CI=0.04–5.37) could prevent the deterioration of FVC after 12 months of follow-up, while the rest of immunosuppressive agents did not show significant efficacy. AZA monotherapy was the option with the worst effectiveness regarding the reduction of deterioration of FVC. Considering the change in DLCO% predicted, they observed that MMF had the greatest probability of reducing the deterioration of DLCO, compared to the rest of therapies; however, no statistical significance was achieved. The use of MTX was associated with more adverse events, in contrast to the use of AZT, which exhibited fewer, compared to the other medications. However, the differences were not statistically significant. They concluded that, although there are no high-quality randomized clinical trials, the available evidence shows that CYC+AZA, and MMF are the best treatment options for patients with SSc-ILD, based on the reduction of FVC deterioration, and DLCO improvement, respectively. After applying the AMSTAR checklist to assess the quality of the study, a score of 8 was obtained indicating that this meta-analysis had a good quality. Among the identified weaknesses is that the authors did not include unpublished data (gray literature) or literature published in languages other than English. Additionally, a complete list of included and excluded studies was not given.
Harvey Barnes and colleagues45 published a Cochrane systematic literature review and meta-analysis regarding the efficacy and safety of CYC for the treatment of CTD-associated ILD. They included randomized controlled clinical trials assessing CTDs-associated ILD. Associated CTDs were: SSc, rheumatoid arthritis (RA), polymyositis/dermatomyositis, Sjogren's syndrome, systemic lupus erythematosus (SLE) and mixed-CTD. The studies compared intravenous or oral CYC used individually or with other immunomodulatory medication for at least 6 months, and follow-up periods of 12 months, compared to any non-cyclophosphamide containing therapy. Treatment effects were calculated based on mean differences and 95% CIs for continuous data, and odds ratio or risk differences for dichotomous data when it was possible. A meta-analysis was developed for pooled quantitative analysis. They identified 1149 studies in the initial search, and 39 studies were selected for full-text review. Finally, 4 studies were included for the meta-analysis. Three studies including only patients with SSc-ILD, and one of them including patients with SSc, RA, polymyositis/dermatomyositis, and SLE. Each study had different treatment protocols as some of them included other medications such as prednisone, AZA, or MMF. Comparison groups varied between placebo and MMF. Regarding the efficacy of CYC vs. placebo, they found that the mean difference in post-treatment FVC% predicted was 2.83 (95% CI: 0.8–4.87; P=0.006) in favor of CYC. Regarding post-treatment DLCO% predicted, the mean difference was 1.68 (95% CI: −4.37 to 1.02; P=0.22) but it was not statistically significant. Comparing the efficacy of CYC vs. MMF, they found that no significant differences were observed regarding the FVC% predicted after 12 months of treatment, or after the end of the study (mean difference −0.68, 95% CI: −5.44 to 4.08; P=0.78). No significant differences were observed for the changes in DLCO% predicted between CYC vs. MMF or placebo, however, heterogeneity was present. Based on these results, the authors concluded that a small benefit was associated with the use of CYC compared to placebo regarding the FVC% predicted after the treatment. Nevertheless, no clear recommendations could be made as the number of studies available was limited, and their quality was low. This study was assessed by applying the AMSTAR Checklist, with a result of 11 points, indicating to be of high-quality regarding its methodological characteristics.
The study conducted by Sabine Adler and colleagues46 is a descriptive retrospective analysis from the EUSTAR database aimed to analyze the current trends of immunosuppressive drugs use, the correlation between drugs use and pulmonary function tests, and establish specific treatments for defined disease characteristics, in patients with SSc-ILD. Data was collected between 2004 and May 6, 2014. The progression of pulmonary function during the treatment was assessed using linear regression analysis of the change in FVC and DLCO since the beginning of the treatment until the end of follow-up. The immunosuppressive regimes used were assessed by calculating the additive and multiplicative effects of single drugs in a group. Results data from 3778 patients were included, with 71% of patients using immunosuppressive therapies. The age and disease duration were higher in patients that never used immunosuppressive therapies. Skin involvement was higher in those who have ever used a medication, as they had higher modified Rodnan scores and presented diffuse SSc with greater frequency. Initial DLCO and FVC were higher in patients who never received medication compared to those who have ever used it (DLCO: 67.4±19.8 vs. 59.9±20, P<0.001. FVC: 94.9±20.9 vs. 84.4±21.5, P<0.001). The history of organ compromise and worse functional class based on NYHA classification was observed in patients who have ever received medication. Anti-centromere antibodies were more frequent in patients who never received immunosuppression, while SCL-70 antibody was common in patients who have ever received medication. In general, immunosuppressive agents were given to patients with more severe and active disease, with glucocorticoids (58.8%) being the most frequently used, followed by CYC (19.1%). Those patients receiving CYC and MMF presented the worst initial values of FVC and DLCO. Considering the evolution of pulmonary function, data were available in 73.6% of patients. The use of CYC and MMF had a negative additive effect in patients with severe lung disease. After adjusting for potential confounders, the use of glucocorticoids and MMF presented multiplicative effects on the course of lung function, while CYC presented additive effects towards lower initial DLCO and FVC values. No specific medication appeared to be superior to the rest regarding the course of pulmonary function. In patients with adequate initial FVC (>75%), the use of glucocorticoids was associated with a reduction in the rate of deterioration. The study concludes that the initial characteristics of the patients play a significant role in the selection of the appropriate medication, with a clear benefit of early detection of pulmonary function changes and early use of immunosuppressive agents. However, no specific recommendations may be given, as these observations are taken from retrospective data in which the changes in prescription patterns and information regarding the decision of changing medication were not available, representing possible confounders, limiting the scope of the study. Another limitation is that the authors did not inform the values of changes in FVC or DLCO, or the absolute FVC or DLCO at the end of the follow-up, in order to have the quantitative data supporting the observations. The only data was given as graphics.
The study by Dinesh Khanna47 is an open-label, international multicenter, phase II randomized clinical trial, aiming to assess the safety and tolerability of Pirfenidone given to patients with SSc-ILD. Previous use of oral CYC or MMF was allowed if they received a stable dose for >3 months before the onset of the study. Patients received Pirfenidone (275mg capsules) at a starting dose of 801mg/day. Patients were randomly assigned to a 2-week titration or 4-week titration in a 1:1 sequence. Randomization was done with a blocked scheme prepared by the sponsor. A total of 63 patients were recruited and randomly assigned to either 2-week titration or 4-week titration. In general, no differences in baseline characteristics were observed, except for a greater proportion of patients with limited SSc in the 2-week titration group, and slightly better pulmonary function parameters in the 4-week titration group. 88.9% of patients completed the study, with 96.8% of patients experiencing at least one adverse event (nausea, headache and fatigue were the most common). Adverse events were more frequent in the 2-week titration group. Also, patients receiving Pirfenidone plus MMF had fewer serious adverse events and compliance, compared to Pirfenidone alone. Regarding the pulmonary function results, no significant changes were observed between the 2- and 4-week titration groups. The change in FVC from baseline was similar in both groups (−0.6, SD: 8.91 vs. −0.6, SD: 5.9), however, differences were identified between those who received vs. those not receiving MMF, favoring the concomitant use of MMF for an improvement in FVC (no MMF: −0.3, SD: 9.5 vs. MMF: 0.6, SD: 5.82). Similar observations could be made regarding the change in predicted DLCO. The authors concluded that Pirfenidone has acceptable tolerability, with lower rates of adverse events with the 4-week titration regime. The authors stated that no real conclusions on behalf of treatment response could be obtained with the data because the design was not meant to assess the efficacy of the used medications. Considering the quality of the study, there is a low risk of selection bias based on an adequate randomization process, however, it is affected by the lack of allocation concealment. Additionally, no blinding strategies were used, due to the aim of the study which is to assess the safety profile.
The final study assessed was published by Elizabeth Volkmann and colleagues,48 aiming to evaluate the association of chemokine CXCL4 levels with the extension of ILD, and to determine whether changes in CXCL4 levels may predict improvement in pulmonary function tests in patients receiving MMF or CYC for the treatment of SSc-ILD. Previous studies have shown that CXCL4 may be associated in the process of perpetuating pro-fibrotic activity in the context of SSc-ILD, as higher levels of this chemokine have been registered in patients with SSc-ILD, compared to those with SSc without lung compromise. This study was conducted in participants from the SLS II trial. Healthy controls were included in order to have an adequate comparison of baseline CXCL4. Plasma samples were collected from SSc-ILD patients at baseline, after 12 months, and after 24 months of follow-up. Plasma samples from healthy controls were only taken at baseline. Two-sample T-test was used to compare continuous variables and X2 test for categorical variables. Pearson correlations were calculated to analyze the relationship between CXCL4 levels with baseline extension of ILD (measured by FVC and DLCO), and for the relationship between changes in CXCL4 levels and the changes in FVC and DLCO from baseline to 12 months of follow-up. Finally, a mixed-effect model was developed to assess the evolution of FVC and the changes in levels of CXCL4 measured every 3 months, between 12 and 24 months of follow-up. A total of 136 patients (71 receiving CYC, and 65 receiving MMF) and 67 healthy controls were included. As expected, the baseline levels of CXCL4 were higher in SSc-ILD patients, compared to healthy controls. No major differences were identified in CXCL4 levels between treatment groups. No correlations were identified between baseline CXCL4 levels and the extension of ILD variables (TLC, FVC, DLCO). Regarding the levels of CXCL4, there was a negative correlation between changes in CXCL4 levels (from baseline to 12 months), and the changes in FVC levels (from baseline to 18 months, and 24 months). Results from the mixed-effect model show that the change in CXCL4 levels from baseline to 12 months was associated with the course of FVC levels from 12 to 24 months of follow-up, after controlling for treatment arm, baseline extension of ILD, and baseline FVC. This study gives evidence of the potential of CXCL4 measurement as a biomarker to predict the response to immunosuppressive therapy (CYC and MMF) for the treatment of SSc-ILD. The authors explain follow-up losses and the management of data from these patients, thus conditioning low risk for attrition bias. Nonetheless, they state at the discussion that the loss to follow-up may affect the statistical power of their results.
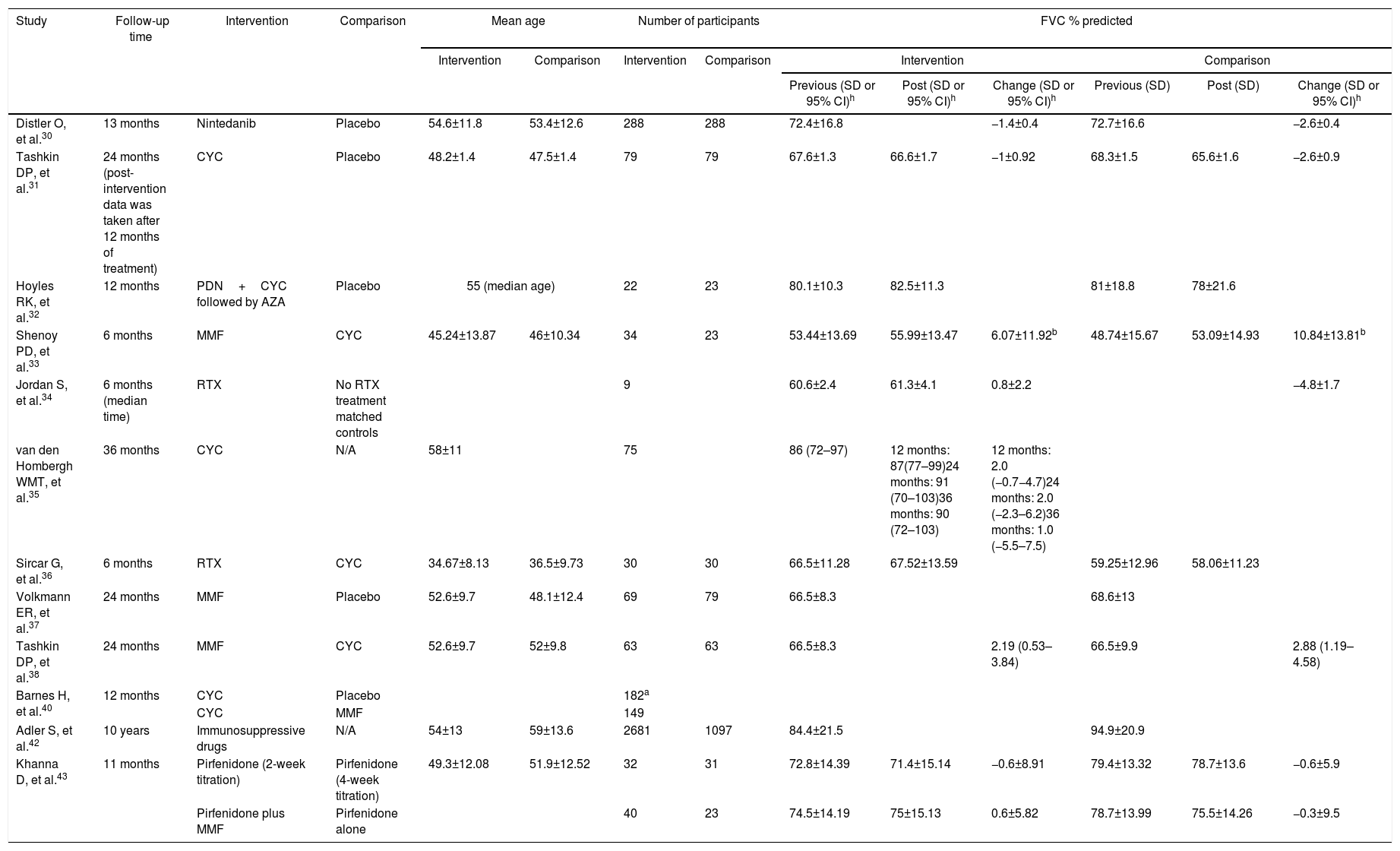
The results associated with the primary outcomes defined for this review are summarized in Table 2. It may be remarked that there was an important heterogeneity regarding the outcomes. Several sections from the chart do not present data, as those were missing from the assessed manuscripts.
Summary of results from studies included in the review. Also, of the results from the calculation of effect measures based on the decline of FVC % predicted.
| Study | Follow-up time | Intervention | Comparison | Mean age | Number of participants | FVC % predicted | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Comparison | Intervention | Comparison | Intervention | Comparison | ||||||||
| Previous (SD or 95% CI)h | Post (SD or 95% CI)h | Change (SD or 95% CI)h | Previous (SD) | Post (SD) | Change (SD or 95% CI)h | ||||||||
| Distler O, et al.30 | 13 months | Nintedanib | Placebo | 54.6±11.8 | 53.4±12.6 | 288 | 288 | 72.4±16.8 | −1.4±0.4 | 72.7±16.6 | −2.6±0.4 | ||
| Tashkin DP, et al.31 | 24 months (post-intervention data was taken after 12 months of treatment) | CYC | Placebo | 48.2±1.4 | 47.5±1.4 | 79 | 79 | 67.6±1.3 | 66.6±1.7 | −1±0.92 | 68.3±1.5 | 65.6±1.6 | −2.6±0.9 |
| Hoyles RK, et al.32 | 12 months | PDN+CYC followed by AZA | Placebo | 55 (median age) | 22 | 23 | 80.1±10.3 | 82.5±11.3 | 81±18.8 | 78±21.6 | |||
| Shenoy PD, et al.33 | 6 months | MMF | CYC | 45.24±13.87 | 46±10.34 | 34 | 23 | 53.44±13.69 | 55.99±13.47 | 6.07±11.92b | 48.74±15.67 | 53.09±14.93 | 10.84±13.81b |
| Jordan S, et al.34 | 6 months (median time) | RTX | No RTX treatment matched controls | 9 | 60.6±2.4 | 61.3±4.1 | 0.8±2.2 | −4.8±1.7 | |||||
| van den Hombergh WMT, et al.35 | 36 months | CYC | N/A | 58±11 | 75 | 86 (72–97) | 12 months: 87(77–99)24 months: 91 (70–103)36 months: 90 (72–103) | 12 months: 2.0 (−0.7−4.7)24 months: 2.0 (−2.3–6.2)36 months: 1.0 (−5.5–7.5) | |||||
| Sircar G, et al.36 | 6 months | RTX | CYC | 34.67±8.13 | 36.5±9.73 | 30 | 30 | 66.5±11.28 | 67.52±13.59 | 59.25±12.96 | 58.06±11.23 | ||
| Volkmann ER, et al.37 | 24 months | MMF | Placebo | 52.6±9.7 | 48.1±12.4 | 69 | 79 | 66.5±8.3 | 68.6±13 | ||||
| Tashkin DP, et al.38 | 24 months | MMF | CYC | 52.6±9.7 | 52±9.8 | 63 | 63 | 66.5±8.3 | 2.19 (0.53–3.84) | 66.5±9.9 | 2.88 (1.19–4.58) | ||
| Barnes H, et al.40 | 12 months | CYC | Placebo | 182a | |||||||||
| CYC | MMF | 149 | |||||||||||
| Adler S, et al.42 | 10 years | Immunosuppressive drugs | N/A | 54±13 | 59±13.6 | 2681 | 1097 | 84.4±21.5 | 94.9±20.9 | ||||
| Khanna D, et al.43 | 11 months | Pirfenidone (2-week titration) | Pirfenidone (4-week titration) | 49.3±12.08 | 51.9±12.52 | 32 | 31 | 72.8±14.39 | 71.4±15.14 | −0.6±8.91 | 79.4±13.32 | 78.7±13.6 | −0.6±5.9 |
| Pirfenidone plus MMF | Pirfenidone alone | 40 | 23 | 74.5±14.19 | 75±15.13 | 0.6±5.82 | 78.7±13.99 | 75.5±14.26 | −0.3±9.5 | ||||
| Study | DLCO % predicted | Effect measurement | HRCT findings | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Comparison | OR decline in FVC (95% CI)i | MD FVC% predicted (95% CI) | MD DLCO% predicted (95% CI) | Intervention | Comparison | ||||||
| Previous (SD or 95% CI)h | Post (SD or 95% CI)h | Change (SD or 95% CI)h | Previous (SD) | Post (SD) | Change (SD or 95% CI)h | >5% | >10% | |||||
| Distler O, et al.30 | 52.9±15.1 | −3.21±0.54 | 53.2±15.1 | −2.77±0.54 | 0.65 (0.44–0.96) | 0.82 (0.44–1.52) | ||||||
| Tashkin DP, et al.31 | 47.2±1.6 | 42.8±1.7 | −4.2±1.16 | 47.9±1.7 | 44.3±2.1 | −3.5±1.0 | 0.36 (0.17–0.78) | |||||
| Hoyles RK, et al.32 | 52.9±11.5 | 49.6±10.7 | 55±12.9 | 51.8±14.9 | 0.45 (0.10–2.07) | 4.19 (0.57–8.95)c | No significant differences reported. | 40%d | 20%d | |||
| Shenoy PD, et al.33 | 1.65 (0.46–5.94) | |||||||||||
| Jordan S, et al.34 | 41.1±2.8 | 44.8±2.7 | 3.7±1.4 | 6.2±6.2 | 3.16 (1–10.03) | |||||||
| van den Hombergh WMT, et al.35 | 42 (32–56) | 12 months: 45 (36–58)24 months: 43 (35–57)36 months: 43 (36–61) | 12 months: −0.5 (−2.8–1.7)24 months: 0.3 (−3–3.5)36 months: 1.6 (−2.5–5.7) | |||||||||
| Sircar G, et al.36 | 1.16 (0.4–3.35) | 9.46 (3.01–15.9) | ||||||||||
| Volkmann ER, et al.37 | 54±11.1 | 46.2±13.3 | 0.22 (0.1–0.5) | 27.2±13.2e | 35.3±16.9e | |||||||
| Tashkin DP, et al.38 | 54±11.1 | −0.4 (−2.81–2.01) | 54.1±14.1 | −2.14 (−4.59–0.31) | 0.64 (0.29–1.43) | 0.95 (−41–2.2)f | 1.84 (−5.16–1.46)f | |||||
| Barnes H, et al.40 | 2.83 (0.8–4.87) | −1.66 (−4.39–1.07) | ||||||||||
| 0.82 (3.95–2.31) | −1.41 (−10.4–7.58) | |||||||||||
| Adler S, et al.42 | 59.9±20 | 67.4±19.8 | 45.5%g | 30.3%g | ||||||||
| Khanna D, et al.43 | 59.3±14.36 | 60.6±19.12 | 0.7±9.57 | 60.1±18.64 | 63.4±18.12 | 3.2±10 | ||||||
| 61.4±17.26 | 64.7±19.8 | 3.2±10.85 | 56.8±14.92 | 57±14.96 | 0.2±7.12 | |||||||
FVC: forced vital capacity; DLCO: diffusion capacity for carbon monoxide; HRCT: high resolution computed tomography; SD: standard deviation; 95% CI: confidence interval 95%; CYC: cyclophosphamide; MMF: mycophenolate mofetil; PDN: prednisolone; AZA: azathioprine; RTX: rituximab.
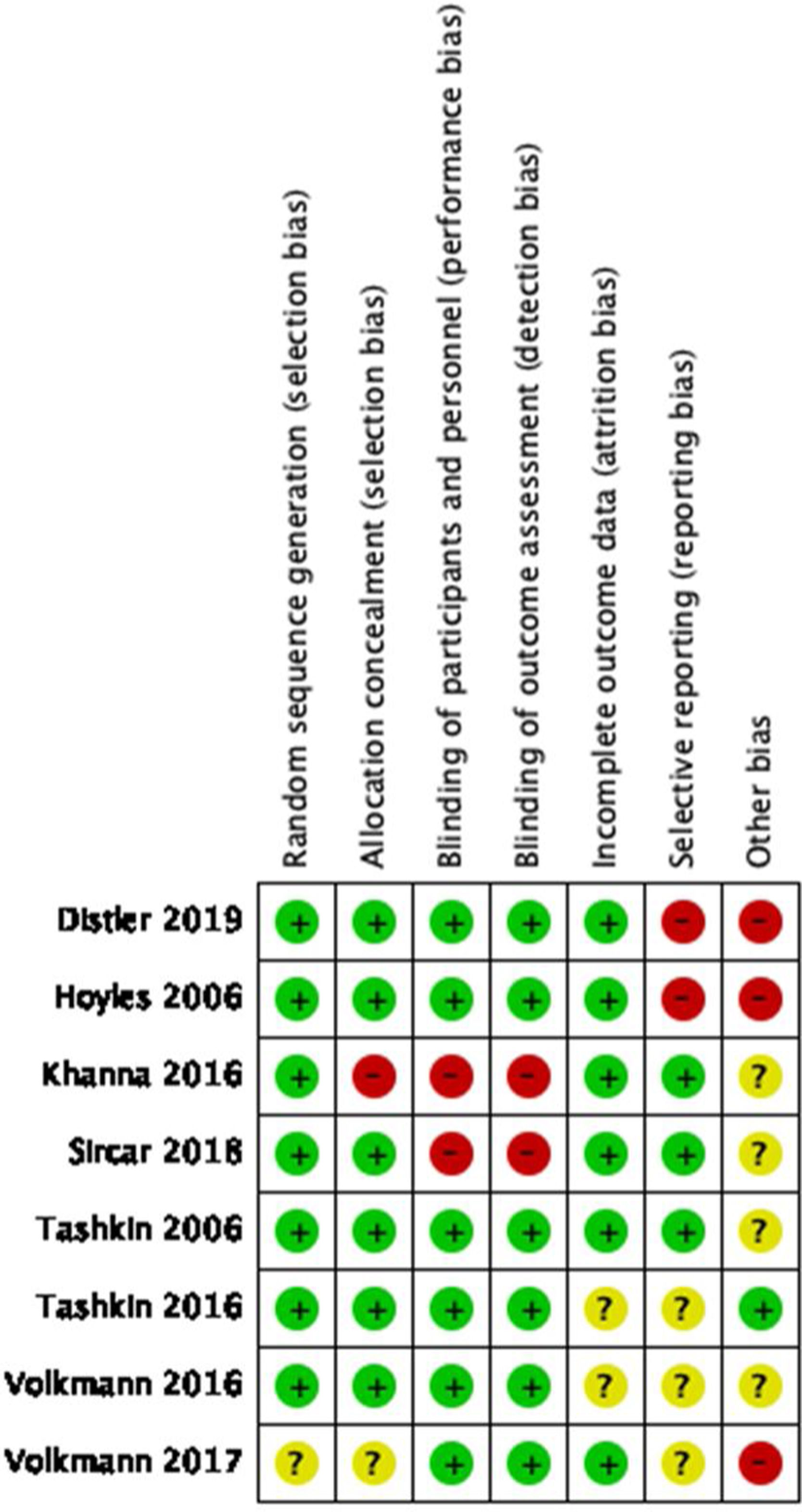
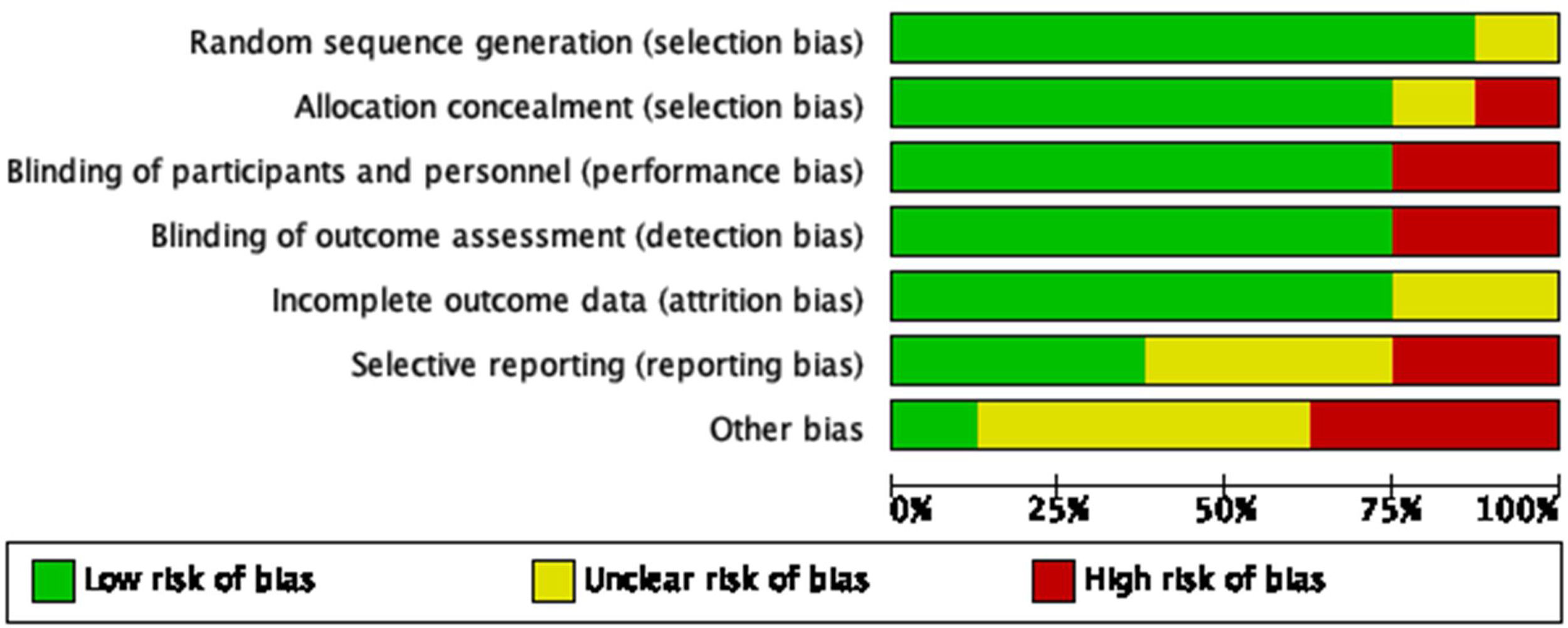
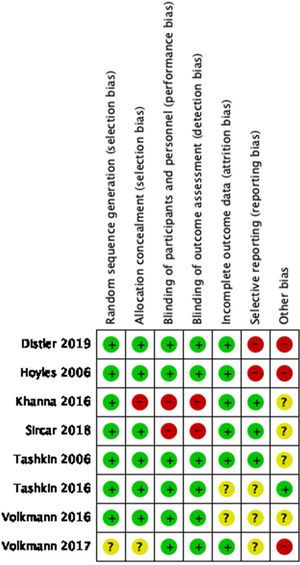
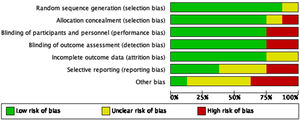
Regarding the quality of the clinical trials that were qualified following the recommendations from the Cochrane Handbook for systematic reviews (see Figs. 2 and 3), there is a low risk of selection and performance bias, considering that most studies used an adequate randomization method and allocations were clearly described (low risk in at least 75% of cases). Also, there was a low risk for performance and detection bias, considering that 6 out of 8 studies were double-blind. Two clinical trials were open-labeled, increasing the risk of bias. Regarding attrition bias, most studies did describe the loss of patients during follow-up, and the statistical treatment given to the missing data, giving a low risk for this bias. In the case of reporting bias, there is a moderate-to-high risk of being present as some studies did not adequately describe the role of sponsors that may influence the publication of certain data. Based on this, the overall quality of clinical trials included could be considered of moderate to high quality.
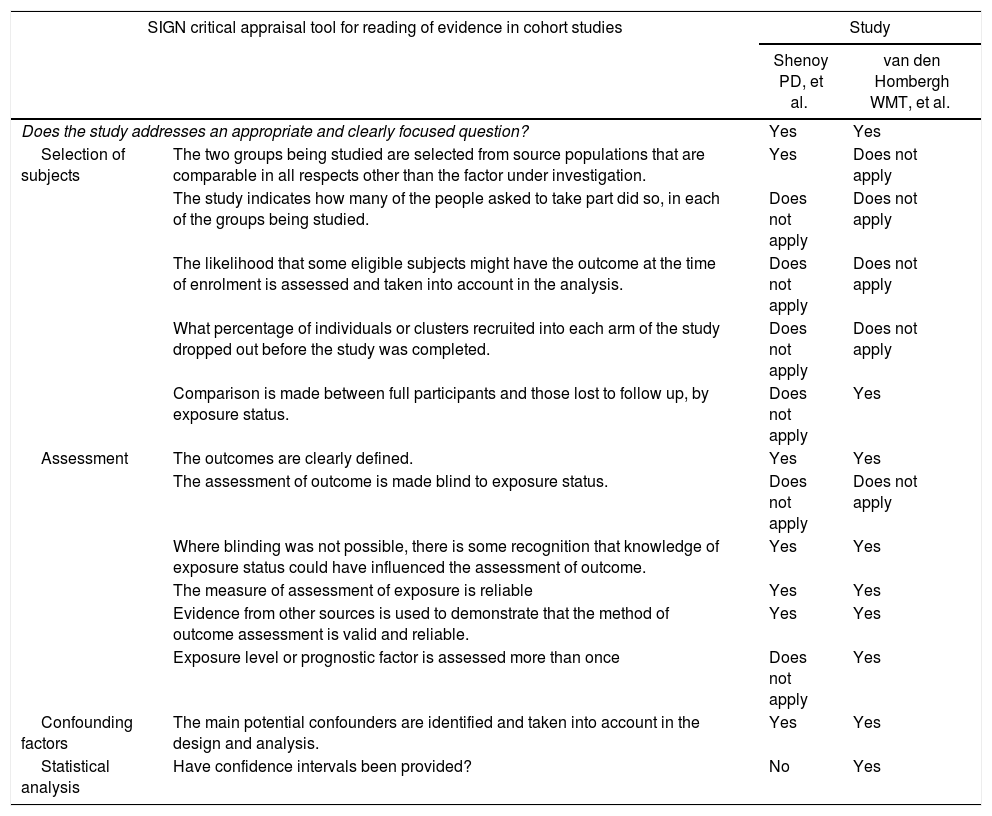
The quality assessment of cohorts was done using the SIGN checklist (Table 3). The studies developed an appropriate research question. They selected the participants from every group from comparable populations. No information was given regarding the number of patients that were asked to participate, as these were retrospective cohorts. One of the studies did compare the outcomes between participants and those patients lost to follow-up. In both studies, the outcomes were clearly defined, and evidence is given regarding the methods used to measure them. As they were retrospective studies, blinding was not possible. Main potential confounders were considered during the analysis. Only one of them reported CIs.
Quality of evidence assessment of cohort studies using the SIGN checklist.
| SIGN critical appraisal tool for reading of evidence in cohort studies | Study | ||
|---|---|---|---|
| Shenoy PD, et al. | van den Hombergh WMT, et al. | ||
| Does the study addresses an appropriate and clearly focused question? | Yes | Yes | |
| Selection of subjects | The two groups being studied are selected from source populations that are comparable in all respects other than the factor under investigation. | Yes | Does not apply |
| The study indicates how many of the people asked to take part did so, in each of the groups being studied. | Does not apply | Does not apply | |
| The likelihood that some eligible subjects might have the outcome at the time of enrolment is assessed and taken into account in the analysis. | Does not apply | Does not apply | |
| What percentage of individuals or clusters recruited into each arm of the study dropped out before the study was completed. | Does not apply | Does not apply | |
| Comparison is made between full participants and those lost to follow up, by exposure status. | Does not apply | Yes | |
| Assessment | The outcomes are clearly defined. | Yes | Yes |
| The assessment of outcome is made blind to exposure status. | Does not apply | Does not apply | |
| Where blinding was not possible, there is some recognition that knowledge of exposure status could have influenced the assessment of outcome. | Yes | Yes | |
| The measure of assessment of exposure is reliable | Yes | Yes | |
| Evidence from other sources is used to demonstrate that the method of outcome assessment is valid and reliable. | Yes | Yes | |
| Exposure level or prognostic factor is assessed more than once | Does not apply | Yes | |
| Confounding factors | The main potential confounders are identified and taken into account in the design and analysis. | Yes | Yes |
| Statistical analysis | Have confidence intervals been provided? | No | Yes |
The quality assessment of the case–control study was also made by applying the SIGN checklist from Table 4. In general, this is a high-quality study considering that it addresses and appropriate research question, cases and controls were recruited from comparable populations, and the same inclusion and exclusion criteria were applied. No information was given regarding the number of patients who were asked to participate, and no comparison was made between participants and no participants. Cases and controls were clearly defined at the design. The exposure was measured in a standard reliable way, and confounding variables were considered during the analysis. No CIs were presented in the manuscript.
Quality of evidence assessment of case–control studies using the SIGN checklist.
| SIGN critical appraisal tool for reading of evidence in case–control studies | Study | |
|---|---|---|
| Jordan S. et al. | ||
| Does the study addresses an appropriate and clearly focused question? | Yes | |
| Subject selection | Are the case and controls taken from comparable populations (except for the factor under investigation)? | Yes |
| Do the same exclusion criteria are used for both cases and controls? | Yes | |
| What percentage of each group (cases and controls) participated in the study? | No information available | |
| Is a comparison made between participants and non-participants to establish similarities or differences? | No | |
| Are the cases clearly defined and differentiated from the controls? | Yes | |
| Is it clearly established that controls are not cases? | Yes | |
| Evaluation | Are measures taken to prevent knowledge of the primary exposure from influencing case ascertainment? | No |
| Is exposure measured in a standard, valid and reliable way? | Yes | |
| Confounding factors | Have potential confounding variables been identified and taken into account in the design and analysis? | Yes |
| Statistical analysis | Are the confidence intervals presented? | No |
To develop the qualitative analysis, it was defined that effect measurements were going to be calculated based on the most frequent outcome reported by the studies included in the review, which in this case was FVC % predicted. The effectiveness outcomes were based on the calculated worsening of FVC% predicted. Two categories were applied: >5% decline in FVC, or >10% decline in FVC. The selected effect measurement, in this case, was the calculation of the odds ratio (OR) for achieving >5% or >10% decline in FVC, depending on the available data from each study included. The OR was calculated by comparing the outcomes between the intervention medication and the comparison (medication or placebo). Given the heterogeneity of data reported by the studies, and the diversity of study designs (clinical trials, cohorts, case–control studies, meta-analyses), the calculation of OR was not possible in all cases. The results of the effect measurements calculated are presented in Table 2.
Based on the final results of effect measurements, the possibility to conduct a meta-analysis was considered. After a consensus between authors, it was decided that the analysis would be focused on assessing the effect of pharmacological treatments vs. placebo, for the development of a >5% or >10% decrease in FVC. A random-effect model was applied for the process given the characteristics of the data.
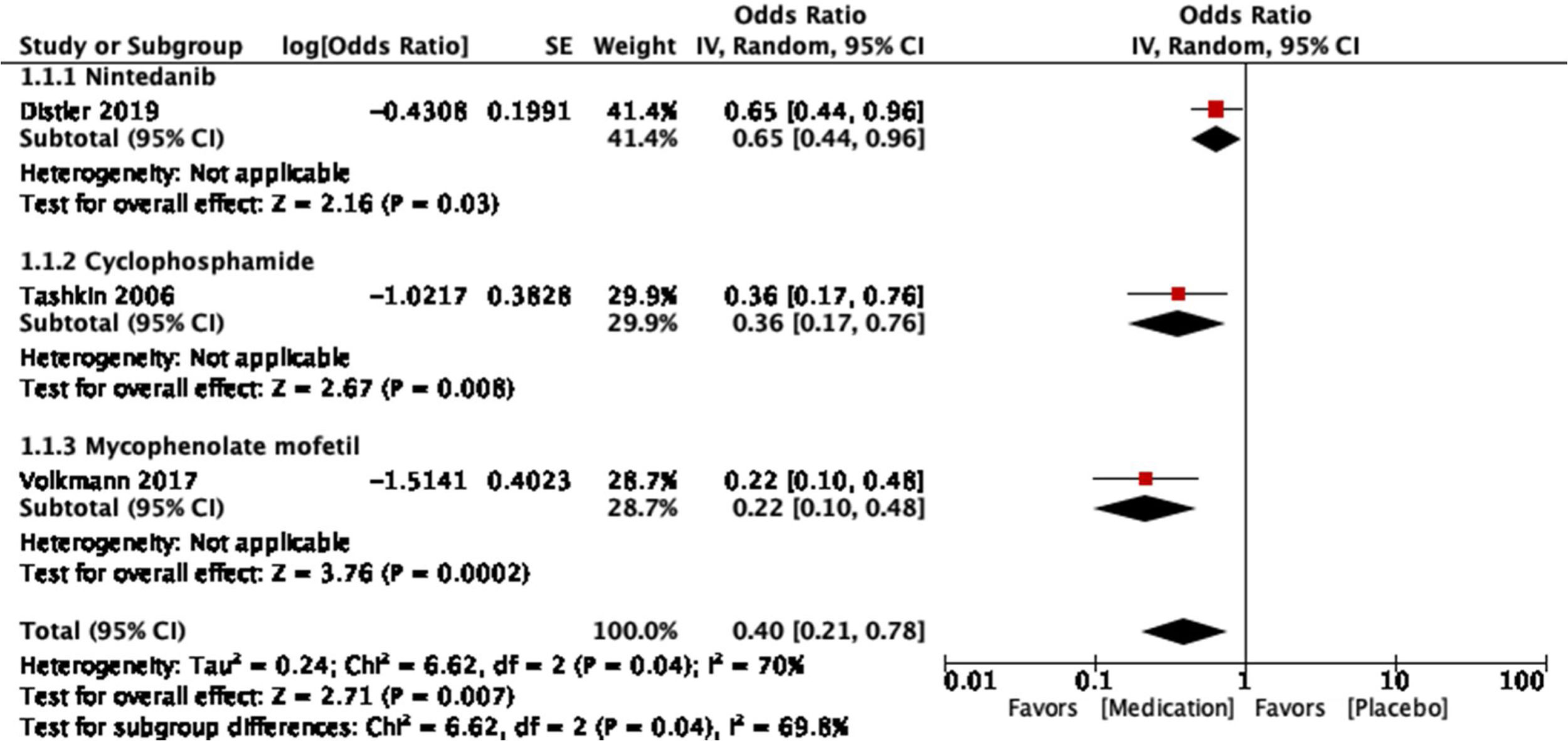
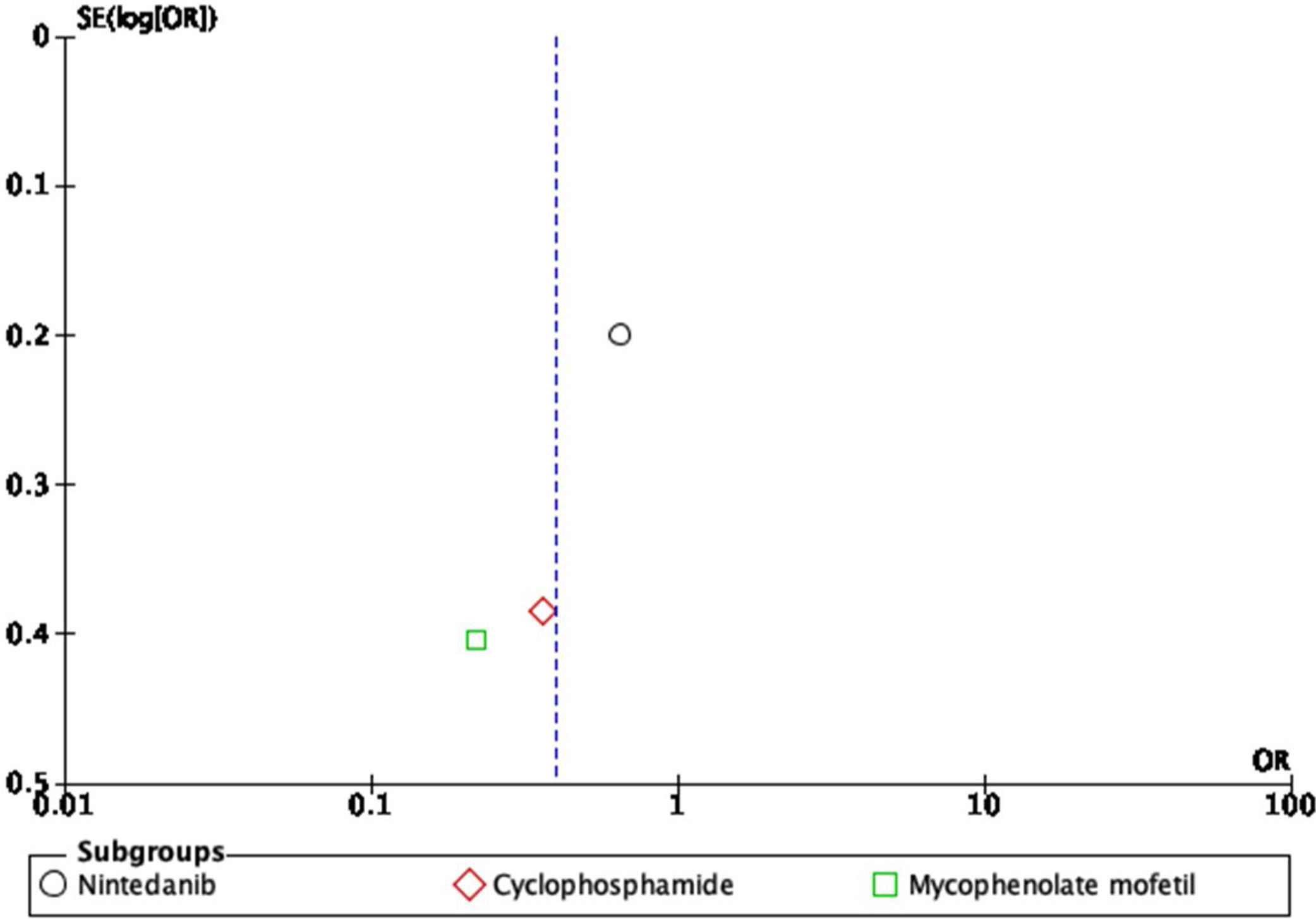
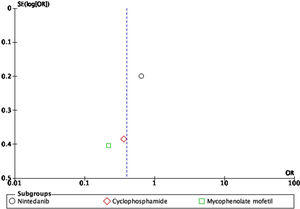
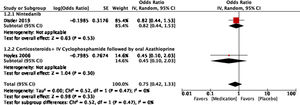
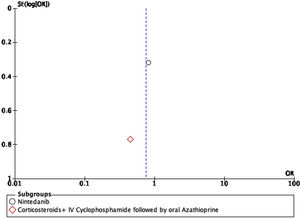
For the outcome of decrease >5% FVC when comparing pharmacological treatment vs. placebo, three studies were selected. The study by Distler and colleagues35 compared nintendanib vs. placebo, with a result favoring placebo for a worsening of FVC values (OR=0.65; 95% CI: 0.44–0.96). Data of CYC vs. placebo was taken from the data reported by Tashkin,36 and the results show that CYC was negatively associated with a >5% decrease in FVC (OR=0.36; 95% CI: 0.17–0.76). Volkmann and colleagues42 reported data comparing MMF vs. placebo, and the effect measurement again favors placebo to a >5% decrease in FVC (OR=0.22; 95% CI: 0.1–0.48). A Forest plot was built with this data (see Fig. 4), and it clearly shows that evidence supports the use of pharmacological medications vs. placebo to prevent a worsening of pulmonary function in patients with SSc-ILD, specifically FVC. The overall result was OR=0.4 (95% CI: 0.21–0.78), indicating that the odds of presenting a >5% decrease in FVC is 0.4 times greater in patients receiving pharmacological agents compared to placebo. MMF was the medication with less association with the outcome. The studies used present a great heterogeneity, which is supported by an I2=70%. The results were statistically significant considering a P=0.007 for the overall effect.
The resulting Funnel plot from the studies included analyzing the decrease in >5% FVC outcome is presented in Fig. 5. It shows that the nintedanib vs. placebo trial presented the greatest sample size and is the closest to the expected overall effect. The studies evaluating CYC and MMF show an inferior sample size, but are still close to the expected effect. Low risk of publication bias may be present.
The second outcome assessed was the decrease >10% of FVC. Following the process described before, two studies were selected for meta-analysis, based on the result of effect measurement (see Fig. 6). Again, the study of Distler and Colleagues,30 comparing nintedanib vs. placebo was selected, and results were an OR favoring nintedanib as being negatively associated to a >10% reduction of FVC, however, the results were not significant (OR=0.82; 95% CI: 0.44–1.53). Hoyles and colleagues compared oral prednisolone+CYC followed by AZA vs. placebo and the results again showed a negative association between the pharmacological regime, compared to placebo, however, no significant difference was obtained (OR=0.45; 95% CI: 0.10–2.03). As expected, pooled results showed no statistical differences in the studies included (OR=0.75; 95% CI: 0.42–1.33). No heterogeneity within studies was detected, based on I2=0%. The results were not statistically significant for the overall effect (P=0.33).
The Funnel plot for this outcome is shown in Fig. 7. Again, the Nintedanib trial presents the greatest sample size and is the one driving the effective measurement of the outcome. The study conducted by Hoyles and colleagues presents a significant lower sample size; however, it remains close to the Funnel axis. Publication bias may exist with this final study, as the plot shows it far from the line of no effect when the expected behavior should be the contrary.
DiscussionILD continues to be a challenging condition in patients with SSc, considering that it is one of the most frequent complications of the disease. Given the nature of this fibrotic condition, its treatment represents great difficulty as limited therapeutic options are available to try to control the progression of the disease. Based on this scenario, this systematic literature review aimed to evaluate the effectiveness of the different pharmacological agents for the treatment of patients with SSc-ILD. Most studies available are clinical trials evaluating several immunosuppressive agents such as CYC and MMF compared to placebo or other therapeutic regimes. The purpose in most of them was to assess objective evidence of stopping disease progression or even the improvement of pulmonary function, which generally were expressed in terms of changes in FVC% predicted.
CYC was the pharmacological agent most frequently used in the trials, especially the parenteral presentation to accomplish the monthly pulse regime during the induction stage. All studies comparing CYC against placebo show improvements in pulmonary function, skin thickness and quality of life of patients. Improvements were supported by changes in FVC% predicted, while changes in DLCO % predicted were not significant. One study presents evidence of the use of CYC followed by AZA as an alternative treatment, which also appears to be beneficial for the improvement of pulmonary function compared to placebo. However, one of the most recurrent doubts regarding the use of CYC is the profile of adverse events associated with its use. That was the reason why other pharmacological alternatives have been studied.
MMF is a frequent medication used as an alternative to CYC for the treatment of these patients. The studies assessed show evidence that MMF presents no significant differences compared to CYC, regarding effectiveness for the treatment of SSc-ILD. However, this medication is better tolerated and is associated with less toxicity compared to CYC. It represents a safer alternative for treatment during induction and maintenance. As expected, studies comparing MMF vs. placebo also favor MMF regarding improvements in FVC% predicted.
Another alternative medication that has been studied is RTX. The available evidence shows that RTX is also associated with stabilization and even improvement of pulmonary function tests (FVC% predicted over DLCO). When compared to CYC, RTX appears to induce a better functional response than CYC, and it was associated with less adverse events. It seems to work adequately in patients with diffuse SSc and positive anti-Scl-70 antibodies. As expected, immunosuppression increasing the risk of infections represents the greatest alarm regarding the use of this medication.
Lately, multiple anti-fibrotic agents used in idiopathic ILD have been studied for their application in patients with SSc-ILD. In this review, nintedanib seems to reduce and slow disease progression measured by reduction of FVC% predicted. However, it has no effect on other manifestations associated with SSc. More evidence is needed regarding the use of this medication in order to define a clear recommendation. Another anti-fibrotic agent that has been studied is pirfenidone. Evidence found during this review was focused on the assessment of the safety and tolerability of this medication in patients with SSc-ILD. Preliminary data was given regarding pulmonary function, showing that concomitant use of pirfenidone with MMF was associated with improvement in pulmonary lung function, compared to pirfenidone alone. However, the design of the study does not allow the development of recommendations. Nevertheless, the medication shows an adequate safety profile and tolerability, which should encourage future studies with pirfenidone, to specifically assess effectiveness.
Additional information besides specific treatment effectiveness was found during the search. Currently, the only available methods to follow-up treatment response in patients with SSc-ILD are based on periodic measurement of pulmonary function tests, HR-CT, and functional assessment through standard validated questionnaires. Recent efforts are directed toward the identification of potential biomarkers that may help in the follow-up of patients without invasive or high-cost interventions. Volkmann and colleagues48 gave some evidence about the use of chemokine CXCL4 levels as a response biomarker during the treatment of SSc-ILD. They showed significant reductions in CXCL4 levels in patients receiving CYC or MMF for 12 months or more, and they were inversely associated with improvements in FVC% predicted levels. More studies are required in order to validate this potential biomarker.
Regarding the overall quality of the studies found during this review, most of them could be considered of high quality, considering that 8 studies were clinical trials with moderate-to-high quality (based on bias assessment), and 2 high-quality meta-analyses (one Cochrane review, and one network meta-analysis). Only 4 observational studies were included, most of them accomplishing a good quality of evidence based on methodological design. Although the evidence presented in this review has an overall adequate quality, there is still a limited amount of studies based on this topic.
This study represents an updated review of the evidence available about the effectiveness of different pharmacological agents used for the treatment of SSc-ILD. Even if systematic literature reviews were previously published, they were focused on single medications or newer studies were not included therein. Also, the inclusion of anti-fibrotic agents and biologic treatments such as RTX gives a benefit to this study. However, limitations were also present in this study. The exclusion of meeting abstracts and case-series could have reduced the scope of other new pharmacological agents used in these patients. Also, the limited amount of studies and the heterogeneity of outcomes and comparisons did not allow the development of a high-quality meta-analysis reducing the option to propose recommendations from this exercise.
ConclusionILD represents one of the most common complications presented by patients with SSc-ILD. Pharmacologic strategies for the treatment of this condition are based especially in the use of immunosuppressive agents, being the most frequently used CYC and MMF; the last one is a preferred option due to a better safety profile. Other medications are being studied as alternatives including RTX, and anti-fibrotic agents such as nintedanib or pirfenidone. Evidence is still limited in this topic, and more future research is needed in order to develop solid recommendations for treatment.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Flórez-Suárez JB, Maldonado-Cañón K, Londoño J, Méndez-Patarroyo P, Quintana-López G. Tratamiento de la enfermedad pulmonar intersticial asociada a la esclerosis sistémica: revisión sistemática de la literatura y meta-análisis. Rev Colomb Reumatol. 2020;27:146–169.