We describe the case of a 24-year-old pregnant woman with no history of note who was admitted with a diagnosis of bilateral pneumonia caused by the new coronavirus.
Due to clinical worsening, she required urgent cesarean section with general anaesthesia and intubation for decubitus intolerance. After extubation, she presented altered mental state that required a differential diagnosis of encephalitis/meningitis secondary to SARS-CoV-2.
CT and CT-angiography were normal, spinal fluid tests were non-specific, and magnetic resonance imaging reported posterior reversible encephalopathy syndrome (PRES) (due to radiological features suggestive of white matter vasogenic edema affecting the parietal, temporal and occipital lobes, along with altered mental state) secondary to gestational hypertension. Eleven days after the cesarean section the patient began to develop hypertension that required treatment.
PRES is associated with certain clinical (headache, altered mental state, visual disturbances and convulsions) and radiological (reversible changes in white substance mainly affecting the parietal, temporal, and occipital lobes) characteristics suggestive of vasogenic oedema In pregnant SARS-CoV-2 patients, the differential diagnosis of hypertension and altered mental state is often extremely complicated because complementary tests can be normal and there is no immediate sign of peripartum hypertension. SARS-CoV-2 genome sequencing in spinal fluid could have provided a definitive diagnosis, but the treatment would not have differed.
Describimos el caso de una gestante de 24 años de edad, sin enfermedades previas, que fue ingresada con diagnóstico de neumonía bilateral por el nuevo coronavirus 2. Por empeoramiento clínico precisó cesárea urgente con anestesia general e intubación orotraqueal por intolerancia al decúbito. Tras la extubación desarrolló un cuadro de obnubilación que obligó al diagnóstico diferencial de encefalitis/meningitis por SARS-CoV-2, con tomografía computarizada (TC) y angioTC normales, bioquímica del líquido cefalorraquídeo (LCR) inespecífica y resonancia magnética informada como «síndrome de encefalopatía posterior reversible» (al presentar características radiológicas sugestivas de edema vasogénico con alteraciones en la sustancia blanca de localización parieto-temporo-occipital, junto con alteración de nivel de conciencia) secundaria a cuadro hipertensivo del embarazo. La paciente 11 días después de la cesárea comenzó a desarrollar un cuadro hipertensivo que requirió tratamiento.
La encefalopatía posterior reversible (PRES) asocia un conjunto de características clínicas (cefalea, alteración del nivel de conciencia, alteraciones visuales y convulsiones) y radiológicas (alteraciones reversibles en la sustancia blanca fundamentalmente en regions parieto-temporo-occipitales) sugestivas de edema vasogénico.
En pacientes gestantes SARS-CoV-2, el diagnóstico diferencial de la patología hipertensiva y las alteraciones de nivel de conciencia puede ser extremadamente complicado, al encontrarnos pruebas complementarias normales y ausencia de hipertensión arterial (HTA) en el periparto inmediato. Tal vez la secuenciación del genoma del SARS-CoV-2 en el LCR nos hubiera permitido un diagnóstico de certeza, aunque el tratamiento no hubiera variado.
SARS-CoV-2 infection is a global public health problem. Although it was initially feared that the immunosuppressive state caused by pregnancy could lead to a higher incidence of complications, case series published so far seem to indicate that the characteristics of COVID-19 in pregnant woman do not differ from the general population.1,2
However, some clinical pictures and symptoms may be aggravated or masked by the physiological alterations inherent to pregnancy. Although not described at the start of the pandemic, the neurotropism of SARS-CoV-2 is now known to affect the central nervous system (CNS), making it difficult to perform differential diagnosis with pregnancy-associated pathology that causes similar symptoms.
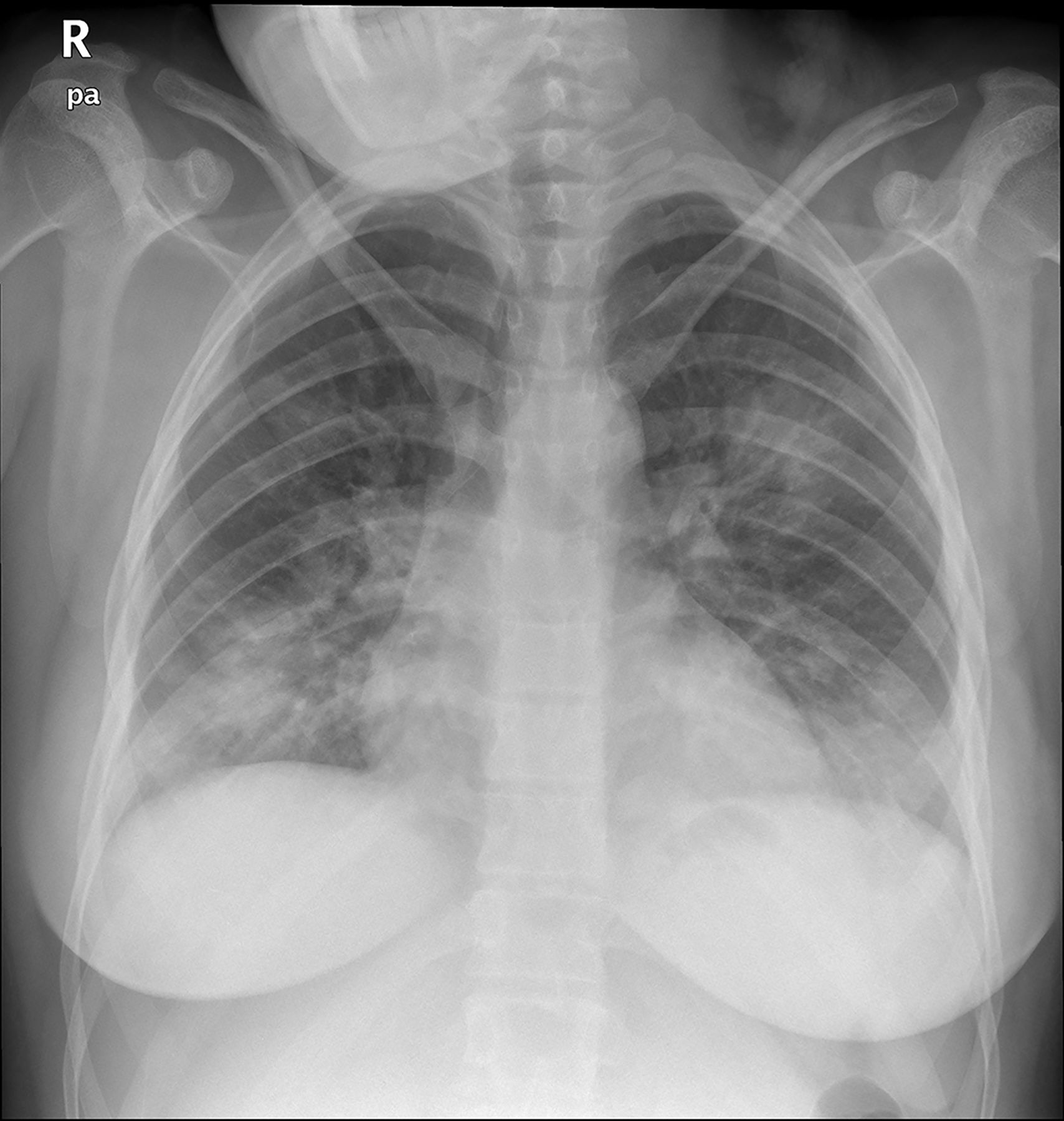
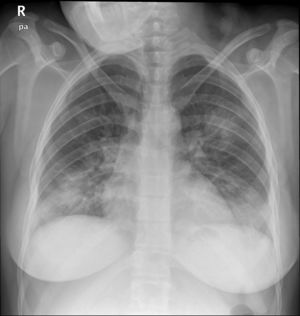
Case reportA 24-year-old pregnant woman at term (height: 1.61 m; weight: 68 kg) was admitted with a 3-day history of fever, cough and dyspnoea. Chest X-ray showed a pattern of bilateral interstitial pneumonia in the middle and lower fields (Fig. 1) and reverse transcriptase polymerase chain reaction (RT-PCR) for COVID-19 was positive. Treatment with hydroxychloroquine, azithromycin, ceftriaxone, lopinavir/ritonavir, and enoxaparin was started.
The day after admission, labour was induced due to clinical worsening (increased cough and dyspnoea). Early epidural analgesia was administered without incident during labour, achieving an optimal level of analgesia up to T8 with no motor block.
Over the following hours, the patient developed dyspnoea at rest with tachypnoea progressing to more than 30 rpm accompanied by deterioration of respiratory mechanics and accessory muscle breathing while remaining in a sitting position with the headrest elevated to 90°. Saturation progressively decreased from 99% with nasal prongs at 2 lpm when induction was started, to 91%–92% with nasal prongs at 6 lpm. Her level of consciousness was optimal at that time, and she remained alert and oriented, with no drowsiness or other disturbances. Because of her rapid respiratory deterioration, an urgent caesarean section was required. The patient, whose SPO2 had fallen to 89%, could not tolerate the supine position required for caesarean section, and required general anaesthesia. After intubation, a recruitment manoeuvre was performed and she was ventilated with an FiO2 of 0.4, PEEP of 12 mmHg, tidal volume of 7 ml/kg, and respiratory rate of 16 rpm, which maintained an O2 saturation of 98%–99%. After surgery, she was transferred to the special COVID-19 intensive care unit (ICU).
Subsequent blood gas measurements in the ICU showed a PaO2/FIO2 ratio of 455. The patient continued to receive ventilatory parameters similar to those in the operating room, and remained sedated with propofol and remifentanil. She made good progress, and was extubated after 20 h. After extubation, she presented a hypoactive confusional syndrome with temporal-spatial disorientation, but no other focal signs.
She was transferred to the ward 2 days later, where she developed mild right hemiparesis, fluctuating levels of consciousness, and increased drowsiness, which progressively worsened to a Glasgow coma score of 10 (M5, O2, V2) and motor aphasia interspersed with periods of agitation, which led to respiratory deterioration. A cranial computed tomography (CT) scan showed no signs of acute pathology.
Given her neurological deterioration, she was admitted to the maternity critical care unit, where she was examined by a neurologist. CT angiography was normal, but lumbar puncture showed xanthochromia, proteins: 0.53 g/L; WBC: 5 cells/μL, glucose: 41 mg/dl. Gram stain, bacterial culture and molecular testing for enterovirus, VZV and HSV 1 and 2 were negative. Treatment with subcutaneous enoxaparin (60 mg/24 h), methylprednisolone (1 mg/kg/24 h) and ceftriaxone was started. Twelve hours after admission she presented respiratory deterioration that required reintubation and transfer to the ICU.
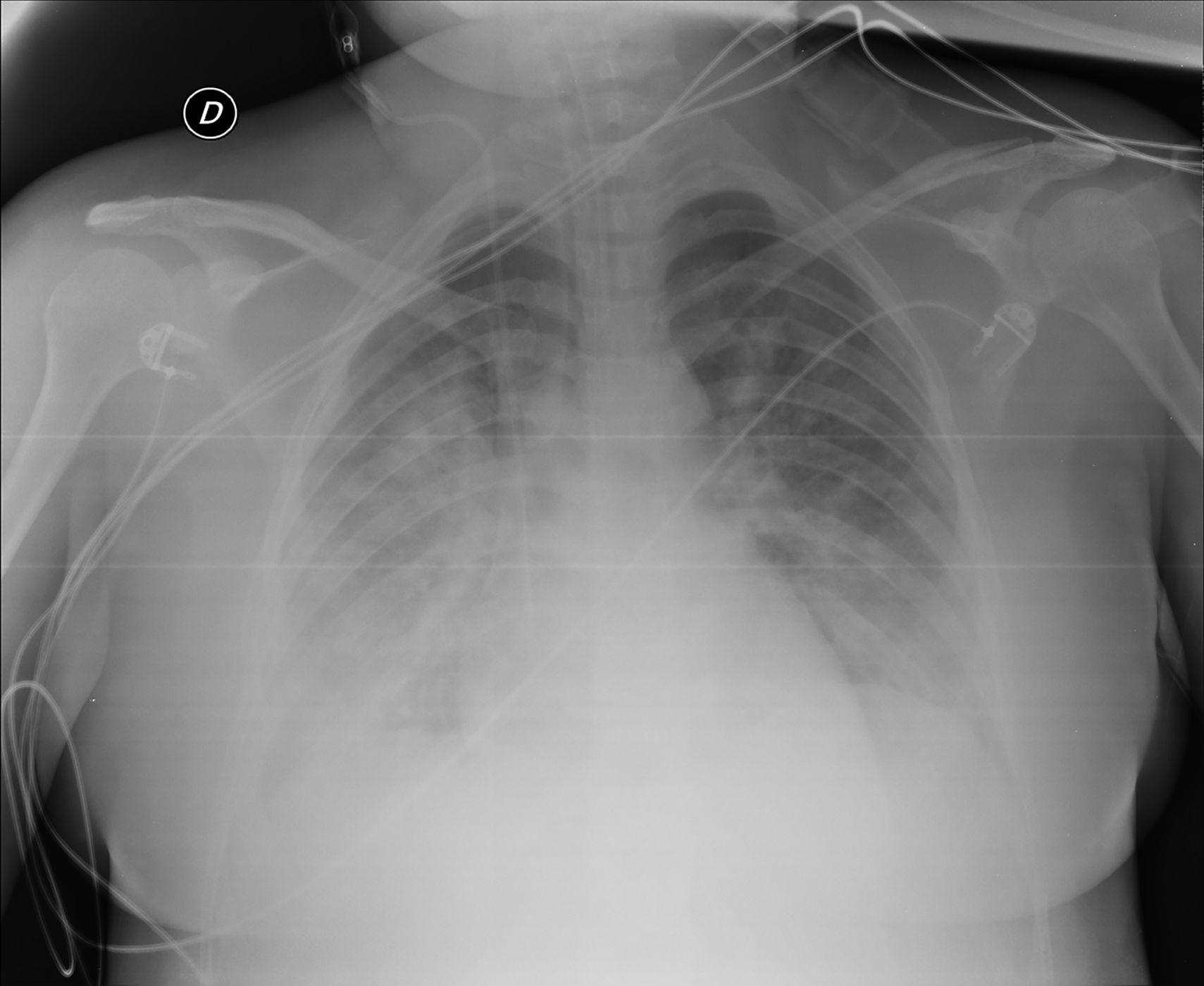
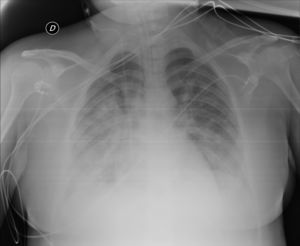
A new chest X-ray showed worsening (Fig. 2) and labs showed a pro-inflammatory state with IL-6: 151 pg/mL, PCR: 13.4 mg/dl, and procalcitonin: 9.3 ng/mL, so tocilizumab was administered. Respiratory mechanics improved, permitting PEEP to be decreased and switching to pressure support ventilation on the third day. Levetirazetam was started due to a suspicion that the neurological deterioration could have been caused by a seizure. An electroencephalogram (EEG) showed a marked slowing pattern and occasional triphasic waves that were more marked at the left temporal level, calling for investigation of a possible underlying lesion. No epileptiform activity was observed. Daily sedation windows were performed, connecting the patient on the fourth day and allowing extubation. During her stay, she presented an isolated episode of hypertension (HT) that responded to 25 mg of iv urapidil.
Anteroposterior chest X-ray in supine position: radiological worsening compared to Fig. 1. Lower lung volumes, increased bibasal opacities. Moderate-severe radiological involvement.
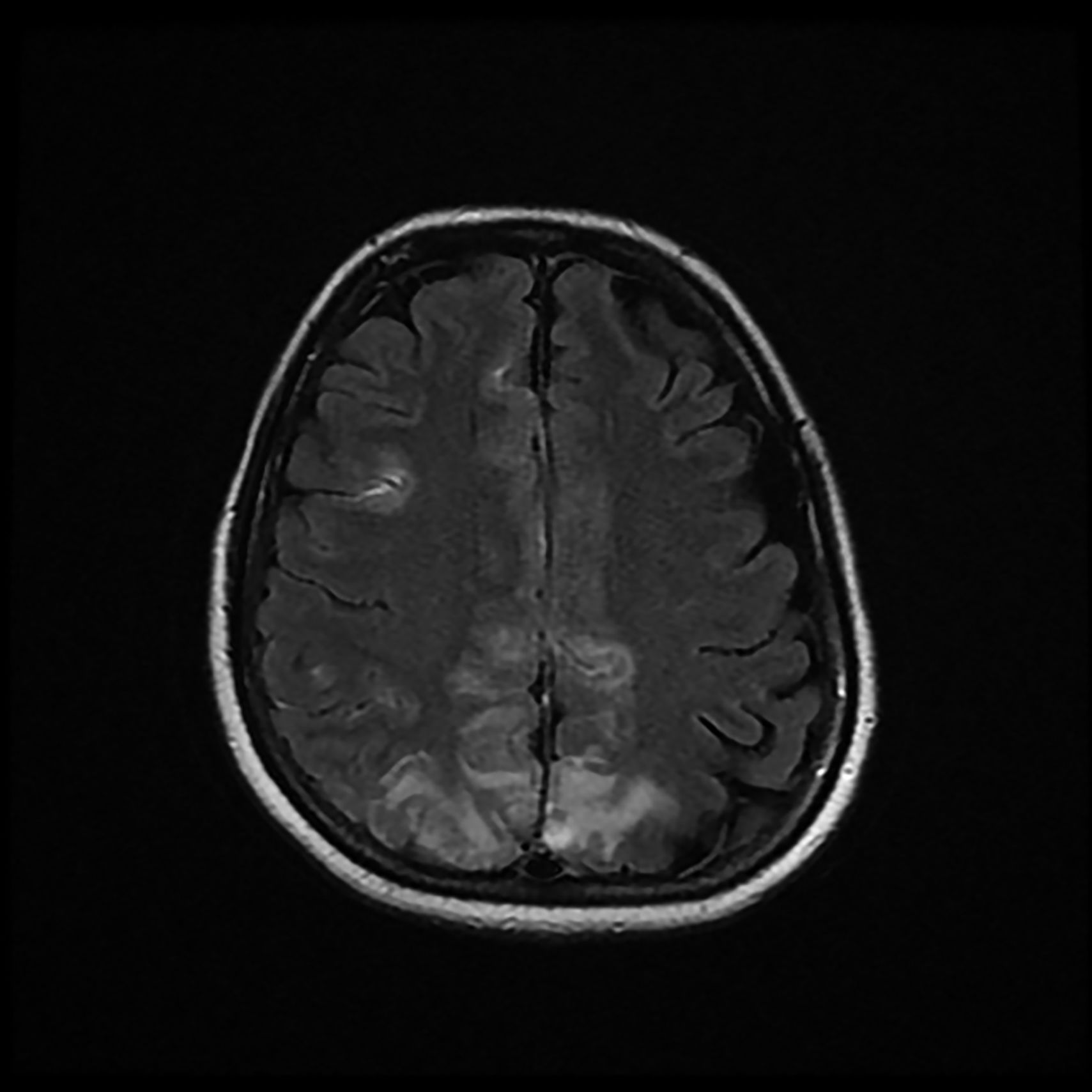
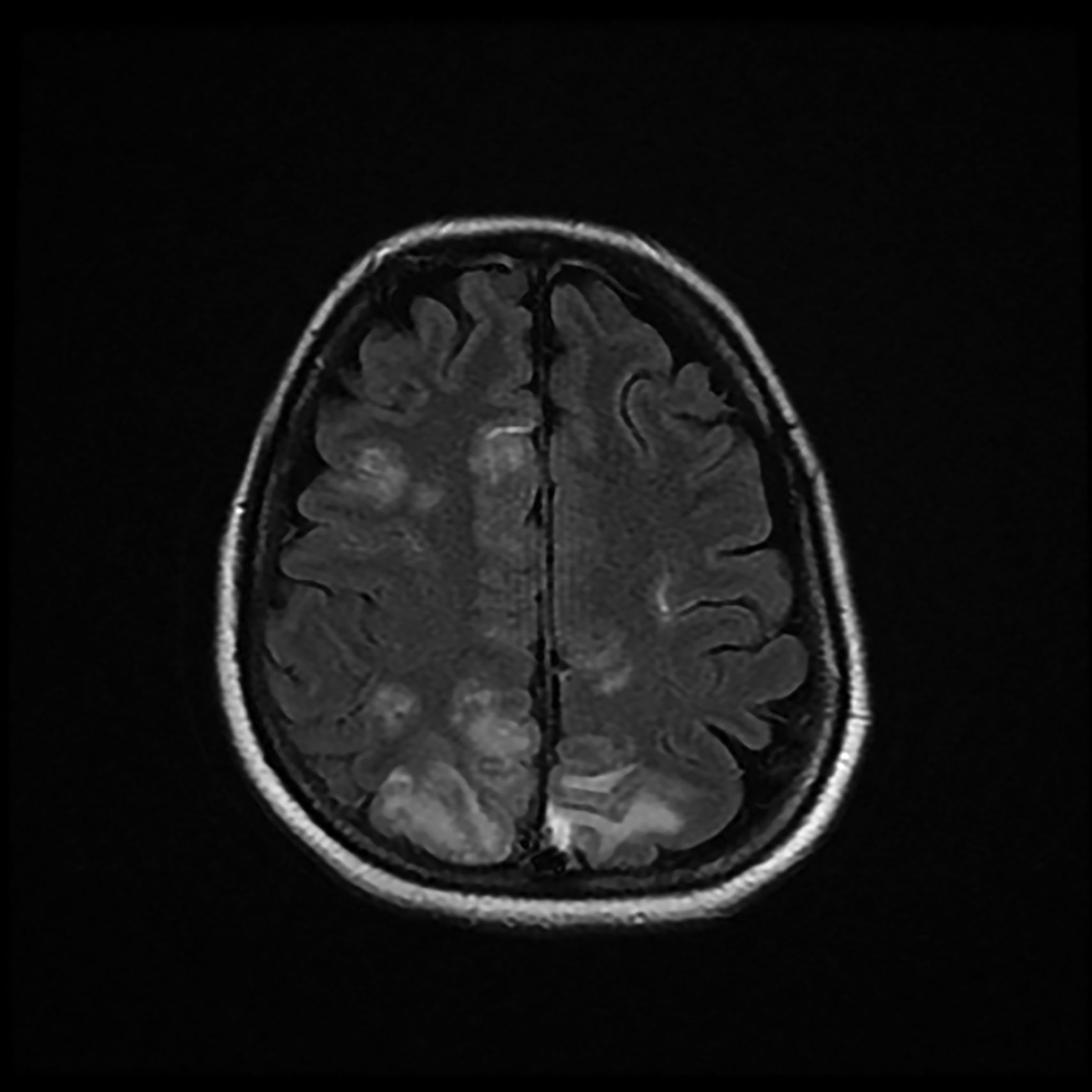
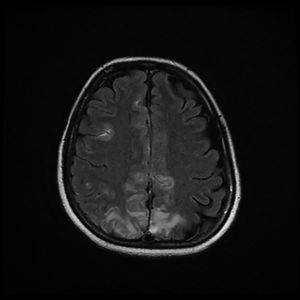
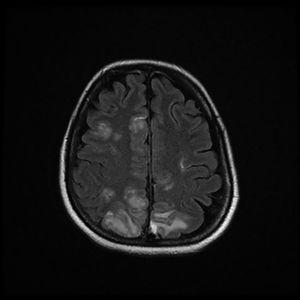
The patient was discharged to the ward on the fifth day, where inattention, apathy, and asymmetric mobilization of the right lower limb were noted. Although she showed steady respiratory and neurological improvement, a magnetic resonance imaging (MRI) study reported extensive hyperintense areas involving both the cortex and subcortical white matter. These areas were bilateral and symmetrical, but with greater extension in the right hemisphere and involvement of the parasagittal region and the bilateral parietal convexity, the bilateral frontal parasagittal region, and areas in the region of the right frontal superior convexity. Small areas of leptomeningeal uptake were identified, corresponding to slower, though unrestricted, intravascular blood flow. The finding was reported as posterior reversible encephalopathy (PRES) (Figs. 3 and 4). After the third day of hospitalisation, the patient presented episodes of HT, which were initially treated with labetalol and later with enalapril and amlodipine, and were diagnosed as peripartum hypertension. Nine days after re-admission to the ward the patient was discharged home after a total hospital stay of 18 days. Enalapril, amlodipine, and levetirazetam were prescribed as outpatient treatment.
Magnetic resonance imaging (T2-weighted and FLAIR images): Axial slices showing hyperintense areas with bilateral cortical and subcortical white matter involvement, more extensive in the right hemisphere. Involvement is seen in the parasagittal region and bilateral parietal convexity, and in the bilateral frontal parasagittal region. The image is suggestive of PRES.
Magnetic resonance imaging (T2-weighted and FLAIR images): Axial slices showing hyperintense areas with bilateral cortical and subcortical white matter involvement, more extensive in the right hemisphere. Involvement is seen in the parasagittal region and bilateral parietal convexity, and in the bilateral frontal parasagittal region. The image is suggestive of PRES.
At the time of writing, the patient has remained at home with clear signs of improvement, although 48 hours after discharge she returned to the emergency room with symptoms of obtundation. A follow-up MRI will be performed within 2 months.
DiscussionSARS-CoV-2 produces neurological involvement at the level of the CNS (tremors, headaches, altered level of consciousness, stroke, ataxia and seizures), the peripheral nervous system (altered taste, smell or vision and neuropathic pain), and the musculoskeletal system. Mao et al. described neurological symptoms in up to 36.4% of patients, associating their appearance with the severity of the disease; 14% of patients with severe disease presented altered level of consciousness.3,4 In Europe, anosmia and ageusia are found in 85% of patients, with olfactory alteration being the initial symptom in 12% of cases.5,6
The pathophysiological mechanisms are not fully understood. As in other viruses, neurological involvement could be caused by direct CNS invasion, by toxic encephalopathy associated with severe systemic infection, or as a manifestation of myelin sheath damage. In addition, patients with COVID-19 tend to present high D-dimer levels, thrombocytopaenia and microangiopathy, which together with endothelial damage increases their propensity to stroke caused by different mechanisms.7
First, several coronaviruses target the angiotensin converting enzyme 2 (ACE2), which is very abundant in type II alveocytes, glial cells and neurons.4 ACE2 mainly regulates blood pressure and controls antisclerotic mechanisms. Interaction between SARS-CoV-2 proteins and the ACE2 of the capillary endothelium causes HT, direct vascular damage, and alteration of the blood-brain barrier (BBB),7 facilitated by inflammatory over-response that originates the cytokine storm that enables the virus to invade the CNS7 and increase the risk of brain haemorrhage.4
Second, some authors have described a mechanism of viral migration along neuronal pathway through infection of the sensory or motor nerve endings, as occurs in the olfactory pathway. The olfactory tract and olfactory bulb nerves in the nasal and cranial cavity are a direct channel between the nasal epithelium and the CNS. SARS-CoV-2 could enter the CNS through the olfactory tract at the onset of infection, thus reaching the brain and cerebrospinal fluid (CSF) and causing inflammation and demyelination. Various analyses have found SARS genomic sequences in neurons.7
In addition, severe SARS-CoV-2-related bilateral pneumonia causes significant hypoxia and promotes anaerobic metabolism in neuronal mitochondria, producing cerebral vasodilation, neuronal involvement, interstitial oedema, blood flow alteration and headache, and can lead to intracranial hypertension, impaired consciousness and coma.
Together with this, the inflammatory response syndrome associated with COVID-19 induces a pro-inflammatory state with significant elevation of IL-6 that can activate glial cells, leading to chronic inflammation and brain damage.7
CSF PCR for the most likely viruses is the primary diagnostic method, but it must be accompanied by neuroimaging tests, EEG, CSF examination, serological antigen detection tests, and even brain biopsy.
The CNS invasion by COVID-19 was first reported in January 2020 in a 56-year-old patient from Wuhan, who presented altered consciousness and a normal CT scan. The diagnosis of encephalitis was confirmed by genomic sequencing of SARS-CoV-2 in CSF.6 In March 2020, another case of a 24-year-old man in Japan with generalized seizures was described. Nasopharyngeal samples were negative for SARS-CoV-2, but CSF RT-PCR was positive, leading to a diagnosis of meningitis/encephalitis due to COVID-19.8 Both cases are significant for presenting altered level of consciousness and normal CT scan with no evidence of cerebral oedema.
Poyiadji et al. reported a case of acute haemorrhagic necrotizing encephalopathy related to the cytokine storm syndrome described in COVID-19, and described the characteristic CT and MRI images.9
In our patient, lumbar puncture was nonspecific, a finding also reported in previous published cases of encephalitis. CSF RT-PCR for SARS-CoV-2 was not performed, as it was not available in the lab.
None of the published cases of encephalitis have reported EEG results. Though nonspecific, the findings in our patient are compatible with viral encephalitis. Thromboembolism (common in COVID-19 patients) and cerebral haemorrhage/ischaemia had previously been ruled out with CT and angioCT without contrast. The MRI performed 2 days later (Figs. 3 and 4) with T1-weighted sequence in the sagittal plane and in T2 and FLAIR in the axial plane was compatible with PRES.
PRES has several clinical (headache, altered level of consciousness, visual alterations and seizures) and radiological (reversible white matter alterations, mainly in parieto-temporo-occipital regions) characteristics suggestive of vasogenic oedema.10 It has been mainly associated with hypertension and immunosuppression.10 The pathophysiological mechanisms include vasoconstriction, increased perfusion, and endothelial damage. In pregnant women, PRES typically appears after pregnancy hypertension, but our patient did not present HT or pre-eclampsia criteria until 10 days after delivery, when she developed high diastolic blood pressure of up to 110 mmHg, which required antihypertensive treatment.
The onset of hypertension in the postpartum is infrequent,10 and it must be borne in mind that SARS-CoV-2 can cause HT, cerebral oedema and encephalopathy, and hypertensive encephalopathy itself should be treated as a sign of infection.4 Therefore, COVID became our first aetiological suspicion, due to the presence of both hypertension and encephalopathy with PRES.
On the other hand, in the context of such a severe case of COVID-19 as that presented by our patient it would be simplistic to associate PRES solely with HT that was not documented until 11 days after admission.
Although neurological symptoms and encephalopathy have seldom been reported in the literature, the subjective feeling of clinicians who treat COVID is that these entities are in fact more common than hitherto suspected. The unavailability of some laboratory tests and the non-specificity of the EEG and MRI alterations make accurate diagnosis difficult in obstetric patients. Larger series will be needed to determine the incidence and real prognosis of this type of complication.
FundingThe authors have not received any funding for the publication of this article.
Conflict of interestsThe authors of this article have no conflicts of interest to declare.
Please cite this article as: López Pérez V, Cora Vicente J, Echeverría Granados C, Salcedo Vázquez ML, Estol F, Tebar Cuesta MY. Alteración del nivel de conciencia puerperal: ¿puede el COVID-19 ser causa de síndrome de encefalopatía posterior reversible? Rev Esp Anestesiol Reanim. 2020. https://doi.org/10.1016/j.redar.2020.06.008