COVID-19 pandemic caused not only many deaths around the world but also made evident technical limitations of hospital and intensive care units (ICU). The growing demand of ICU ventilators in a short lapse of time constitutes one of the main community concerns. The main goal of this communication is to give simple solutions to transform a noninvasive ventilator in an invasive one for intubated patients. The proposal can be applied in two well defined strategies for the COVID-19 pandemic: To replace anesthesia workstations, leaving those machines to be used in patients. To apply this option in COVID-19 patients by way of a therapeutic “bridge”, waiting for the release of a ventilator in the ICU.
La pandemia del COVID-19 ha hecho estragos, no solo en el número de víctimas fatales sino también en la infraestructura de los hospitales y unidades de cuidados intensivos. El número limitado de respiradores es una preocupación de toda la comunidad dada la demanda masiva y a muy corto plazo de estos equipos. Esta presentación tiene como fin dar soluciones sencillas para ventilar pacientes intubados de modo mandatorio utilizando equipos de ventilación no invasiva. Las soluciones propuestas permiten dos estrategias claras frente al COVID-19: Reemplazar las máquinas de anestesia para disponer de ellas en pacientes. Usar la opción de equipos de ventilación no invasiva para pacientes con COVID-19 a modo de “puente” y a la espera de la liberación de un respirador específico en la unidad de cuidados críticos.
The COVID-19 pandemic caused by the SARS-CoV-2 coronavirus that originated in the city of Wuhan, China, has left numerous victims around the world in a short period of time.1–4 COVID-19 is a predominantly respiratory condition, ranging from flu-like symptoms to acute respiratory distress syndrome (ARDS). Approximately 10%–15% of patients have moderate respiratory failure, while 5% of cases progress to severe forms that require invasive ventilatory support in intensive care units (ICU).2–4
The highly contagious nature of the virus, shown by its high basic reproduction number (Ro: 2.2-3.5)5 explains why hospitals are crowded with critically ill patients requiring ventilatory support. The supply of respirators currently far outstrips demand, leading to a shortage of these advanced life support devices. This critical health situation forms the basis of the quarantine and social isolation measures implemented to control the rate of infection and minimise, among other things, the need for respirators in the general population. This shortage could become even more acute and dramatic in emerging and underdeveloped countries with severely limited infrastructure and scarce economic resources.
We propose a solution to the shortage of respirators for the treatment of COVID-19. The idea is based on transforming a non-invasive ventilation (NIV) device into a critical care ventilator for intubated patients. We describe the basic configuration of our models, and then describe a simulation performed in a patient with healthy lungs and another with ARDS.
Proposed solutionsTraditional NIV devices are not designed to ventilate intubated patients - one reason for this is rebreathing the expiratory carbon dioxide (CO2) accumulated in the single tube. In other words, as these devices do not have separate inspiratory and expiratory limbs or one-way valves, part of the CO2 exhaled into the single circuit will be rebreathed on the next inspiration, causing hypercapnia. In order to use an NIV device in a ventilated patient, therefore, hypercapnia must be avoided without depressurizing the system or affecting the patient's alveolar ventilation.
In order to use an NIV device in pressure-controlled continuous mandatory ventilation (PC-CMV) mode it must be equipped with a time cycle and back up respiratory rate. This allows the NIV device to be used in both mandatory and spontaneous ventilation modes.
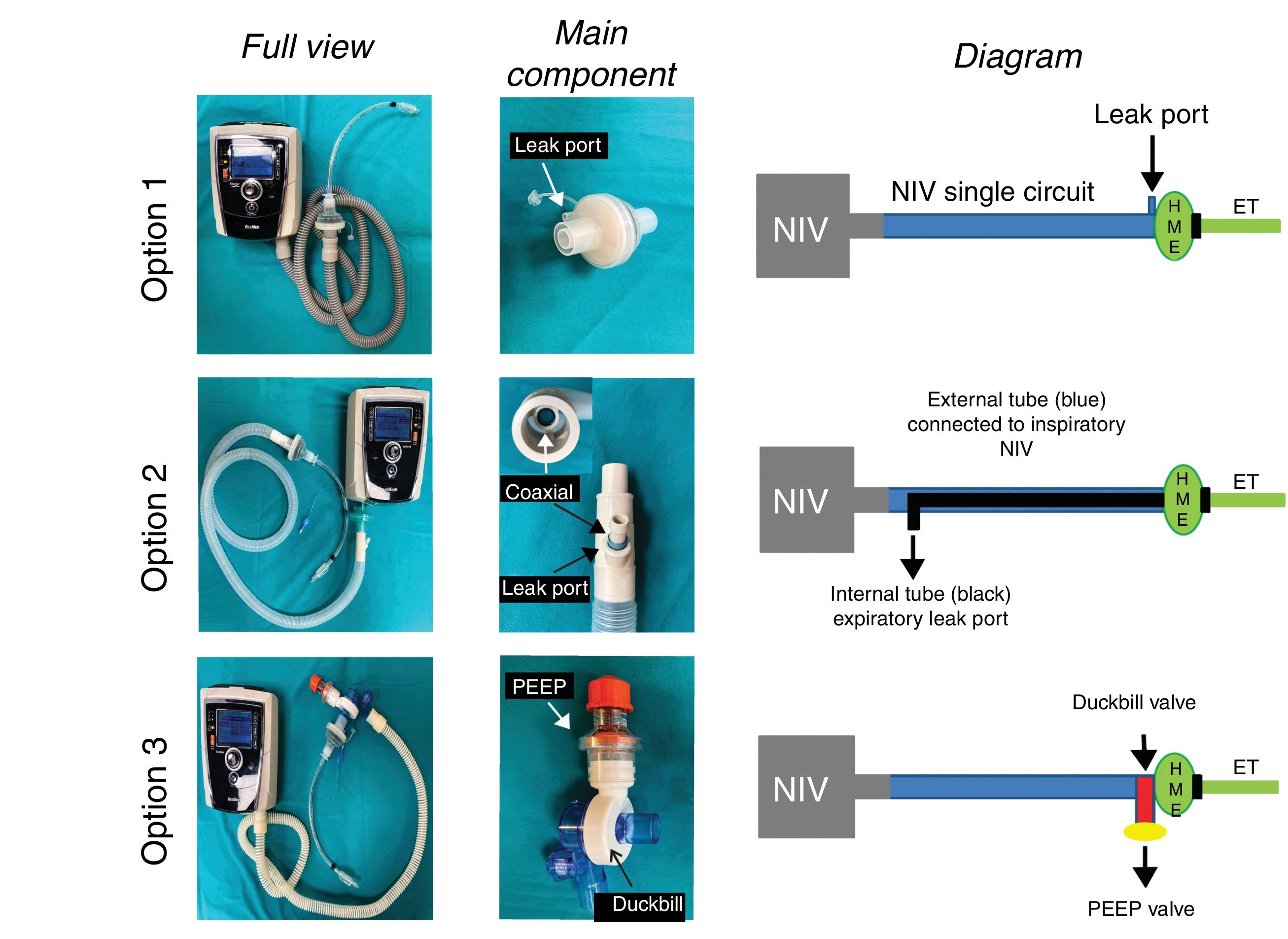
We propose the following solutions (Fig. 1). In all these modifications an antibacterial/viral filter (HMEF) is placed at the end of the endotracheal tube6 to avoid dispersion of SARS-CoV-2 and to conserve temperature and humidity.
Models tested to turn NIV systems into intensive care ventilators. Model 1 uses a single circuit non-invasive ventilator (NIV) with a leak valve proximal to the HMEF filter and endotracheal tube (ET). Model 2 is to add a Bain anaesthesia circuit with double coaxial tubing. The external tube adapts to the NIV equipment and the internal tube serves vents expired gases. Model 3 is a standard NIV circuit with a duckbill non-rebreathing valve located at the distal end connected to the patient, behind the HMEF filter. Expired gases escape through the valve, where an external PEEP valve has been added.
This is the simplest configuration, in which a leak port in the HMEF or the leak port used in this type of device allows a continuous flow of gas that flushes the expired CO2 (Fig. 1, top). In other words, the patient exhales through the HMEF while the NIV device provides enough gas to not only ventilate the patient but also to flush the CO2 trapped in the circuit. This can only be achieved if the NIV device has a highly efficient flow generator to maintain pressurisation. CO2 rebreathing will depend on the size of the leak and the pressure setting (the higher the pressure, the greater the leak flow and the more efficient the CO2 flushing.
Model 2This solution consists of replacing the single branch circuit of the NIV device with a Bain or modified Mapleson D circuit with a double coaxial tube.7,8 The bag of the Bain circuit and fresh gas flow are eliminated. The external tube of the Bain circuit is connected to the NIV device, while the internal branch is left open to allow expired gases to escape (Fig. 1, middle). The potential advantages of this configuration are: 1) expired CO2-rich gas is eliminated through the internal tube, since it is prevented from passing through the external tube by the flow/pressure provided by the NIV device, and 2) some of the temperature and humidity within the circuit is conserved.
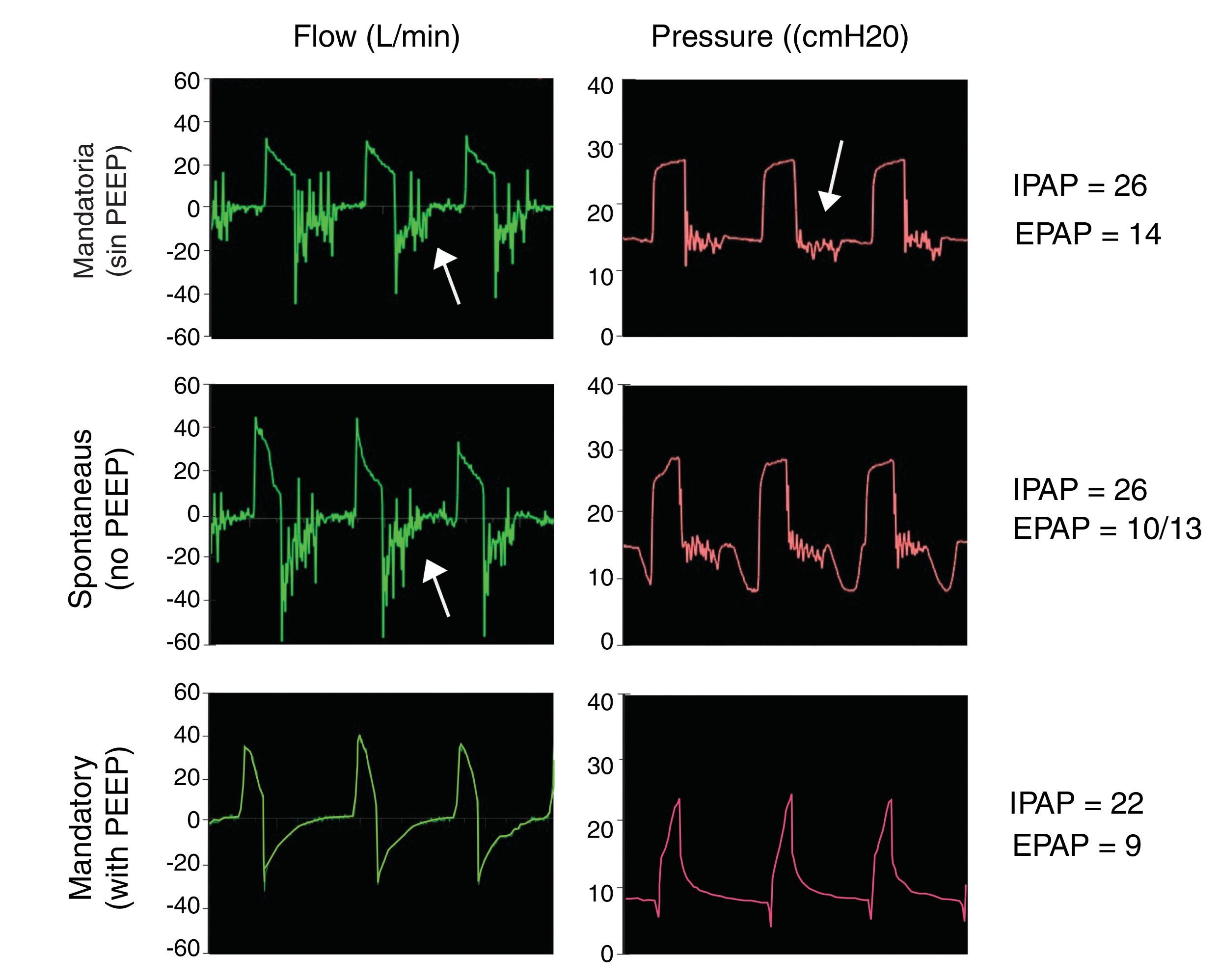
Model 3Another solution is to completely avoid CO2 rebreathing with a Duck-Bill one-way valve placed at the distal end of the single NIV single tube, behind the HMEF filter and the endotracheal tube (Fig. 1, bottom). Mapleson C non-rebreathing ventilation systems (e.g. Ambu® resuscitation bag) use a duck-bill valve in which gas is delivered to patient at one end and CO2 is exhaled into the environment through an external PEEP valve at the other.9 The external PEEP must be set to the same level as the expiratory positive airway pressure (EPAP) of the NIV device in order to reduce expiratory resistance of the duck bill valve, which is not designed for spontaneous ventilation (Fig. 2).
Duck bill valve operation. Simulations with Model 3 setting IPAP to 22 cmH2O and EPAP to 8 cmH2O. Mandatory (top ) and spontaneous (middle) ventilation without external PEEP valve shows flutter in the flow and pressure curves during expiration (arrows). This flutter of the valve on expiration was associated with increased resistance to expiratory flow. This phenomenon was reversed by using an external PEEP valve with the same EPAP settings (bottom).
The proposed configurations (Fig. 1) were evaluated at the Simulation Centre of the Association of Anaesthesia, Analgesia and Post Anaesthesia Care in the city of Buenos Aires. The systems were tested on an ASL 5000 respiratory simulator® (IngMar Medical, Pittsburgh, US ) connected to the NIV device through a number 8 endotracheal tube°. The NIV device used was the Stellar® 150 (ResMed Inc., Sydney, Australia), which includes back up breath rate and time cycling. Pressure, flow and capnography were monitored using the FluxMed monitor® (MBMED, Buenos Aires, Argentina) with sensors placed behind the HMEF filter. The fraction of inspired oxygen (FiO2) was measured with a gas analyser placed between the HMEF and the endotracheal tube.
The simulations were merely intended to show whether the proposed modifications allowed the NIV system to function correctly, maintained adequate pressure during operation, and avoided CO2 rebreathing while achieving FiO2. Given the urgency to publish the results of this due to the COVID-19 pandemic, we did not test different ventilatory parameter combinations or other potential modifications to the NIV circuit.
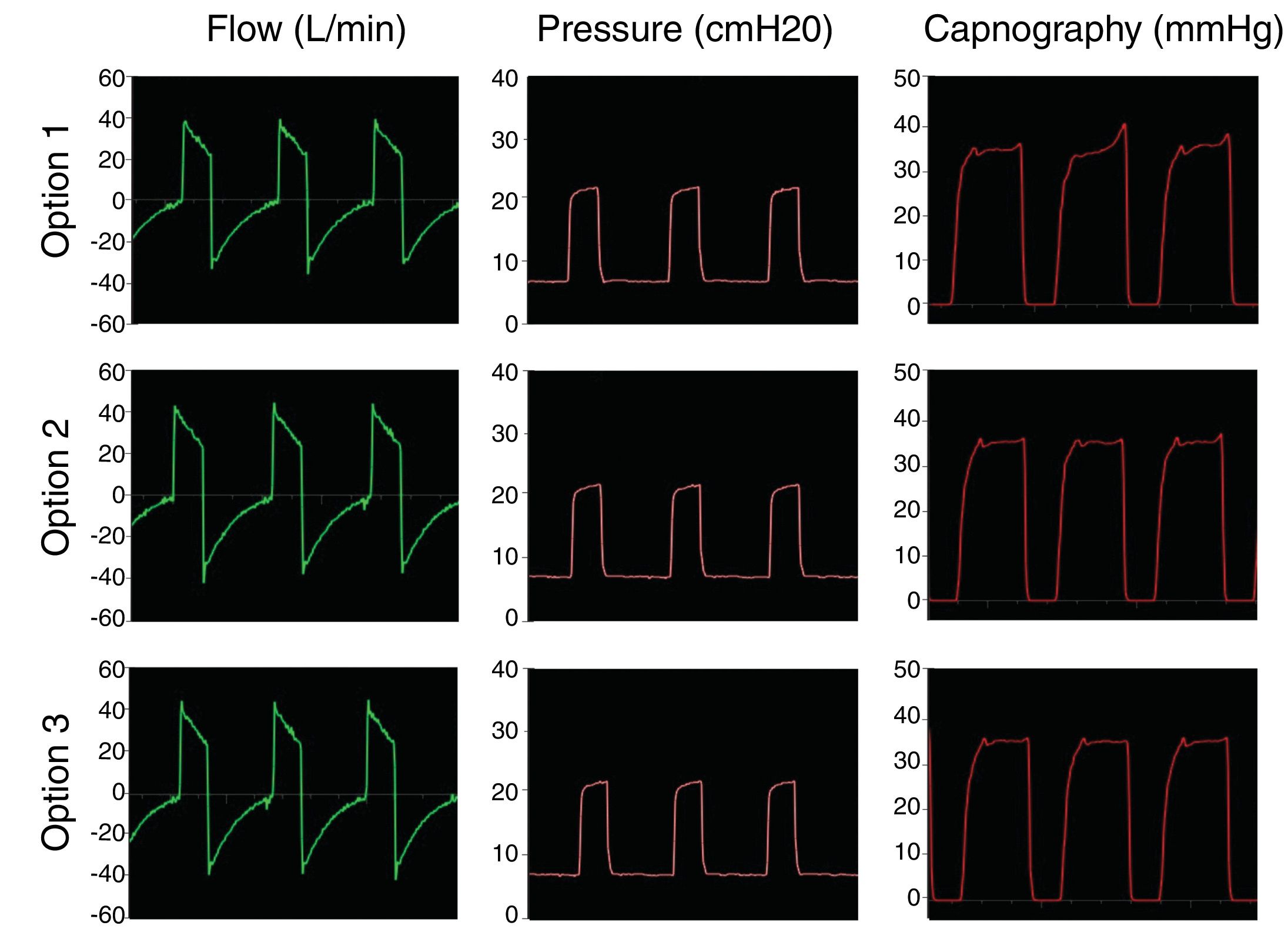
The first step was to simulate controlled ventilation in a patient with healthy lungs producing 170 ml/min CO2, with 50 ml/cmH2O of compliance and airway resistance of 8 cmH2O/l/s. Inspiratory positive airway pressure (IPAP) was set at 20 cmH2O and expiratory positive airway pressure (EPAP) at 8 cmH2O, with a respiratory rate of 18 bpm (inspiratory time; 1 s; expiratory time: 2.3 s). Fig. 3 shows that the proposed modifications maintain circuit pressure and eliminate CO2 correctly, without rebreathing, i.e., the CO2 returns to 0 during inspiration.
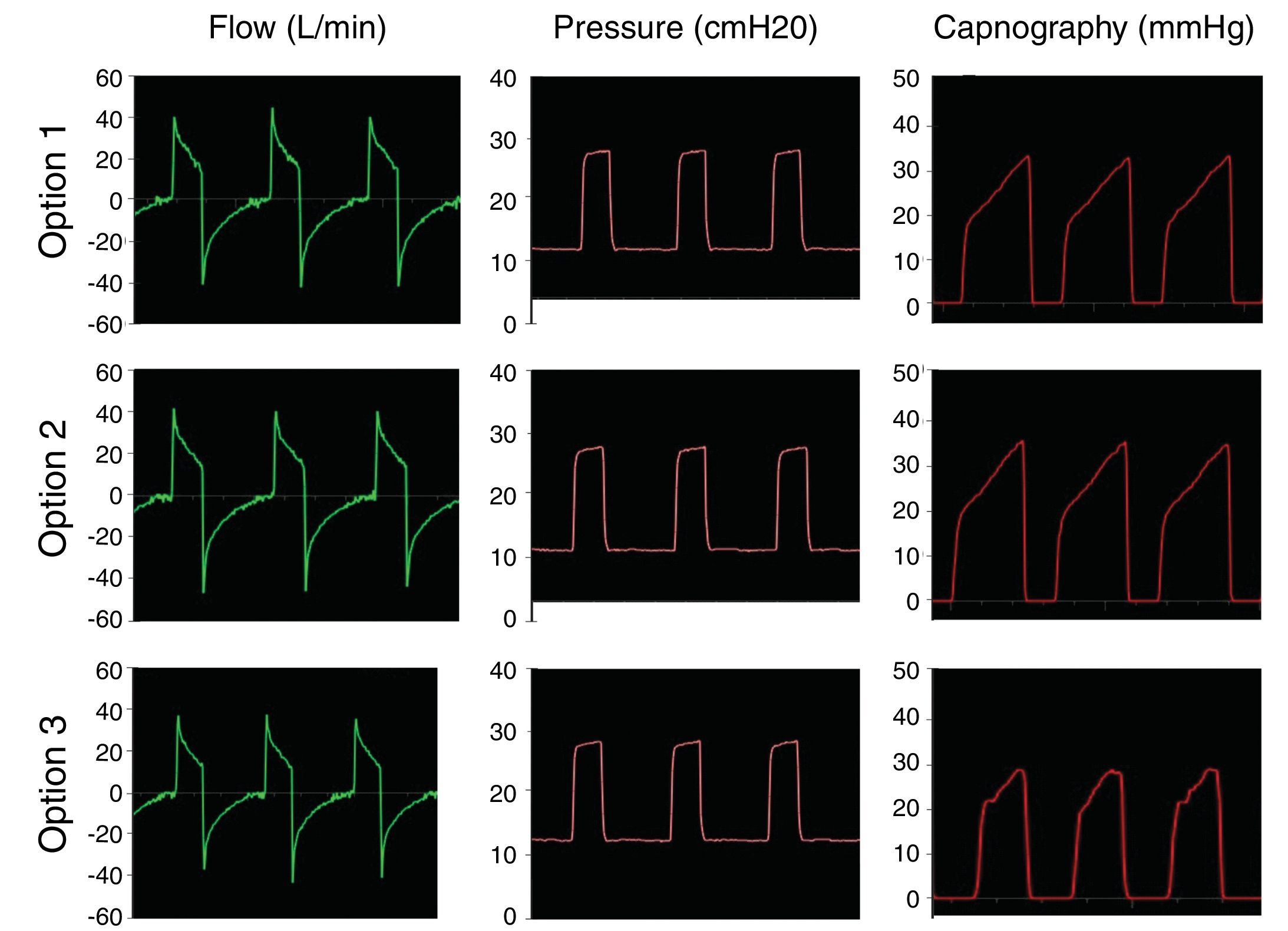
The second step was to simulate controlled ventilation in a patient with ARDS producing 170 ml/min CO2, with 30 ml/cmH2O of compliance and airway resistance of 18 cmH2O/l/s. IPAP was set to 25 cmH2O and EPAP to 12 cmH2O with a respiratory rate of 18 bpm (inspiratory time: 1 s; expiratory time: 2.3 s). Fig. 4 shows that in the pathological lung simulation the configurations tested also conserve pressure settings and prevent rebreathing of carbon dioxide.
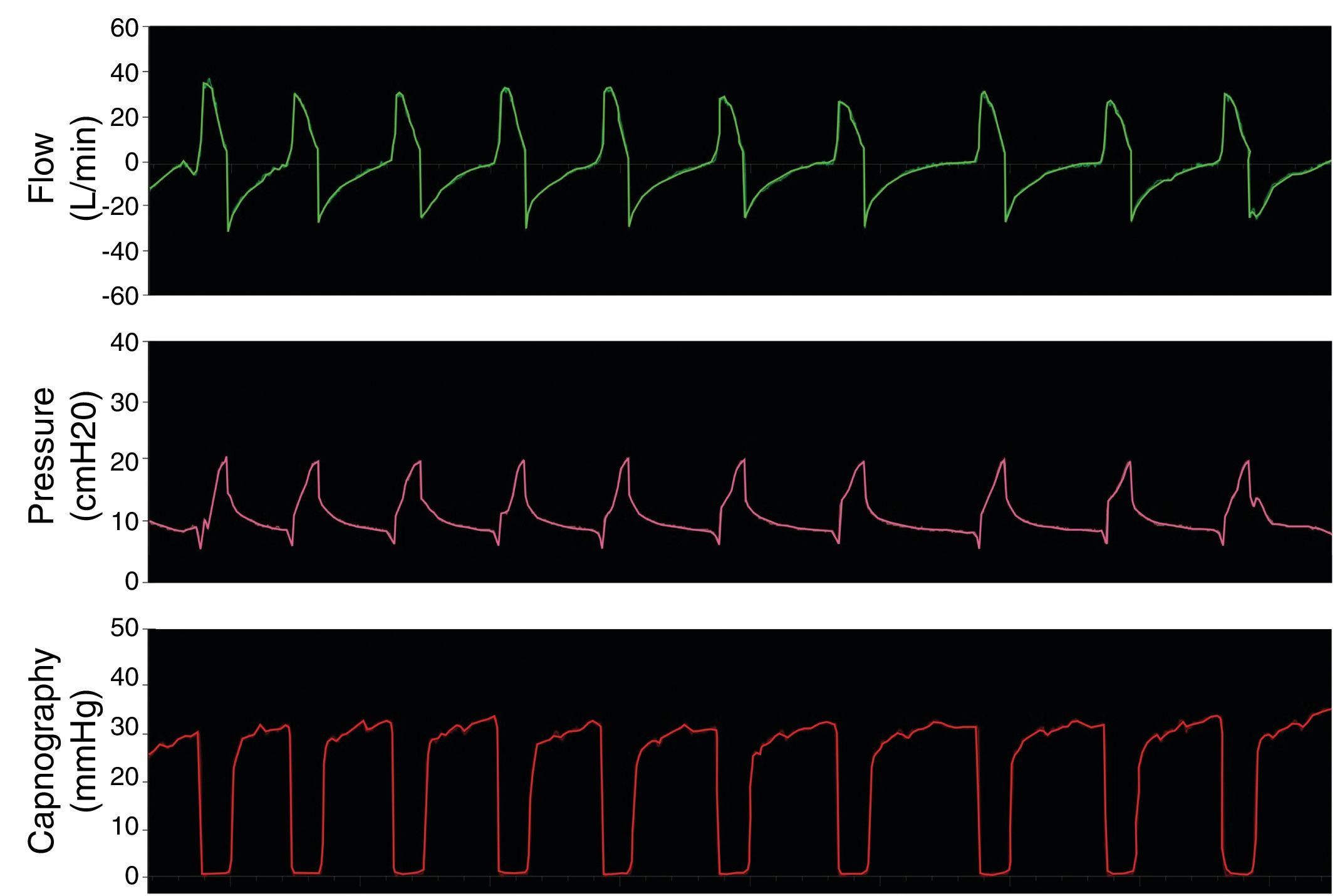
The final step was to test the systems by simulating a healthy patient breathing spontaneously. Fig. 5 shows the flow, pressure and capnography curves obtained with modification 3, as an example. The curves clearly show absence of CO2 rebreathing and conservation of the pressure settings. Similarly results were obtained with models 1 and 2.
Supplementary O2 to adjust FiO2 — at a fixed minute volume of 7 l/min — was administered in 2 positions:
- 1
Supplementary O2 delivered via the specific socket located on the rear panel of the Stellar® 150. A flow of 5 l/min results in an FiO2 of almost 90%.
- 2
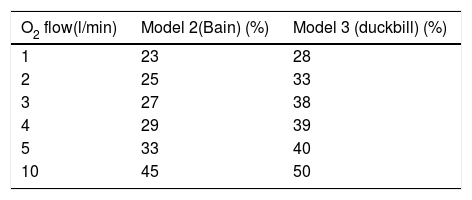
Supplementary O2 delivered via the outlet of the NIV device where the single circuit is connected. We tested one of the proposed models with leak valve and without a non-rebreathing valve (Model 2) and Model 3 with the one-way valve at different O2 flow rates. Table 1 shows the results of the tests using mandatory ventilation.
Table 1.FiO2 ratio according to supplementary oxygen flow for each proposed model.
O2 flow(l/min) Model 2(Bain) (%) Model 3 (duckbill) (%) 1 23 28 2 25 33 3 27 38 4 29 39 5 33 40 10 45 50 Inspired fraction of oxygen (FiO2) achieved at the distal end of the NIV circuit when supplementary O2 was delivered at the proximal end of the NIV circuit.
- 1
The proposed models can be used to ventilate patients of any age, weight and size in mandatory or spontaneous mode.
- 2
The airway pressures achieved by these NIV devices are within protective ventilation ranges.
- 3
The circuit, adapters and valves are all easily sterilised.
- 4
NIV systems are more readily available than intensive care invasive ventilators.
- 1
FiO2 cannot be precisely controlled because it depends on supplementary O2 flow, where the O2 is administered, the leak, and the respiratory minute volume settings. However, the information obtained from the simulations indicates that this would not pose a significant problem.
- 2
Poor monitoring compared to critical care ventilators.
- 3
Many NIV systems are not battery-powered.
- 4
Alarms may not be as comprehensive and/or sensitive as in intensive care ventilators. This problem will depend on the technical characteristics of the NIV device used for this purpose, and will require closer monitoring.
Note: Because of the wide range of NIV devices on the market, the operating characteristics of the particular system available should be checked: ventilatory modes, pressure limits/range, alarms, delivery of supplementary O2, etc. We recommend preparing some of the proposed configurations and testing them on an artificial lung to ensure they function correctly before using them on patients.
Strategy behind the proposed solutionThese modifications have been developed for 2 purposes. The first is to replace anaesthesia machines so that they can be used in patients with COVID-19. This would compel us to perform total intravenous anaesthesia in the operating room, because all the proposed options are "open" ventilation circuits. The second is to ventilate COVID-19 patients with this device when ICU ventilators are busy. This will allow these modified NIV devices to be used as a "ventilatory bridge" until an ICU ventilatory device becomes available.
Beyond the COVID-19 pandemic, the simple modifications involved will allow these models (battery-powered) to be used to transport ventilated patients not only within the hospital but also from remote locations.
ConclusionsWe propose a number of solutions to transform an NIV device into an intensive care ventilator for intubated patients. These configurations are particularly important for remote regions and emerging and underdeveloped countries where resources are usually scarce and infrastructure is limited.
FundingNone of the authors has received grants or scholarships related to this presentation.
Conflicts of interestThe authors declare that they have no conflicts of interest.
We would like to thank bioengineer Ariel Alberto Bonardi and kinesiologist Hiromi Kakisu for their technical help, Medtronic for facilitating the ASL 5000 simulator®, and JAEJ SA for providing the NIV device for the simulation.
Please cite this article as: Tusman G, Campos M, Gogniat E. COVID-19: cómo transformar un ventilador de no invasiva en un ventilador de críticos. Rev Esp Anestesiol Reanim. 2020;67:367–373.