COVID-19 induces coagulopathy associated with an increase of thromboembolic events. Due to the lack of agreement on recommendations for thromboprophylactic management, the aim of this study was to study the dosages of LMWH used in critically ill COVID-19 patients assessing the effect on their outcome.
MethodsWe evaluated data of the Reg-COVID19. According to LMWH dose two groups were analyzed: prophylaxis and treatment. Primary outcome was the relationship of LMWH dosage with mortality. Secondary outcomes included the incidence of thrombotic and bleeding events, length of ICU stay, invasive mechanical ventilation, and thrombotic and inflammatory parameters.
ResultsData of 720 patients were analyzed, 258 in the prophylaxis group and 462 in the treatment group. C Reactive Protein, invasive mechanical ventilation, tocilizumab and corticosteroid treatments were related with the choice of LMWH dose. Hemorrhagic events (66/720, 9.2%) and thrombotic complications (69/720, 9.6%) were similar in both groups (p = .819 and p = .265), as was the time course of the thrombotic events, earlier than hemorrhagic ones (9 [3–18] and 12 [6–19] days respectively). Mortality was lower in prophylaxis group (25.2% versus 35.1%), but once an inverse probability weighting model was applied, we found no effect of LMWH dose.
ConclusionWe found no benefit or harm with the administration of therapeutic or prophylactic LMWH dose in COVID19 critically ill patients. With a similar rate of hemorrhagic or thrombotic events, the LMWH dose had no influence on mortality. More studies are needed to determine the optimal thromboprophylaxis protocol for critically ill patients.
Los pacientes COVID-19 presentan una coagulopatía caracterizada por una elevada incidencia de complicaciones tromboembólicas. Ante la controversia existente sobre el manejo de la tromboprofilaxis, se llevó a cabo un estudio con el objetivo de analizar el efecto de las diferentes dosis de heparina de bajo peso molecular (HBPM) utilizadas en los pacientes críticos con COVID-19.
Material y métodosSe evaluaron datos del Reg-COVID19. Se compararon dos grupos de pacientes según la dosis de HBPM administrada: profilaxis y tratamiento. El objetivo primario fue determinar si había relación de la dosis de HBPM con la mortalidad. Los objetivos secundarios incluyeron la incidencia de eventos trombóticos y hemorrágicos, la duración de la estancia en UCI, la ventilación mecánica invasiva y parámetros trombóticos e inflamatorios.
ResultadosSe analizaron datos de 720 pacientes, 258 en el grupo de profilaxis y 462 en el de tratamiento. La proteína C reactiva, la ventilación mecánica invasiva, y el tratamiento con tocilizumab o corticosteroides se relacionaron con la elección de la dosis de HBPM. La incidencia de complicaciones hemorrágicas (66/720, 9.2%) y trombóticas (69/720, 9.6%) fue similar en ambos grupos, al igual que el curso temporal de los eventos trombóticos, que ocurrieron antes que los hemorrágicos (9 [3-18] y 12 [6-19] días respectivamente). La mortalidad fue menor en el grupo de profilaxis (25.2% frente a 35.1%), pero al aplicar un modelo de ponderación de probabilidad inversa, no se encontraron diferencias entre los grupos.
ConclusiónNo se encontraron efectos beneficiosos ni perjudiciales relacionados con la administración de dosis profilácticas o terapéuticas de HBPM en pacientes críticos COVID-19, con una tasa similar de complicaciones hemorrágicas o trombóticas. A partir de estos resultados, consideramos que son necesarios más estudios para determinar el protocolo óptimo de tromboprofilaxis en estos pacientes.
Clinical observations have revealed the role of systemic inflammation in the pathophysiology of SARS-CoV-2 infection. SARS-CoV-2 binds to the cellular anti-inflammatory receptor resulting in increased signalling at thrombin receptors and platelet activation1,2. It has been suggested that COVID-19-induced coagulopathy may be due to an uncontrolled immunothrombotic response to COVID-19, with a massive inflammatory response as the main mechanism responsible, including cytokine storm and neutrophil extracellular traps (NETs)3.
The synergy between inflammation and thrombosis has been well described in the literature. Initially, anti-inflammatory pathways were described specifically aimed at mitigating cardiovascular disease in patients with inflammatory diseases, but also during infections4. Thirty percent of patients with COVID-19 admitted to intensive care units (ICU) develop macrovascular thrombotic complications despite thromboprophylaxis3. Even under prophylactic antithrombotic treatment (unfractionated heparin or low-molecular-weight heparin [LMWH] capable of altering NETs among other anti-inflammatory effects4), an unexpectedly high number of pulmonary embolism (20.6%) has been observed in critically ill patients. This represents up to twice the frequency found in patients with similar severity scores. Microvascular thrombosis has also been linked to disease progression, with pulmonary clots being proposed to contribute to respiratory failure4,5 and clots in other vascular beds to multiple organ failure6.
These clinical observations have led to empirical treatment of hospitalised patients with COVID-19 with LMWH thromboprophylaxis at higher than usual doses7. Although some guidelines suggest that intermediate-dose anticoagulation may be considered for critically ill patients8, it is unclear whether the most critically ill COVID-19 patients have a different level of risk of venous thromboembolism (VTE) than other patients admitted to the ICU. In addition, LMWH has been shown to have anti-inflammatory properties that may be of additional benefit in COVID-19, with increased pro-inflammatory cytokines9.
In vitro studies aimed at investigating the coagulation profile in patients with COVID-19 show a dramatic increase in thrombin generation ex vivo. Indeed, despite anticoagulation, thrombin generation capacity is not decreased, thrombin peak and endogenous thrombin potential (ETP) are in the normal range10. Concomitantly, COVID-19 is associated with fibrinolytic abnormalities with elevated levels of PAI-1, TAFI and tPA (released due to endothelial injury) resulting in hypofibrinolysis, which plays an important role in COVID-19-associated coagulopathy11.
At the beginning of the pandemic, according to early published studies, there was no unified recommendation for the prevention and treatment of VTE in patients with COVID-19, and therefore the recommended dose of thromboprophylaxis varied from the current standard prophylaxis to intermediate (LMWH bidosis or weight-based dose escalation) or full anticoagulation dose. However, individualised decisions on anticoagulant dosing had been made in all studies and the risk of thrombotic or haemorrhagic events when treated with standard or higher than usual remained unclear. In addition, the risk of bleeding in critically ill patients on COVID-19 is unknown and conflicting data have been reported suggesting a fragile balance in the haemostatic status of these patients12.
The discrepancy between clinical data together with new laboratory findings is interesting. Anticoagulation strategies to safely and effectively manage these patients remain a challenging unsolved problem for clinicians. Therefore, our aim was to study the doses of LMWH used in critically ill COVID-19 patients and the effect of these on outcomes.
Material and methodsStudy designReg-COVID.19 (CoVid19.ubikare.io) is a registry operated by a Spanish collaborative working group (UCI-Network) in which data were prospectively collected from the electronic medical records of patients admitted to 36 ICUs in Spain and Andorra for acute respiratory failure due to SARS-COV-2 infection, following a standardised protocol that protects patient confidentiality through pseudo-anonymisation of the data.
In the present study, an analysis of the Reg-COVID-19 cohort of patients admitted between 12 March and 1 September 2020 was conducted, having been registered at clinicaltrials.com NCT04623177. The study protocol was approved by a reference Ethics Committee (Ethics Committee of Euskadi, Spain) and by the Ethics Committee of each participating hospital. Verbal informed consent was obtained from the patients' relatives by the investigators, in the presence of an independent witness in some centres. In other centres, such informed consent was not considered necessary by decision of the Ethics Committee.
Study populationAll patients over 18 years of age diagnosed with acute respiratory failure due to SARS-COV-2 infection (confirmed by polymerase chain reaction study in samples obtained from respiratory smears) admitted to the ICU, who received LMWH and were registered in the Reg-COVID-19 registry were included. The follow-up period was the time from admission to discharge to the COVID ward of the hospital or until death. Exclusion criteria were absence of confirmation of SARS-COV-2 infection, refusal to participate in the study, absence of clinical or laboratory data on the first day of ICU admission, existence of "do not resuscitate" indications on ICU admission and failure to meet pre-specified outcomes or to be discharged from the ICU at the time of study closure.
Data collection, variables and assessment criteriaPatients were analysed in 2 groups according to the dose of LMWH administered: prophylaxis group (less than 100 IU/kg/24 h) and treatment group (150 IU/kg/24 h or more).
The primary objective was to assess the effects of LMWH dose on mortality during ICU stay. The secondary objectives were to assess the effects of LMWH dose on the incidence of thrombotic and bleeding events, on length of ICU stay and invasive mechanical ventilation, and on thrombotic and inflammatory parameters (Appendix B, see Supplementary material for details of the parameters investigated).
Statistical analysisFollowing the study protocol, patients who met the inclusion criteria were included. The variables in this population were described using, according to their nature, percentages, means and standard deviations or medians and interquartile ranges. To compare the distribution of these variables between the 2 groups (either defined by the degree of exposure to LMWH or by the outcome death or survival), we used: a) for variables measured on an interval scale, Student's t-test or Mann-Whitney U test, depending on whether or not they were normally distributed; b) for categorical variables, the Chi-square or Fisher's exact test, depending on the expected frequency of their categories. In all contrasts, the alternative hypothesis was 2-tailed and a p < .05 was used as the threshold for rejecting the null hypothesis. Analyses were performed with the statistical software STATA® version 16 (StataCorp LLC, Texas, USA).
The data included in the analysis were demographic and clinical: prior to ICU admission (age, BMI, diabetes, thrombotic events, antiplatelet or anticoagulant therapy and date of admission); in the first 48 h of ICU admission (APACHE II and SOFA scores, PaO2/FiO2 ratio, ferritin, D-dimer and C-reactive protein [CRP]/lymphocyte ratio) and during ICU stay (maximum dose of noradrenaline, number of days on mechanical ventilation, need for renal replacement therapy, peak procalcitonin and plasma creatinine levels).
The relationship between LMWH doses (prophylactic versus treatment) and mortality were analysed using inverse-probability-of-treatment weighting techniques to construct equivalent groups in terms of the variables prior to and associated with the decision on the type of dose to be used. A multivariate logistic model was fitted with dose category as the dependent variable, and those variables associated with dose in the bivariate analyses as explanatory variables. Each patient was finally weighted by the inverse of the probability of being included in the treatment dose and in this population a logistic model was applied to determine the association between anticoagulation strategy and in-hospital mortality.
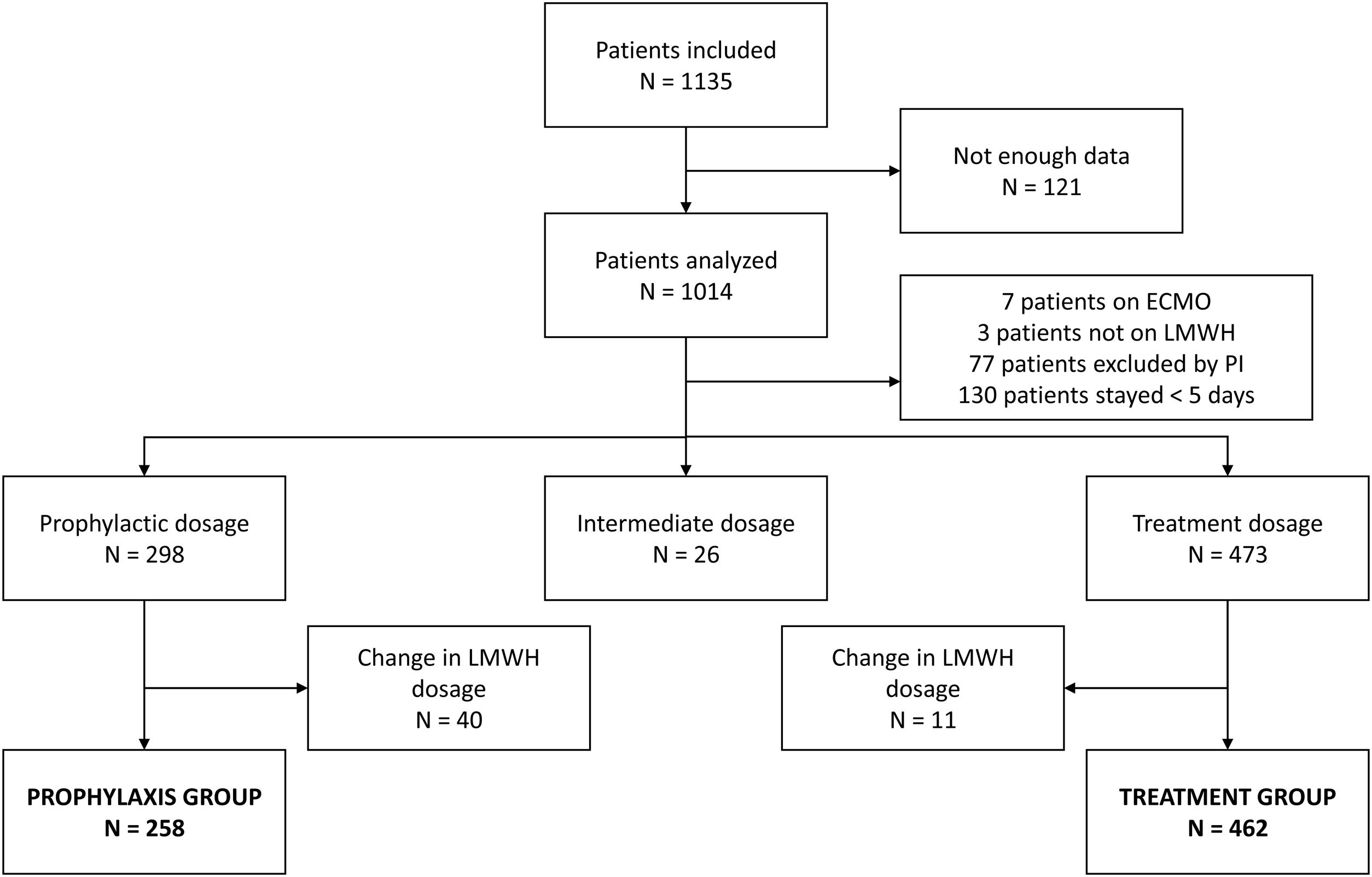
ResultsSeven hundred and twenty patients were analysed, 258 in the prophylaxis group and 462 in the treatment group (Fig. 1). Baseline and ICU evolution data of the thromboprophylaxis and anticoagulation groups are described in Table 1. Most of the data and the use of respiratory support with high-flow oxygen therapy as first choice at ICU admission were similar between groups. Invasive mechanical ventilation requirements, recruitment manoeuvres and prone sessions were significantly lower in the thromboprophylaxis group in the first 48 h. The use of neuromuscular blockers, tocilizumab and corticosteroids was significantly lower in the thromboprophylaxis group.
Demographic data and ICU management data.
| GlobalN = 720 | ProphylaxisN = 258 | TreatmentN = 462 | P value | MortalityN = 227 | SurvivalN = 493 | P value | |
|---|---|---|---|---|---|---|---|
| ICU admission data | |||||||
| Age (years) | 63 [56−71] | 63 [55−70] | 63.50 [56.00−71.00] | .196 | 67 [61−72] | 61 [54–69] | <.001 |
| Sex: women | 233 (33) | 84 (33) | 149 (32) | 1.000 | 85 (38) | 148 (30) | .05 |
| Body mass index (kg/m2) | 29 [26−32] | 29 [26−32] | 29.00 [26.00−32.00] | .803 | 28 [26−32] | 29 [26−32] | .834 |
| Chronic treatment: | 25 (11.0) | ||||||
| Anti-aggregate | 69 (10) | 30 (12) | 39 (8) | .207 | 16 (7.0) | 44 (8.9) | .454 |
| Anticoagulant | 43 (6) | 16 (6) | 27 (6) | .976 | 40 (17.6) | 27 (5.5) | .511 |
| Combined | 110 (15) | 45 (17) | 65 (14) | .272 | 70 (14.2) | .283 | |
| Days of symptoms till hospital admission | 7 [5–9] | 7 [5–9] | 7 [5–9] | .658 | 7 [4–8] | 7 [5–9] | .228 |
| Days of symptoms till ICU admission | 10 [7–13] | 9 [7–12] | 10 [7–13] | .547 | 10 [7–13] | 9 [7–13] | .797 |
| Admission in March 2020 | 574 (80) | 203 (79) | 371 (80) | .673 | 192 (84.6) | 382 (77.5) | .036 |
| ICU stay (days) | 15 [9–20] | 15 [8–26] | 16 [10–26] | .292 | 16 [10–26] | 15 [9–26] | .413 |
| APACHE II | 1 [9–17] | 12 [8–16] | 12 [9–17] | .775 | 15 [12–20] | 11 [8–16] | <.001 |
| SOFA | 7 [4–9] | 7 [4–8] | 7 [4–9] | .228 | 8 [6–11] | 6 [4–8] | <.001 |
| PaO2/FiO2 (%) ratio: | |||||||
| 41−100 | 96 (14) | 25 (10.8) | 71 (15.9) | .112 | 31 (14.6) | 65 (13.9) | .011 |
| 100−200 | 361 (53) | 134 (57.8) | 227 (50.8) | 129 (60.6) | 232 (49.8) | ||
| 200−486 | 222 (33) | 73 (31.5) | 149 (33.3) | 53 (24.9) | 169 (36.3) | ||
| Lab | |||||||
| Ferritin (ng/mL) | 1564 [854−3718] | 1474 [780−3732] | 1613 [907−3655] | .380 | 1706 [1077−7124] | 1514 [810−3151] | .065 |
| D-dimer (ng/mL) | 2088 [897−7185] | 2200 [800−6500] | 2040 [936−7675] | .177 | 3801 [1183−14,400] | 1694 [814−5438] | <.001 |
| C-reactive protein (mg/l) | 29 [13−162] | 19.76 [1041−4472] | 44.75 [16.22−196.00] | <.001 | 36.15 [14.97−190.50] | 25.57 [12.97−147.54] | .017 |
| Lymphocyte count (×109/l) | .7 [.5−1.08] | .70 [.50−1.00] | .74 [.50–1.10] | .388 | .70 [.48−1.04] | .74 [.50−1.00] | .221 |
| CRP/lymphocyte count | 42.8 [15.86–195.6] | 29.45 [13.39−69.36] | 66.67 [17.56−254.75] | <.001 | 66.34 [20.52−291.03] | 39.40 [15.10−167.74] | .006 |
| Platelets (×109/l) | 272 [204−607] | 275 [209−390] | 269 [202−434] | .768 | 295 [217−1.408] | 266 [201−376] | .003 |
| Interleukin 6 (pg/l) | 773 [140−1.732] | 276 [102−1.328] | .076 | 277 [74−1.246] | 353 [154−1.575] | .239 | |
| Lactate deshydrogenase (IU/l) | 477 [383−607] | 471 [372−614] | 479 [386−603] | .552 | 525 [417−683] | 456 [363−582] | <.001 |
| Procalcitonin (ng/mL) | .26 [.12–.6] | .28 [.13−.67] | .24 [.12−.60] | .304 | .43 [.16−1.00] | .23 [.11−.51] | <.001 |
| Creatinine clearance (ml/min) | 95 [61−120] | 89.54 [63.61−123.00] | 87.01 [60.13−116.50] | .443 | 73.05 [48.66−101.69] | 94.73 [70.66−126.88] | <.001 |
| Extracorporeal support | |||||||
| High flow oxygen therapy | 28 | 13 (5.0) | 15 (3.2) | .321 | 11 (4.8) | 17 (3.4) | .488 |
| Invasive ventilation: | |||||||
| <3 days stay | 598 (83) | 193 (74.8) | 405 (87.7) | <.001 | 214 (94.3) | 384 (77.9) | <.001 |
| >2 days stay | 24 (3.3) | 6 (2.3) | 18 (3.9) | 8 (3.5) | 16 (3.2) | ||
| Recruitment manoeuvres: | |||||||
| <3 days stay | 333 (46) | 93 (36.0) | 240 (51.9) | <.001 | 122 (53.7) | 211 (42.8) | .006 |
| >2 days stay | 84 (12) | 27 (10.5) | 57 (12.3) | 29 (12.8) | 55 (11.2) | ||
| Prone sessions: | |||||||
| <3 days stay | 402 (56) | 119 (46.1) | 283 (61.3) | <.001 | 147 (64.8) | 255 (51.7) | |
| >2 days stay | 106 (15) | 34 (13.2) | 72 (15.6) | 49 (21.6) | 57 (11.6) | <.001 | |
| CO2 extraction | |||||||
| <3 days stay | 1 (.14) | 0 (.0) | 1 (.2) | .742 | 1 (.4) | 0 (.0) | <.001 |
| >2 days stay | 13 (1.8) | 5 (1.9) | 8 (1.7) | 12 (5.3) | 1 (.2) | ||
| Kidney replacement therapy: | |||||||
| <3 days stay | 20 (2.8) | 6 (2.3) | 14 (3.0) | .559 | 11 (4.8) | 9 (1.8) | <.001 |
| >2 days stay | 68 (9.4) | 21 (8.1) | 47 (10.2) | 46 (20.3) | 22 (4.5) | ||
| Pharmacological treatment | |||||||
| Neuromuscular relaxation: | |||||||
| <3 days stay | 430 (60) | 136 (52.7) | 294 (63.6) | <.001 | 169 (74.4) | 261 (52.9) | <.001 |
| >2 days stay | 71 (9.9) | 18 (7.0) | 53 (11.5) | 32 (14.1) | 39 (7.9) | ||
| Lopinavir/ritonavir: | |||||||
| 423 (59) | 150 (58.1) | 273 (59.1) | .967 | 125 (55.1) | 298 (60.4) | .087 | |
| >2 days stay | 8 (1.1) | 3 (1.2) | 5 (1.1) | 5 (2.2) | 3 (.6) | ||
| Hydroxychloroquine: | |||||||
| <3 days stay | 615 (85) | 212 (82.2) | 403 (87.2) | .109 | 187 (82.4) | 428 (86.8) | .063 |
| >2 days stay | 18 (2.5) | 6 (2.3) | 12 (2.6) | 10 (4.4) | 8 (1.6) | ||
| Remdesivir: | |||||||
| <3 days stay | 12 (.02) | 6 (2.3) | 6 (1.3) | .179 | 3 (1.3) | 9 (1.8) | .627 |
| >2 days stay | 9 (1.3) | 1 (.4) | 8 (1.7) | 4 (1.8) | 5 (1.0) | ||
| Interferon: | |||||||
| <3 days stay | 164 (22.8) | 53 (20.5) | 111 (24.0) | .301 | 67 (29.5) | 97 (19.7) | .005 |
| >2 days stay | 10 (1.4) | 2 (.8) | 8 (1.7) | 5 (2.2) | 5 (1.0) | ||
| Tocilizumab: | |||||||
| <3 days stay | 280 (38.9) | 93 (36.0) | 187 (40.5) | .006 | 80 (35.2) | 200 (40.6) | .393 |
| >2 days stay | 49 (6.8) | 9 (3.5) | 40 (8.7) | 16 (7.0) | 33 (6.7) | ||
| Immunomodulators: | |||||||
| <3 days stay | 382 (53.0) | 126 (48.8) | 256 (55.4) | .122 | 129 (56.8) | 253 (51.3) | .262 |
| >2 days stay | 2 (.3) | 0 (.0) | 2 (.4) | 0 (.0) | 2 (.4) | ||
| Azithromycin: | |||||||
| <3 days stay | 495 (68.8) | 181 (70.2) | 314 (68.0) | .630 | 140 (61.7) | 355 (72.0) | .019 |
| >2 days stay | 38 (5.3) | 11 (4.3) | 27 (5.8) | 16 (7.0) | 22 (4.5) | ||
| Corticosteroids: | |||||||
| <3 days stay | 419 (58.2) | 135 (52.3) | 284 (61.5) | .053 | 121 (53.3) | 298 (60.4) | .010 |
| >2 days stay | 151 (21) | 60 (23.3) | 91 (19.7) | 63 (27.8) | 88 (17.8) | ||
| Vasopressor treatment | 701 (97.4) | 248 (96.1) | 453 (98.1) | .192 | 224 (98.7) | 477 (96.8) | .213 |
Data are presented as median [IQR] for continuous variables and as n (%) for dichotomous variables.
CRP: C-reactive protein; ICU: Intensive Care Unit.
Factors related to early selection of LMWH dose were CRP levels, invasive mechanical ventilation requirements, and treatment with tocilizumab or corticosteroids (Table 2).
Relationship between risk or COVID-19 severity factors and thromboprophylaxis doses.
| Predictors | Odds ratio | CI | P value |
|---|---|---|---|
| Age | 1.01 | .99−1.02 | .226 |
| Males | .99 | .70−1.40 | .977 |
| C-reactive protein | 1.00 | 1.00−1.01 | <.001 |
| Mechanical ventilation | 2.58 | 1.63−4.12 | <.001 |
| Recruitment manoeuvres | .84 | .49−1.46 | .521 |
| Prone sessions | 1.00 | .62−1.64 | .993 |
| Neuromuscular relaxation | 1.76 | .96−3.37 | .075 |
| Tocilizumab | 2.57 | 1.25−5.85 | .015 |
| Corticosteroids | .63 | .42−.94 | .022 |
CI: Confidence Interval.
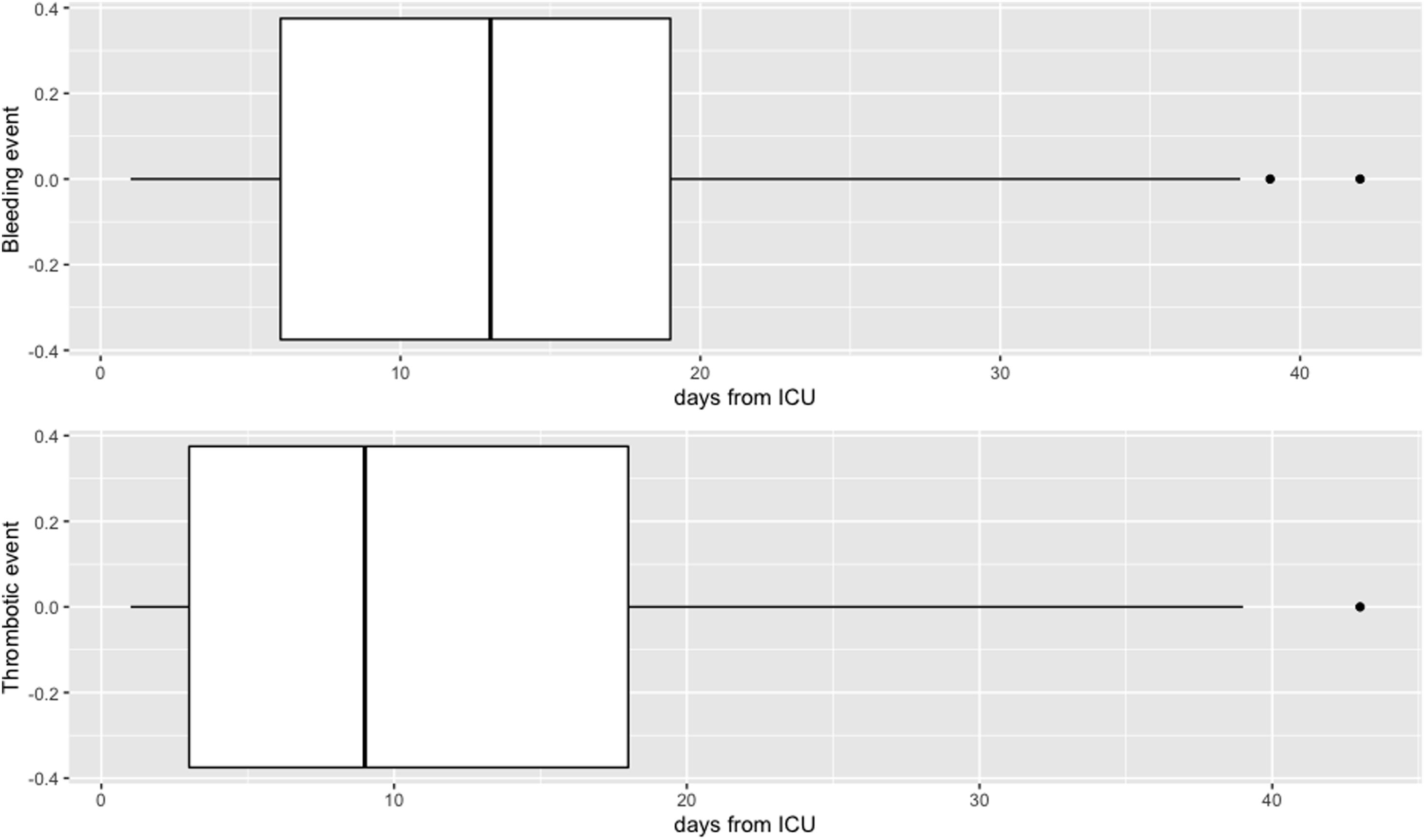
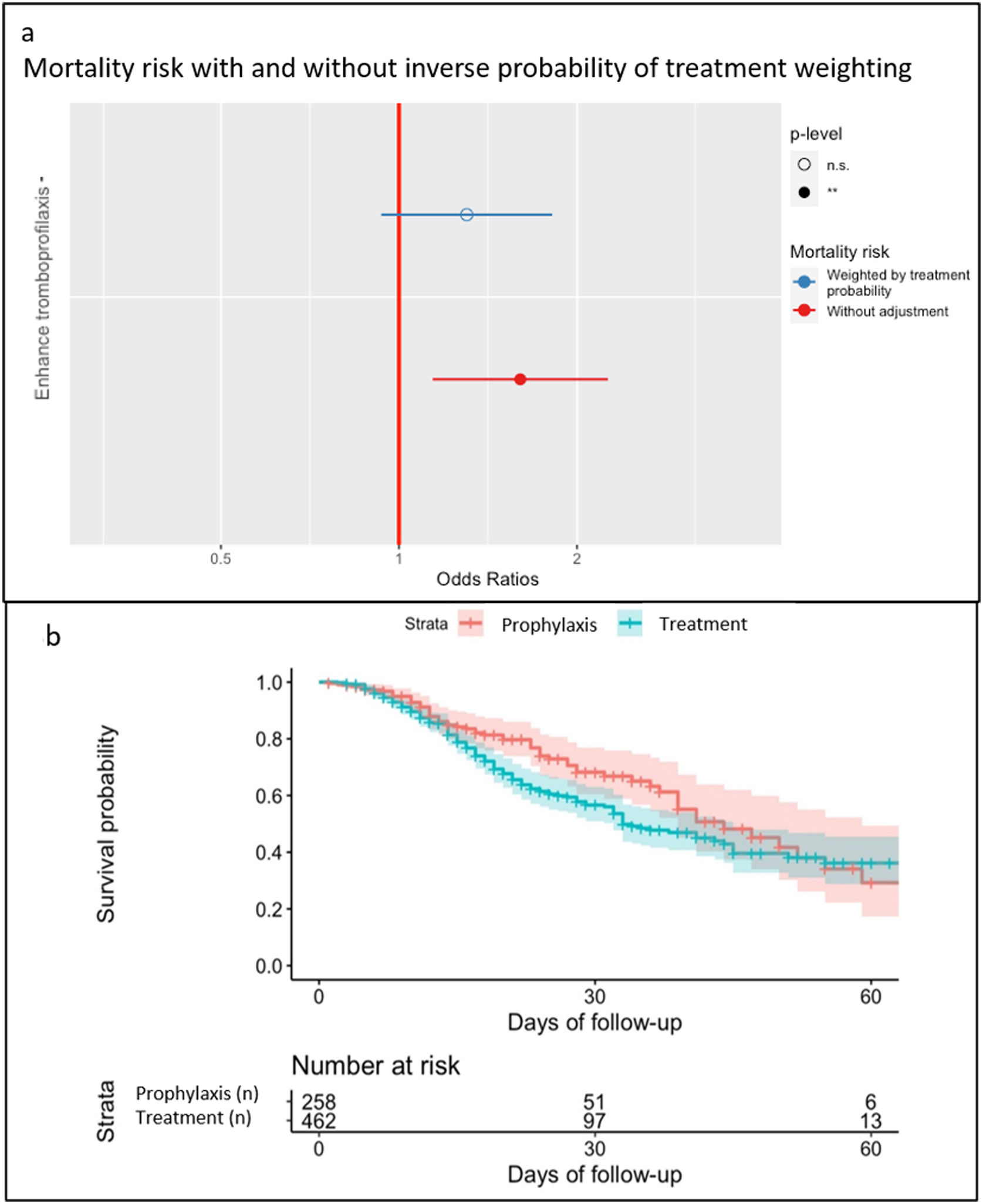
There were 66 haemorrhages (9.2%, 37 minor and 29 major) and 69 (9.6%) thrombotic complications. Haemorrhagic and thrombotic events (Appendix B-see Supplementary material 2) had a different presentation over time, although without statistically significant differences (Fig. 2). LMWH doses did not influence complications, with a similar incidence of haemorrhagic and thrombotic events in both groups, although mortality was significantly lower in the thromboprophylaxis group (Table 3). In the thromboprophylaxis group 65/258 (25.2%) patients died and in the treatment group 162/462 (35.1%). However, when the inverse probability weighting model was applied, it was determined that there was no effect of LMWH dose on mortality (OR: 1.14, CI: .82–1.59, p = .429) (Fig. 3a). Septic shock was the main cause of mortality in both groups (Appendix B-see Supplementary material 3). The probability of survival during ICU stay is shown in Fig. 3b.
By including more than 1000 patients, this study describes routine clinical practice in critically ill COVID-19 patients admitted to the ICU in Spain. The results show the absence of significant benefit or harm in terms of cumulative incidence of mortality and thrombotic or haemorrhagic complications when comparing patients who received LMWH at therapeutic doses versus those who received prophylactic doses.
On admission to the ICU, the decision on the dose of LMWH depended on the responsible physician and the protocol of each hospital. Surprisingly, some analytical parameters described in COVID-19 patients, such as increases in D-dimer or ferritin, did not influence the decision on the dose of LMWH administered. However, serum CRP levels, CRP/lymphocyte ratio and the need to initiate mechanical ventilation or to perform pronation manoeuvres in the first 2 days of admission to the ICU, prior treatment with tocilizumab or corticosteroids, were factors that apparently conditioned the choice of LMWH dose.
Several trials have attempted to define the optimal anticoagulation protocol in critically ill patients, comparing prophylactic, intermediate or therapeutic doses of LMWH13–16 highlighting the uncertainty that exists in this regard. Moreover, during the first wave of the pandemic, when this registry was completed, the doubts were even greater. The existing guidelines did not coincide in recommending one or another anticoagulation protocol, varying from prophylactic doses for all patients to anticoagulation at therapeutic doses for those patients with a high suspicion of having developed a thrombotic event due to the rapid and unexpected increase in prothrombotic analytical parameters, or only for those in whom thrombosis had been diagnosed17–21.
Our analysis included 720 of the 1135 patients collected in the UBIKARE registry, with the aim of comparing the 2 main strategies for the prevention of thrombotic events in critically ill COVID-19 patients: 64% of patients received a treatment dose (462/720) and 36% a prophylactic dose (258/720). To reduce bias and allow reliable comparison between groups taking into account confounding factors, only patients on LMWH treatment were analysed and those receiving intermediate doses and those with a change in LMWH dose (from prophylactic to therapeutic or vice versa) were excluded from the analyses.
The results obtained suggest that the systematic administration of therapeutic doses of LMWH in patients admitted to an ICU without a confirmed diagnosis of a thrombotic event does not reduce its incidence, although it does not influence the incidence of haemorrhagic events either. However, although a higher mortality rate was observed in the group of patients who received a therapeutic dose of LMWH (35% versus 25% of patients who received the prophylactic dose), after applying an inverse probability weighting model, the dose of LMWH was not found to have an influence on mortality. This result would support a possible absence of benefit or harm directly related to LMWH dose.
These data are consistent with those reported from randomised trials22,23, which demonstrate the ineffectiveness of administering therapeutic doses of anticoagulation compared to prophylactic doses in critically ill patients, with the aim of reducing multi-organ deterioration and/or mortality. Along the same lines, other recent recommendations24,25 also advise against the systematic administration of therapeutic doses of anticoagulation. Finally, a meta-analysis26 also suggests that increasing the dose of LMWH would not have any benefit on the final outcome.
Confirmation of these results probably requires further studies, as controversial conclusions appear in other articles. Nadkarni et al.27, in an observational analysis comparing 3 arms with different doses of anticoagulation (therapeutic, prophylactic or no anticoagulation), concluded that any dose implies lower mortality and better outcome in patients hospitalised for COVID-19. They found a non-significant association of therapeutic anticoagulation with an improvement in these outcomes compared to prophylactic dosing. Meizlish et al.15, in a propensity analysis comparing in-hospital mortality in patients receiving intermediate versus prophylactic doses of anticoagulation (retrospective data including a cohort of 1624 patients hospitalised in both the ward and ICU), concluded that intermediate dose was associated with a lower cumulative incidence of mortality. The study by Lavinio et al.,13 conducted with the aim of finding the optimal dose, concluded that a strategy based on so-called "enhanced thromboprophylaxis" could decrease mortality in patients admitted to the ICU without increasing haemorrhagic complications. In the Reg-COVID-19 registry, from which the data for this study were extracted, only 33 patients received intermediate doses, so it was not possible to perform a comparative analysis with this group.
In the group receiving therapeutic doses, there was no higher incidence of bleeding events and no lower incidence of thrombotic events. Severe COVID-19 causes an increase in proinflammatory cytokines and a broad spectrum of cells such as neutrophils and platelets that have an important influence on mononuclear leukocytes and endothelial cells, which could lead to a "loop" activation of coagulation. Moreover, hypoxia would also play an important role in hypercoagulability through activation of the endothelium, with a decrease in thrombomodulin and protein S. Thus, the pathophysiology of COVID-19, which combines thromboinflammation, immunothrombosis and endotheliopathy, could call into question the real role of therapeutic anticoagulation with the exception of patients with an established diagnosis of VTE.
Thrombotic complications appeared earlier than haemorrhagic complications (9 [3–18] and 12 [6–19] days, respectively), similar to the results obtained by Godier et al. (9 [3–11] days versus 17 [14–23] days, respectively)28. Although it is not possible to draw any definitive conclusions, even with the low rate of bleeding complications observed in our study (4%, 29/720 major bleeding complications), once the inflammation subsides following the disease course itself, reducing the dose of LMWH might also reduce the associated bleeding risk.
The incidence of clinically significant thrombotic complications found in our registry was 9.6% (69/720 patients), with no differences between groups (7.8 vs. 10.6%; p = .265). Early studies in COVID-19 patients in which VTE screening was systematically performed reported a higher rate of thrombotic complications, even reaching 70%–80%29,30 in critically ill patients, raising the possibility of undiagnosed cases that might have been missed in our registry. In the INSPIRATION trial14 comparing intermediate and standard doses of enoxaparin, an overall VTE rate of 3.4% (3.3% and 3.5%, respectively) was reported, suggesting that the lack of a targeted search for thrombotic complications in Reg-COVID-19 probably had no influence on the final outcome of thrombotic complications.
This study has some strengths. Firstly, it is a prospective, multicentre, nationwide study of more than 1000 patients from 36 ICUs providing a detailed description of all data collected from ICU admission to death or discharge. Secondly, we used the statistical tool of inverse probability of treatment weighting to reduce bias by controlling for the previously specified demographic, comorbidities and severity confounders.
On the other hand, it is also necessary to point out the limitations of the study. The registry data come from patients in the first wave (March–May 2020), when the disease in many cases exceeded the capacity of medical care and there was insufficient knowledge about aspects such as inflammation and its pathophysiological involvement in the development of thrombosis. An obvious limitation is the observational nature of the study, which may imply certain biases. Although, as mentioned above, potential confounders were adjusted for, it is possible that other factors were not taken into account. Therefore, the main weakness of our work is the registry itself, with the loss of various data from some patients. As this was a real registry, outside a trial, with many hospitals involved, the decision on the administration of the dose of LMWH depended on each responsible physician, individually following the local protocol accepted in each hospital, which was modified according to the guidelines of the health authorities. Therefore, we could also find another limitation in the non-differentiation of results between the participating hospitals. Finally, there was also insufficient information on prophylaxis or anticoagulant treatment prior to ICU admission and its possible impact on ICU admission or on the overall results of the study. However, we believe that, at the time of data collection, most protocols considered prophylactic treatment of patients admitted to the wards to be more appropriate.
Finally, as this is an observational data set and a retrospective analysis, no cause-effect relationship can be derived from this study. The results should be interpreted as an approximation of what could be the ideal thromboprophylaxis strategy for critically ill patients with COVID-19.
ConclusionIn this prospective observational study of critically ill COVID19 patients admitted to the ICU, routine administration of a therapeutic dose of LMWH compared with a prophylactic dose showed no benefit or harm. Our data support that systemised therapeutic anticoagulation does not improve outcome and should perhaps be reserved for patients with a confirmed or highly suspected thrombotic complication, adapted individually according to clinical course. The dose of LMWH did not influence mortality or haemorrhagic or thrombotic complications. It is necessary to await the definitive conclusions of randomised trials, mainly in critically ill patients, to determine the optimal thromboprophylaxis protocol in each case.
Conflict of interestsThe authors have no conflict of interests to declare.
Our special thanks to all patients and relatives who participated in the study. We would also like to thank all the researchers of the COVID-19 Spanish ICU Network Group for their indispensable work, and would be grateful if their names could be registered in PubMed.