Postoperative pulmonary complications (PPCs) vary amongst different surgical techniques. We aim to compare the incidence of PPCs after laparoscopic non–robotic versus laparoscopic robotic abdominal surgery.
Methods and analysisLapRas (Risk Factors for PPCs in Laparoscopic Non–robotic vs Laparoscopic robotic abdominal surgery) incorporates harmonized data from 2 observational studies on abdominal surgery patients and PPCs: ‘Local ASsessment of VEntilatory management during General Anaesthesia for Surgery’ (LAS VEGAS), and ‘Assessment of Ventilation during general AnesThesia for Robotic surgery' (AVATaR). The primary endpoint is the occurrence of one or more PPCs in the first five postoperative days. Secondary endpoints include the occurrence of each individual PPC, hospital length of stay and in–hospital mortality. Logistic regression models will be used to identify risk factors for PPCs in laparoscopic non–robotic versus laparoscopic robotic abdominal surgery. We will investigate whether differences in the occurrence of PPCs between the two groups are driven by differences in duration of anesthesia and/or the intensity of mechanical ventilation.
Ethics and disseminationThis analysis will address a clinically relevant research question comparing laparoscopic and robotic assisted surgery. No additional ethical committee approval is required for this metanalysis. Data will be shared with the scientific community by abstracts and original articles submitted to peer-reviewed journals.
RegistrationThe registration of this post-hoc analysis is pending; individual studies that were merged into the used database were registered at clinicaltrials.gov: LAS VEGAS with identifier NCT01601223, AVATaR with identifier NCT02989415.
Las complicaciones pulmonares postoperatorias (CPP) varían en las diferentes técnicas quirúrgicas. Nuestro objetivo fue comparar la incidencia de CPPs tras la cirugía laparoscópica no robótica frente a la cirugía laparoscópica robótica abdominal.
Métodos y análisisLos análisis LapRas (Factores de riesgo de CPPs en la cirugía laparoscópica no robótica frente a la cirugía abdominal robótica laparoscópica) armonizaron datos de dos estudios observacionales sobre los pacientes de cirugía abdominal y CPPs: ‘Local ASsessment of VEntilatory management during General Anaesthesia for Surgery’ (LAS VEGAS), y ‘Assessment of Ventilation during general AnesThesia for Robotic surgery' (AVATaR). El criterio de valoración primario es la aparición de uno o más CPP dentro de los 5 primeros días postoperatorios. Los criterios de valoración secundarios incluyen la aparición de cada CPP individual, la duración de la estancia hospitalaria, y la mortalidad hospitalaria. Se utilizarán modelos de regresión logística para identificar los factores de riesgo de CPP en cirugía laparoscópica no robótica frente a la cirugía laparoscópica robótica abdominal. Estudiaremos si las diferencias en cuanto a aparición de CPP entre los grupos están impulsadas por las diferencias en cuanto a duración de la anestesia y/o la intensidad de la ventilación mecánica.
Ética y divulgaciónEste análisis abordará una cuestión de investigación clínicamente relevante, comparando las cirugías laparoscópica y robótica. No es necesaria aprobación adicional del comité de ética para la realización de este metaanálisis. Se compartirán los datos con la comunidad científica en forma de resúmenes y artículos originales presentados a las revistas para su revisión por pares.
RegistroEl registro de este análisis post-hoc está pendiente; los estudios individuales que se integraron en la base de datos del estudio fueron registrados en clinicaltrials.gov: LAS VEGAS con identificador NCT01601223, y AVATaR con identificador NCT02989415.
Postoperative pulmonary complications (PPCs) occur often and are associated with worse outcome in patients undergoing open abdominal surgery.1 Minimally invasive abdominal surgery is increasingly replacing open abdominal surgery, and more recently these procedures are often assisted by a robot.2 In patients undergoing robotic–assisted surgery (RAS), the incidence of PPCs is higher3 than in patients undergoing non–robotic laparoscopic procedures.4 The higher incidence could be due to the longer duration of anesthesia, and therefore a longer duration of intraoperative ventilation. Besides, patient positioning during RAS, which is more extreme than with non–robotic laparoscopic surgery, could add to the harmful effects of ventilation.
To gain a better insight and understanding of the incidence and factors that have an association with PPCs in patients undergoing abdominal surgery, we will harmonize and merge the individual patient data of two worldwide prospective studies, named ‘Local ASsessment of VEntilatory management during General Anaesthesia for Surgery’ (LAS VEGAS4 and ‘Assessment of Ventilatory management during general AnesThesia for Robotic surgery and its effects on postoperative pulmonary complications’ (AVATaR3 into a new pooled database, the LapRas (Laparoscopic and Robot-assisted surgery).
The aim is to compare the incidence of PPCs after laparoscopic non–robotic versus RAS abdominal surgery, and to determine which patient, surgery and anesthesia factors have associations with PPCs. We hypothesize that differences in the occurrence of PPCs between the two groups are more driven by differences in duration of anesthesia rather than by the intensity of mechanical ventilation.
MethodsStudy designThis is a preplanned analysis of a pooled database that merged the individual data of patients included in two worldwide observational studies of ventilation and PPCs. The study protocols for each study were approved by a central Institutional Review Board, and subsequently by local ethics committees if needed. Written informed consent was obtained whenever it was deemed necessary according to local legislation. The two studies, and the pooled database are registered at clinicaltrials.gov (NCT01601223, NCT02989415). LapRas will be registered at clinicaltrials.gov (pending). The creation of a pooled database, including specific modifications for patients’ categories, neither requires additional ethical approval nor informed consent. We will follow the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement.5
PatientsLAS VEGAS included consecutive patients undergoing general anesthesia with invasive ventilation for elective and non–elective surgeries. In LAS VEGAS, patients were excluded from participation if younger than 18 years, or scheduled for pregnancy–related surgery, surgical procedures outside the operating room, or procedures involving cardiopulmonary bypass. Data from patients undergoing thoracic surgery, or who required one–lung ventilation during surgery, and those who had received ventilation at any time in the previous 30 days were excluded. AVATaR included consecutive patients aged older than 18 years and undergoing RAS for abdominal procedures. Patients were excluded from participation if pregnant and if the procedure was converted to open or laparoscopic surgery.
For this current analysis we will additionally exclude patients not undergoing laparoscopic surgery in LAS VEGAS, and not undergoing abdominal RAS in AVATaR. Patients undergoing combined thoracic–laparoscopic surgery and patients in whom the intervention was converted from laparoscopic or RAS to open surgery will be excluded. Patients that received recent ventilation before surgery, patients with incomplete ventilation datasets not allowing the computation of intensity of ventilation, and patients with an incomplete follow–up for PPCs will be also excluded.
Patients will be categorized as ‘laparoscopic patients’ in case of laparoscopic surgery without RAS, all other patients will be categorized as ‘RAS patients’.
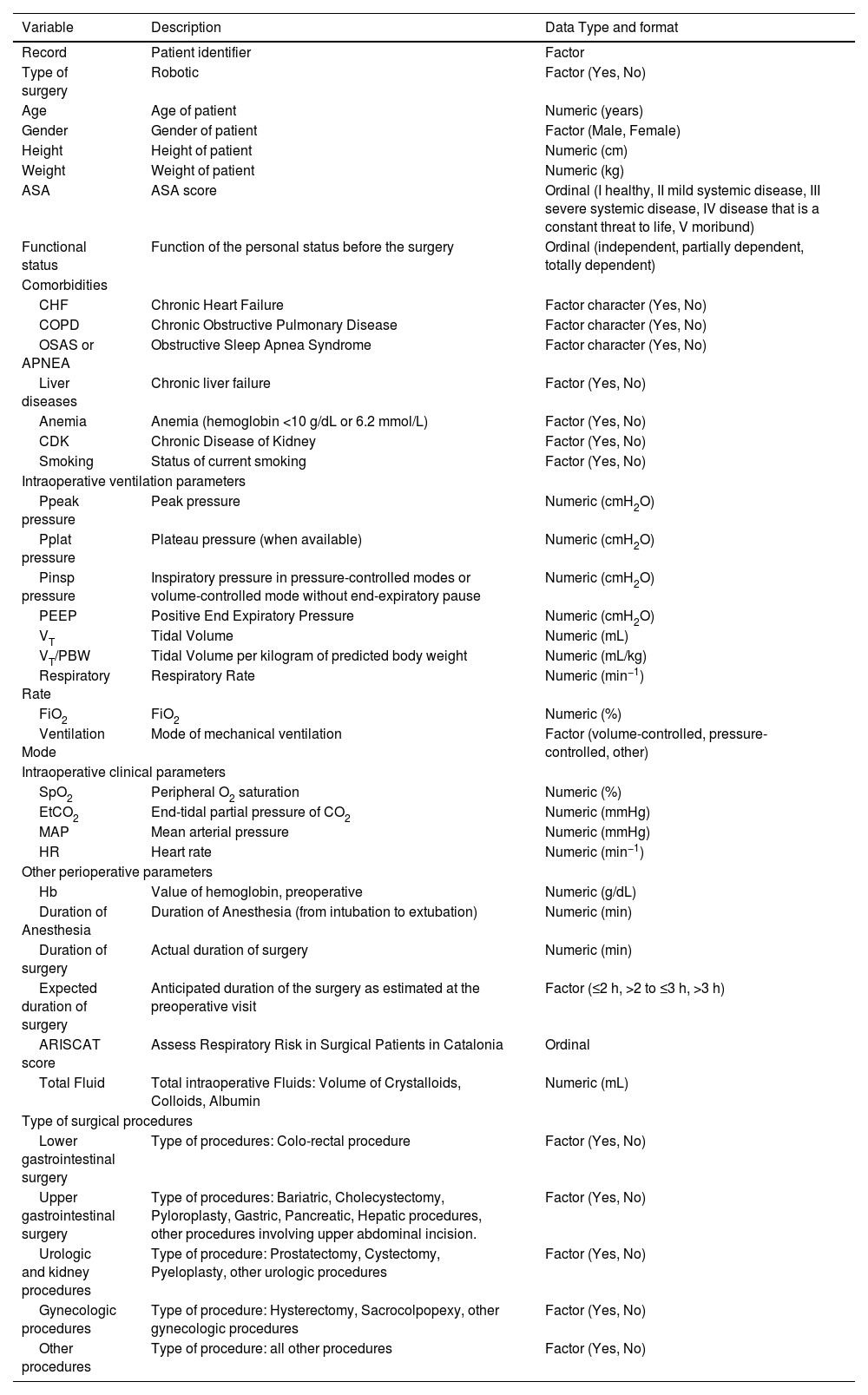
Baseline patients’ characteristicsThe key variables extracted from the LAS VEGAS and AVATaR studies are listed in Table 1. Data on patient demographic and baseline characteristics include age, sex, height, weight, current state of smoking, comorbidities such as chronic heart disease, obstructed sleep apnea syndrome (OSAS), anemia, chronic kidney disease. Furthermore, data regarding anesthesia and surgery will be collected from the parent studies datasets including anticipated duration of surgery, actual duration of anesthesia, actual duration of surgery, non-surgical ventilation time, total fluid, type of surgery.
Definition of the minimum expected set of variables resulting from the merging of the LAS VEGAS and AVATaR datasets.
| Variable | Description | Data Type and format |
|---|---|---|
| Record | Patient identifier | Factor |
| Type of surgery | Robotic | Factor (Yes, No) |
| Age | Age of patient | Numeric (years) |
| Gender | Gender of patient | Factor (Male, Female) |
| Height | Height of patient | Numeric (cm) |
| Weight | Weight of patient | Numeric (kg) |
| ASA | ASA score | Ordinal (I healthy, II mild systemic disease, III severe systemic disease, IV disease that is a constant threat to life, V moribund) |
| Functional status | Function of the personal status before the surgery | Ordinal (independent, partially dependent, totally dependent) |
| Comorbidities | ||
| CHF | Chronic Heart Failure | Factor character (Yes, No) |
| COPD | Chronic Obstructive Pulmonary Disease | Factor character (Yes, No) |
| OSAS or APNEA | Obstructive Sleep Apnea Syndrome | Factor character (Yes, No) |
| Liver diseases | Chronic liver failure | Factor (Yes, No) |
| Anemia | Anemia (hemoglobin <10 g/dL or 6.2 mmol/L) | Factor (Yes, No) |
| CDK | Chronic Disease of Kidney | Factor (Yes, No) |
| Smoking | Status of current smoking | Factor (Yes, No) |
| Intraoperative ventilation parameters | ||
| Ppeak pressure | Peak pressure | Numeric (cmH2O) |
| Pplat pressure | Plateau pressure (when available) | Numeric (cmH2O) |
| Pinsp pressure | Inspiratory pressure in pressure-controlled modes or volume-controlled mode without end-expiratory pause | Numeric (cmH2O) |
| PEEP | Positive End Expiratory Pressure | Numeric (cmH2O) |
| VT | Tidal Volume | Numeric (mL) |
| VT/PBW | Tidal Volume per kilogram of predicted body weight | Numeric (mL/kg) |
| Respiratory Rate | Respiratory Rate | Numeric (min−1) |
| FiO2 | FiO2 | Numeric (%) |
| Ventilation Mode | Mode of mechanical ventilation | Factor (volume-controlled, pressure-controlled, other) |
| Intraoperative clinical parameters | ||
| SpO2 | Peripheral O2 saturation | Numeric (%) |
| EtCO2 | End-tidal partial pressure of CO2 | Numeric (mmHg) |
| MAP | Mean arterial pressure | Numeric (mmHg) |
| HR | Heart rate | Numeric (min−1) |
| Other perioperative parameters | ||
| Hb | Value of hemoglobin, preoperative | Numeric (g/dL) |
| Duration of Anesthesia | Duration of Anesthesia (from intubation to extubation) | Numeric (min) |
| Duration of surgery | Actual duration of surgery | Numeric (min) |
| Expected duration of surgery | Anticipated duration of the surgery as estimated at the preoperative visit | Factor (≤2 h, >2 to ≤3 h, >3 h) |
| ARISCAT score | Assess Respiratory Risk in Surgical Patients in Catalonia | Ordinal |
| Total Fluid | Total intraoperative Fluids: Volume of Crystalloids, Colloids, Albumin | Numeric (mL) |
| Type of surgical procedures | ||
| Lower gastrointestinal surgery | Type of procedures: Colo-rectal procedure | Factor (Yes, No) |
| Upper gastrointestinal surgery | Type of procedures: Bariatric, Cholecystectomy, Pyloroplasty, Gastric, Pancreatic, Hepatic procedures, other procedures involving upper abdominal incision. | Factor (Yes, No) |
| Urologic and kidney procedures | Type of procedure: Prostatectomy, Cystectomy, Pyeloplasty, other urologic procedures | Factor (Yes, No) |
| Gynecologic procedures | Type of procedure: Hysterectomy, Sacrocolpopexy, other gynecologic procedures | Factor (Yes, No) |
| Other procedures | Type of procedure: all other procedures | Factor (Yes, No) |
Abbreviations: ARISCAT, Assess Respiratory Risk in Surgical Patients in Catalonia; ASA, American Society of Anesthesiologist; CDK, Chronic Kidney Disease; CHF, Congestive Heart Failure; COPD, Chronic Obstructive Pulmonary Disease, OSAS, Obstructive Sleep Apnea Syndrome or Apnea; EtCO2, End-tidal Carbon Dioxide; FiO2, Fraction of Inspired Oxygen; Hb, hemoglobin; HR, Heart Rate; MAP, Mean Arterial Pressure; SpO2, Peripheral Oxygen Saturation; PEEP, Positive End-Expiratory Pressure; Pinsp, Inspiratory Pressure; Ppeak, Peak Inspiratory Pressure; Pplat, Plateau Pressure; PBW, Predicted Body Weight; RR, Respiratory Rate; TV, Tidal Volume.
The following variables will be extracted from the database of the two studies, expressed as median of hourly measurements (see Table 1): inspiratory pressure (Pinsp), plateau pressure (Pplat), positive end expiratory pressure (PEEP), tidal volume (VT) as absolute value and its ratio to the predicted body weight, respiratory rate (RR), fraction of inspired oxygen (FiO2), peripheral oxygen saturation (SpO2), end-tidal carbon dioxide (EtCO2). Mode of ventilation will be classified as volume controlled (VC), pressure controlled (PC, including volume-guarantee pressure-controlled) ventilation and others (all other ventilation modes).
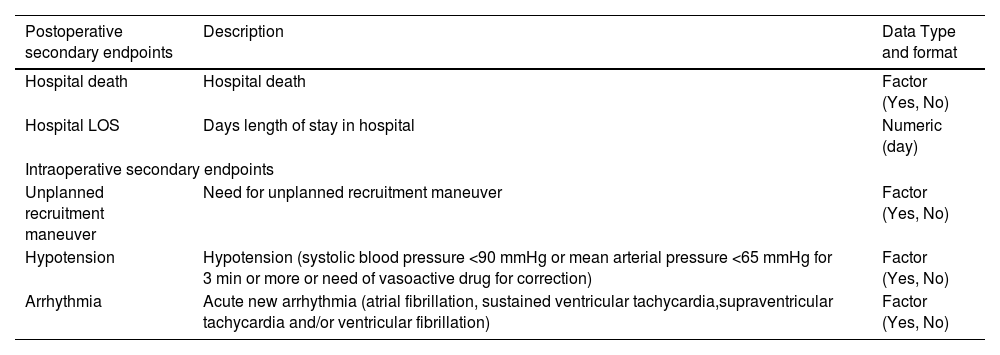
Study endpointsThe primary endpoint is the occurrence of one or more PPCs in the first five postoperative days. Secondary endpoints are the occurrence of each individual PPC, hospital length of stay (LOS) and in–hospital mortality. Data on primary and secondary endpoints will be reported in a dedicated table.
Classification of surgical proceduresIn LAS VEGAS, patients were classified into five broad surgical procedure categories, ‘lower abdominal surgery’, ‘upper abdominal surgery’, ‘urological and kidney surgery’, ‘gynecologic surgery’ and ‘other’. In AVATaR, patients were classified in more detail. To harmonize classification, we used the following criteria. Colorectal procedures will be considered as ‘lower abdominal surgery’; bariatric, cholecystectomy, pyloroplasty, gastric, pancreatic, hepatic as ‘upper abdominal surgery’; prostatectomy, cystectomy, pyeloplasty, and other urologic procedures as ‘urological and kidney surgery’; and hysterectomy, sacrocolpopexy, and other gynecological procedures as ‘gynecologic surgery’. All other surgical procedures will be considered ‘other’.
Alignment of outcome measuresIn both studies, PPC was considered as at least one of the following clinical conditions: new onset of acute respiratory distress syndrome (ARDS), pneumonia, pneumothorax, acute respiratory failure, need of oxygen therapy, need for postoperative invasive or non-invasive mechanical ventilation. Detailed definitions adopted in the two studies were equivalent and are reported in Table 2. LAS VEGAS had a longer follow–up time for the primary endpoint. For the purpose of harmonizing the primary outcome to AVATaR, PPCs occurring after the fifth postoperative day in LAS VEGAS will be ignored. Table 3 illustrated the secondary intra- and post-operative endpoints that will be extracted from the LAS VEGAS and AVATaR datasets.
Definitions of the primary composite endpoint of postoperative pulmonary complications in the LAS VEGAS and AVATaR studies.
| Type of PPC | LAS VEGAS | AVATaR |
|---|---|---|
| Need for oxygen therapy | Unplanned supplementary oxygen (oxygen administered due to PaO2 <8 kPa or SpO2 <90% in room air, but excluding oxygen supplementation given as standard care, e.g., directly after arrival in the post-anesthetic care unit) or prolonged invasive mechanical ventilation (after discharge from the operating room) | Need for oxygen therapy (defined as supplementary oxygen used due to PaO2 <60 mmHg or SpO2 <92% in room air [in individuals with no prior pulmonary disease] or SpO2 <88% [in individuals with prior pulmonary disease]); |
| Respiratory failure | Respiratory failure (PaO2 <8 kPa or SpO2 <90% despite oxygen therapy, or a need for noninvasive positive pressure ventilation (NIPPV) | Acute respiratory failure (defined as PaO2 <60 mmHg or SpO2 <92%, despite treatment with oxygen, or need for non-invasive mechanical ventilation [NIV]) |
| Pneumonia | Pneumonia (presence of a new or progressive radiographic infiltrate and at least two of three clinical features; fever >38 C or >100.4 F, leukocytosis or leukopenia (WBC count >12 000 cells/mm3 or <4000 cells/mm3 and purulent secretions) | Pneumonia (defined by the presence of a new or progressive radiographic infiltrate in addition to at least two of the three clinical characteristics: fever >38 °C, leukocytosis or leukopenia (WBC count >12000 cells/mm3 or <4000 cells/mm3, respectively), and purulent secretion) |
| ARDS | ARDS (defined according to the Berlin definition of ARDS) | ARDS (defined as per Berlin Definition) |
| Pneumothorax | Pneumothorax (air in the pleural space with no vascular bed surrounding the visceral pleura on the chest X-Ray). | Pneumothorax (defined as the presence of air between the visceral and parietal pleura; diagnosis can be made by clinical examination and chest X-Ray) |
Abbreviations: AVATaR, ‘Assessment of Ventilatory management during general Anesthesia for Robotic surgery and its effects on postoperative pulmonary complications’; ARDS, acute respiratory distress syndrome; LAS VEGAS, ‘Local ASsessment of VEntilatory management during General Anaesthesia for Surgery’; NIPPV: non-invasive positive pressure ventilation; NIV: non-invasive ventilation; PaO2, arterial partial pressure of oxygen; SpO2, peripheral saturation of oxygen; WBC: white blood cells.
Definition of the minimum expected set of secondary endpoints resulting from the merging of the LAS VEGAS and AVATaR datasets.
| Postoperative secondary endpoints | Description | Data Type and format |
|---|---|---|
| Hospital death | Hospital death | Factor (Yes, No) |
| Hospital LOS | Days length of stay in hospital | Numeric (day) |
| Intraoperative secondary endpoints | ||
| Unplanned recruitment maneuver | Need for unplanned recruitment maneuver | Factor (Yes, No) |
| Hypotension | Hypotension (systolic blood pressure <90 mmHg or mean arterial pressure <65 mmHg for 3 min or more or need of vasoactive drug for correction) | Factor (Yes, No) |
| Arrhythmia | Acute new arrhythmia (atrial fibrillation, sustained ventricular tachycardia,supraventricular tachycardia and/or ventricular fibrillation) | Factor (Yes, No) |
Abbreviations: LOS, length of stay.
From patient baseline characteristics, the body mass index (BMI), the predicted body weight (PBW) and the assess respiratory risk in surgical patients in Catalonia (ARISCAT) score will be calculated. Intensity of mechanical ventilation will be assessed using three different estimators: the driving pressure (ΔP),6 driving pressure multiplied by four plus respiratory rate (4DPRR7 and the mechanical power of ventilation (MP). ΔP will be calculated by subtracting PEEP from the Pplat in volume–controlled modes of ventilation and subtracting the PEEP from Pinsp in pressure–controlled modes of ventilation and when volume–controlled modes are used without end-expiratory pause. The 4DPRR will be calculated as 4 * ΔP + RR.7 The mechanical power (MP) will be computed with the following simplified formula: MP (J/min) = 0.098 * VT * RR * (Pplat–0.5 * ΔP) (in volume–controlled modes of ventilation), and (J/min) = 0.098 * VT * RR * (Pinsp–0.5 * ΔP) (in pressure–controlled modes of ventilation and in volume–controlled modes applied without end-expiratory pause). These formulas represent a surrogate of the original proposed formula8 to allow computation in both ventilation modes, but do not account for the resistive component in volume–controlled modes. In the contest of anesthesia, the non-surgical ventilation time will be calculated using the following formula: actual duration of anesthesia minus actual duration of surgery.
Statistical analysesDemographics, baseline characteristics, clinical and outcome variables will be presented as medians with interquartile ranges (IQR), or as number with percentages, as appropriate. Differences in baseline characteristics between patients who underwent laparoscopic surgery and patients received RAS will be analyzed using the Pearson Chi–squared or Fisher exact tests for categorical variables and with a Wilcoxon rank–sum test for continuous variables. Cumulative distribution plots will be used to show the differences between the two groups for key variables such as: actual duration of anesthesia, actual duration of surgery, non-surgical ventilation time, ΔP, 4DPRR and MP.
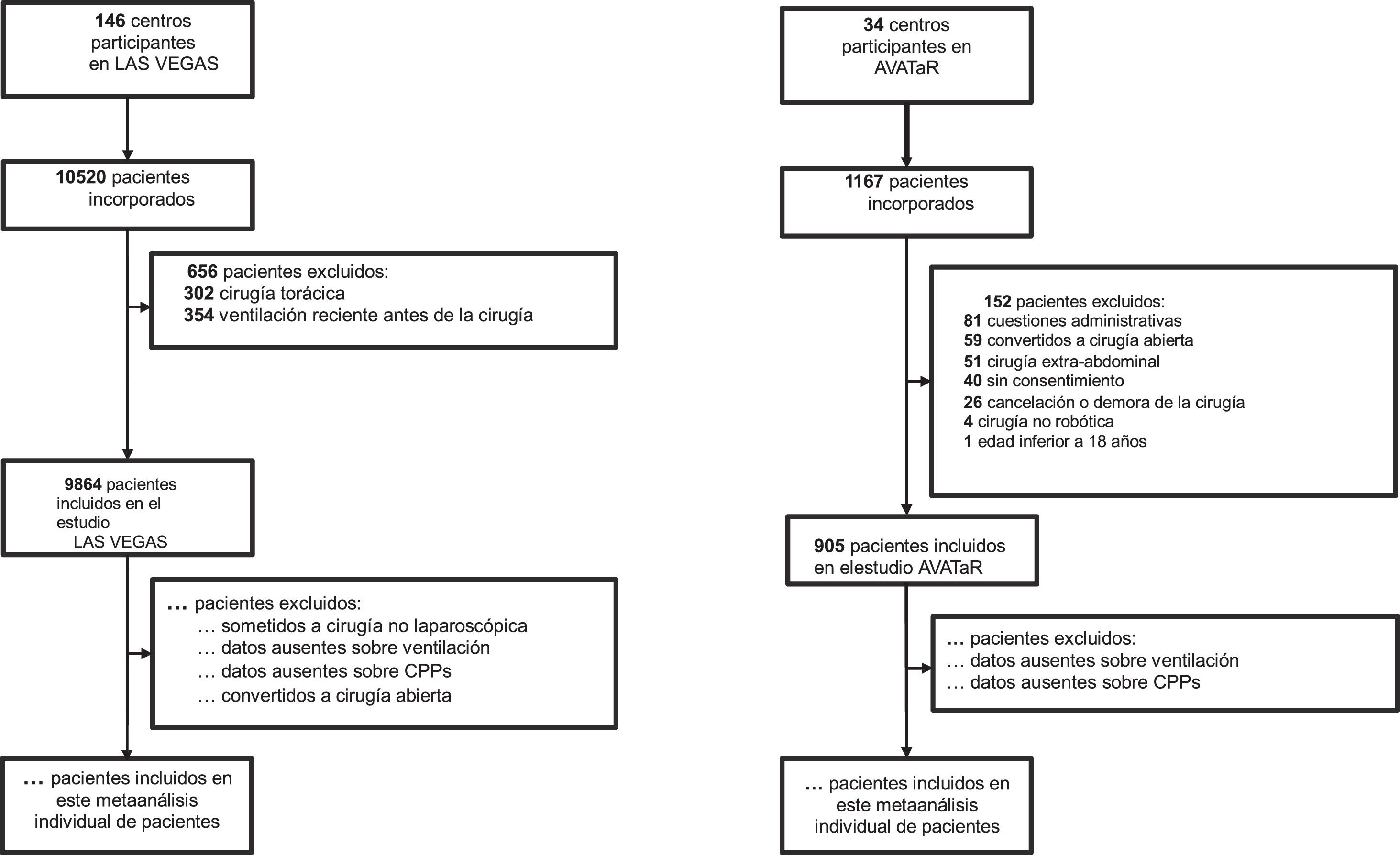
Sample size calculationWe will not perform a formal sample size calculation, as the number of included patients is based on the number of available patients. LAS VEGAS included 1737 patients undergoing laparoscopic surgery, AVATaR included 905 patients undergoing RAS abdominal surgery (see Fig. 1). Estimating a drop-out of 10% due to the application of the additional exclusion criteria, we aim to include at least 2378 patients. The total number of observed PPC events will determine the maximum number of co–variates that will be entered in the logistic regression models, maintaining a maximum of 1 co-variate every 10 observed PPC events.9
Inclusion flow outline of the present individual patient data meta-analysis. LAS VEGAS: ‘Local ASsessment of VEntilatory management during General Anaesthesia for Surgery’; AVATaR: ‘Assessment of Ventilatory management during general Anesthesia for Robotic surgery and its effects on postoperative pulmonary complications’; PPCs: postoperative pulmonary complications.
The main analysis will be an univariable followed by a multivariable model including known risk factors for PPCs, intensity of ventilation and group (laparoscopic versus RAS). The association of PPC with duration of anesthesia and intensity of ventilation will be examined using a multivariable mixed–effects logistic regression model, including a random term for center clustering. Covariates with a clinically plausible effect on outcome will be entered in the model. Additionally, hospital LOS will be assessed through mixed-effects Cox regression models. These models will be adjusted for the same baseline variables. In case of relevant co-linearity of independent variables, separate models will be built excluding co-linear variables and the performances of the different models will be assessed using the corrected Akaike information criterion (AICc).
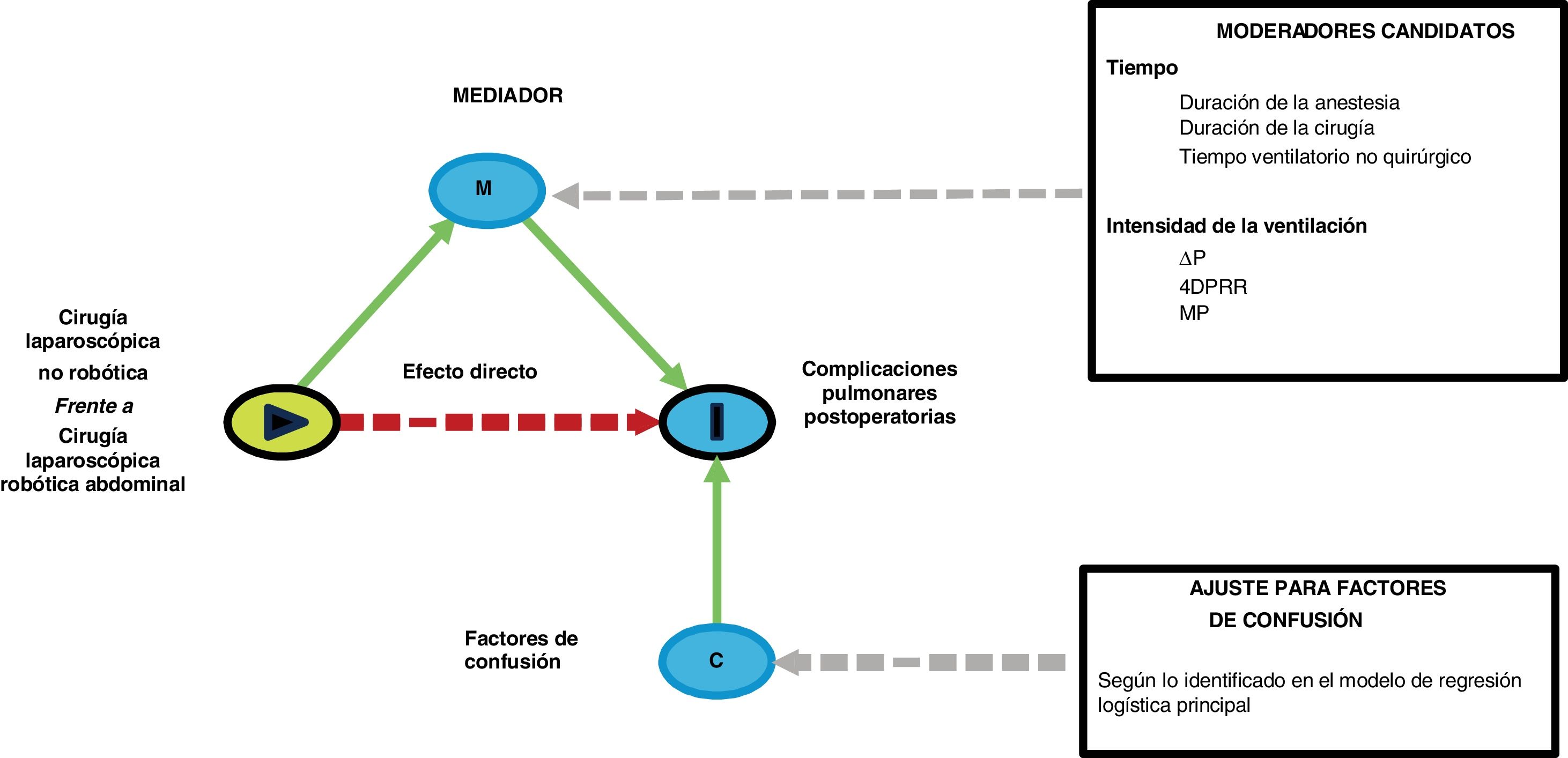
Sensitivity analysesThe following preplanned sensitivity analyses will be conducted in case an association between RAS and PPCs is observed: (1) a mediation analysis to investigate whether the relationship between PPC and surgical approach (RAS or conventional laparoscopy) is mediated by time estimated by duration of surgery, duration of anesthesia and non-surgical ventilation time or intensity of ventilation estimated by ΔP, 4DPRR and MP; (Fig. 2) (2) a matched-cohort analysis in patients with similar length of mechanical ventilation; (3) a matched-cohort analysis in patients with similar intensity of ventilation.
Directed acyclic diagram (DAG) showing the structure of the sensitivity mediation analyses. In each model, a single candidate moderator will be entered, corrected for the confounding factors associated with the development of PPCs as identified in the main logistic regression model. PPCs: postoperative pulmonary complications; ΔP: driving pressure; 4DPRR driving pressure multiplied by four plus respiratory rate; MP: mechanical power.
One group of sensitivity analyses will be performed using mediation analysis to investigate whether duration of surgery, duration of ventilation and intensity of ventilation estimators act as mediators between the type of surgical procedure (conventional laparoscopic versus RAS) and occurrence of PPCs, while correcting for key variables as identified in the main statistical models. Mediation analysis aims to identify the intermediate variables that explain the relationship between the independent variable (surgical approach) and the dependent variable (PPCs).10 The goal is to determine whether a specific variable, strongly influenced by the type of surgical approach, will influence the incidence of PPCs that may explain in whole or in part the effects resulting from the type of procedure. To determine if the mediator is truly playing a role in the outcome, an association between the mediator and the outcomes should be observed. The mediation proportion quantifying how the surgical approach induces a change in the candidate mediators, which subsequently affects the incidence of PPCs. The mediation analysis will be reported according to the “A Guideline for Reporting Mediation Analyses” (AGReMA) statement.11
Data will be analyzed using R 4.0.3 is (R Project for Statistical Computing: www.r-project.org). P-value < 0.05 will be considered statistically significant. When appropriate, the statistical uncertainty will be expressed by 95% confidence intervals.
DiscussionThis study is preplanned analysis of pooled database that harmonized and merged the individual patient data of 2 previously performed worldwide observational studies of intraoperative ventilation and PPCs (Table 3). This analysis will address a clinically relevant research question related to the comparison of laparoscopic surgery and RAS. The study will try to explore the mechanisms underlying the higher incidence of PPCs observed in patients undergoing RAS,3 as compared to those receiving non-robotic surgery.4 This analysis will help to identify potential targets for intervention to reduce the risk of PPCs in patients undergoing RAS. This might have relevant clinical implications and guide the development of future physiological and clinical studies.
Limitations and strengthThe strengths of this analysis are the large sample size and the participation of centers across several countries. The analysis employs robust statistical methods including multivariable models, matched-cohort models and mediation analysis to control for predictors and evaluate the association between surgical approach and outcomes. Furthermore, the predefined statistical analysis plan, which will be strictly followed, will limit the possibility of deviations from the initial research hypotheses.
Limitations are as follows. First, the two parent studies ran separately and in different years. However, the definitions of outcome measures were equivalent as were the time-points for intraoperative data acquisition. Second, as this is a post-hoc analysis of previously acquired observational data, the findings of this current analysis can only be used for generating hypotheses to be confirmed in prospective randomized trials. Third, the heterogeneity of mechanical ventilation modes used in the clinical practice in the two parent studies require the adoption of surrogate formulas to compute ΔP, 4DPRR and MP.
In conclusion, the here proposed analysis will allow to provide an insight on the clinical phenomena related to the development of PPCs in patients receiving invasive mechanical ventilation for laparoscopic surgery or RAS. The results of this study will allow generating new hypotheses on possibly modifiable clinical factors influencing the occurrence of PPCs in this high-risk surgical population.
FundingThe funding of studies used for this individual patient data analysis have been reported with the original publications. This analysis was performed without additional funding.
Conflict of interestNone.