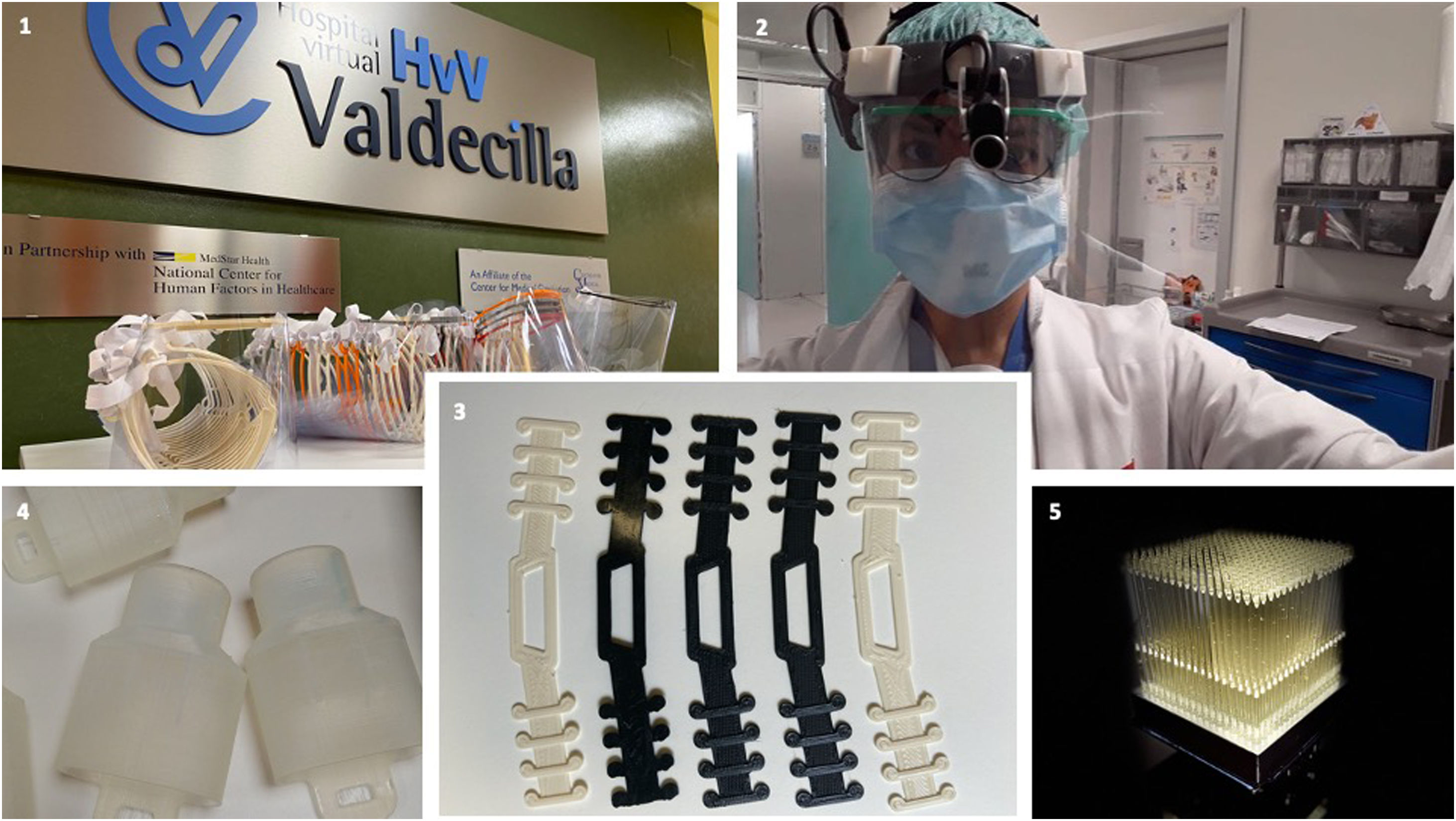
There is a shortage of supplies for the protection of professionals during the COVID-19 pandemic. 3D printing offers the possibility to compensate for the production of some of the equipment needed. The objective is to describe the role of 3D printing in a health service during the COVID-19 pandemic, with an emphasis on the process to develop a final product ready to be implemented in the clinical environment.
MethodsA working group was formed between the healthcare administration, clinicians and other public and private institutions in Cantabria, Spain coordinated by the Valdecilla Virtual Hospital. The process included receiving the printing proposals, learning about the printing resources in the region, selecting the devices, creating a team for each project, prototyping, evaluation and redesign, manufacturing, assembly and distribution.
ResultsThe following supplies are produced: 1) devices that help protect providers: face protection screens (2400 units), personalized accessories for photophores (20 units) and ear-protection forks for face-masks (1200 units); 2) products related to the ventilation of infected patients: connectors for non-invasive ventilation systems; and 3) oral and nasopharyngeal swabs (7500 units) for the identification of coronavirus carriers with the aim of designing action protocols in clinical areas.
Conclusions3D printing is a valid resource for the production of protective material for professionals whose supply is reduced during a pandemic.
Durante la pandemia de COVID-19 se produce una reducción del material para la protección de los profesionales. La impresión 3D ofrece la posibilidad de compensar la escasez de algunos de los suministros. El objetivo es describir el papel de la impresión 3D en un servicio de salud durante la pandemia de COVID-19, con énfasis en proceso para desarrollar un producto final listo para ser implementado en el entorno clínico.
Materiales y métodosSe formó un grupo de trabajo entre la administración sanitaria, clínicos y otras instituciones público-privadas de Cantabria coordinado en el Hospital virtual Valdecilla. El proceso incluyó la recepción de las propuestas de impresión, el conocimiento de los recursos de impresión en la región, la selección de los dispositivos, la creación de un equipo para cada proyecto, diseño de prototipos, evaluación y rediseño, fabricación montaje y distribución.
ResultadosSe producen 1) dispositivos que ayudan a prevenir el contagio de los profesionales: pantallas de protección facial (2.400 unidades), accesorios personalizados para fotóforos (20 unidades) y horquillas salvaorejas para mascarillas (1.200 unidades); 2) productos relacionados con la ventilación de pacientes infectados: conectores de sistemas de ventilación no invasive entre tubuladura y mascarilla; y 3) hisopos oro y nasofaríngeos (7.500 unidades) para la identificación de portadoras del coronavirus con el objetivo de diseñar protocolos de actuación en las área clínicas.
ConclusionesLa impresión 3D es un recurso válido para la producción de material de protección de los profesionales cuyo suministro está reducido durante una pandemia.
Estimates suggest that up to 30% of patients infected with the COVID-19 coronavirus may require hospital admission. The risk of contagion among hospital staff directly involved in the care of these patients is particularly high during aerosol-generating procedures.1 Up to 15%–20% of hospitalised patients need some form of oxygen therapy and ventilation, and many require airway management to perform upper respiratory or digestive tract endoscopic studies or surgical interventions.2
Anaesthesiologists, together with personnel from the surgical and critical care units, are at the forefront of patient care in all these interventions, and this increases the demand for personal protective equipment against droplets and aerosols, such as face shields and masks, and for sampling kits to detect COVID-positive patients.3
As the virus spreads to a greater percentage of the population and the risk of exposure and contagion among healthcare personnel increases, the demand for such equipment has risen not only in Spain, but also worldwide, and stocks are rapidly becoming depleted. This, in combination with a slow-down in production, the interruption of global and off-shore transport and distribution chains, and more stringent customs control between countries, has meant that the demand for such resources can no longer be met. Despite the efforts of governments and businesses to intervene in purchase and distribution chains and increase and redirect and increase the production of supplies needed at the national level, some equipment, such as face shields, swabs for nasopharyngeal sampling, ventilators parts, and other materials such as masks or gowns, are still in short supply.4
Three-D printing is a flexible robotic technology that uses computer-aided design systems to create layer-by-layer customised designs with controlled architecture and composition. Advances in 3D printing technology and the development of strong, flexible, biocompatible and sterilisable polymers can help hospitals overcome shortages of some critical supplies.5
Several international initiatives, such as the NIH 3D Print Exchange, have created libraries of 3D-printable models of critical medical devices.6 This, combined with the availability of 3D printers in numerous hospital services, simulation centres, other public institutions and private companies, facilitates the production of some parts or medical equipment that are in short supply during the pandemic.7 However, we were unable to find studies describing the entire process of selection and production of the protective equipment needed by anaesthesiologists and other surgical and critical care staff, particularly in terms of transforming the ideas generated into solutions that can be used directly in clinical practice. Neither were we able to find any references to the procedure used to combine public and private efforts during the pandemic.
The purpose of this study is to describe our experience with 3D printing in a tertiary hospital during the COVID-19 pandemic, with emphasis on public-private coordination and the design, evaluation and development of end products for surgical and critical care units using biocompatible polymers.
Material and methodsSettingThe study was conducted in the hospitals run by the Cantabrian Health Service in Spain during the COVID-19 pandemic between 9 March and 24 April 2020.
Sponsors and objectivesThe initiative was promoted by the Spanish Ministry of Health and coordinated through the Valdecilla Virtual Hospital (HvV). The HvV is a high-performance training and innovation centre for health professionals run by the Government of Cantabria and accredited by the American College of Surgeons.8
The board, management and nursing staff of the Marqués de Valdecilla hospital also participated, and a member of the clinical services was also involved in certain projects.
Our vision has been to provide staff in surgical and critical care units in the autonomous community of Cantabria with personal protective equipment not available elsewhere during the COVID-19 pandemic.9 Our mission has been to create a procedure for coordinating the 3D printing proposals received at the regional level was created.
Production processThe following steps were taken to produce an end product that was suitable for use in clinical practice:
- 1
Creation of the coordination team at the Valdecilla virtual Hospital.
- 2
Reception of 3D printing proposals at the regional level.
- 3
Communication with regional institutions and companies offering 3D printing services.
- 4
Selection of equipment and parts that can be produced.
- 5
Creation of a specific interprofessional team for each project.
- 6
Design of a prototype (geometry and selection of materials).
- 7
Evaluation and redesign of the prototype following usability tests and clinical evaluation.
- 8
Final design.
- 9
Manufacture, assembly, sterilization and distribution of the equipment produced.
The participants in these project received no financial compensation and did not claim any intellectual property rights. The instructions, computer files and images required for manufacturing the equipment have been published in open source format both on the HvV website10 and in international and private repositories.
Selected projects and participantsThree products related to the protection of clinical staff that are in short supply worldwide during the pandemic were identified. First, products that help protect staff from infection, such as face shields, accessories for surgical headlights, and ear clips for masks. Second, products related to the ventilation of patients infected with the coronavirus, such as parts for turning Decathlon diving masks© into ventilators for patients with respiratory failure. Third, nasopharyngeal swabs that are needed to identify people who are carriers of the coronavirus, with the aim of designing action protocols for surgical and other units using as much real data as can be collected. The specific participants of each project are shown in Table 1. An industrial engineer participated in the HvV, and 35 private sector companies and 4 public institutions also took part in the project.
Project participants.
| Participants | Shields | headlights | Clips | Connectors | Swabs |
|---|---|---|---|---|---|
| Hospital virtual Valdecilla | DI,P,M,E, | P,M,E | P,E | DI, P,E | DI,P,M,E,D |
| Anaesthesiology | DI,E,D | DI, P,E | |||
| ENT | E | DI,D | DI,E | ||
| Pulmonology | DI,E,D | ||||
| Nursing | P,E,D | ||||
| Health and Safety | DI,E,D | E | E,D | ||
| Microbiology | DI, E | ||||
| Valdecilla Research Institute | MA | ||||
| University of Cantabria | M | AR | |||
| Regional Ministry of Health | D | ||||
| Private companies | M | E | |||
| Other public institutions | M |
AR = Additional resources; D = Distribution; DI = Design; E = Evaluation; M = Manufacturing; P = Prototyping.
The material and printing equipment shown in Table 2 were used for creating the prototype and printing all the personal protective equipment and sampling swabs.
Material and equipment used for 3D printing.
| Material | Brand | 3D printer | 3D printing technology | Pros | Cons | Characteristics |
|---|---|---|---|---|---|---|
| Polylactic acid (PLA) | Eolas prints | DT60Ultimaker 2+Ultimaker 3 | FDM | Ease of printingBiodegradable | Finish qualityPrinting of complex parts | Tensile strength: 59 MPaElongation at break: 7%Young modulus 1.2 GPaGlass transition temperature: 55 °C |
| Acrylonitrile butadiene styrene (ABS) | Smart material | DT60Ultimaker 2+Ultimaker 3 | FDM | Resistant to high temperatureLightness | Printing difficulty | Young modulus 1.7−2.8 GPaElongation at break: 3%Bending modulus: 2.1–7.6 GPaFlexural strength: 69–97 MPaGlass transition temperature: 100 °C |
| Surgical Guide Resin | Formlabs | Form 2 | SLA | BiocompatibleSterilizable in autoclave | Cost | Young modulus 0.73 GPaElongation at break: 12.3%Flexural strength: 103 MPa |
| Draft resin | Formlabs | Form 2 | SLA | Printing speedLarge pieces | Printing precision | Young modulus 1.6 GPaElongation at break: 7%Flexural strength: 90 MPa |
| White resin | Formlabs | Form 2 | SLA | Quality of details | Opaque | Young modulus 2, 2 GPaElongation at break: 6.2%Flexural strength: 2.2 GPa |
MPa: megapascal; GPa: gigapascal.
Face shields and accessories for surgical headlights increase protection against infection by droplets. The fasteners make long-term use of face masks more comfortable for hospital staff, and in this way help limit their exposure to infectious particles.9
The production of face shields has been coordinated with regional organisations offering the use of their 3D printers. In total, 1800 units have been distributed in the Valdecilla Hospital. The Preventive Medicine and Occupational Risks Service prepares instructions for disinfecting the equipment before the first use and after subsequent uses (Table 3).
Use and dilutions of bleach for disinfection of goggles and face shields.*
| Wash the goggles or shields with soap and water (neutral or enzymatic depending on availability) and rinse under running water |
| For each litre of water, add 100 mL of commercial bleach (concentration 1:10). Very important: measure the amount of bleach exactly |
| Fully immerse goggles or face shields for 5 minutes |
| Rinse and dry. They can now be re-used |
| Discard the solution after use. The prepared solution can be used again if it is used immediately, and as a general rule up to a maximum of 3 consecutive uses. |
Laser cut face shields, which give healthcare personnel greater protection, are also being produced. This shield, which has been granted the national certificate of sanitary use, has been developed by the consortium formed by HvV, ENSA, Metacrilatos y grabados, and Textil Santanderin, and 700 units have so far been produced.
Customised connectors for attaching face shields to surgical headlights can also be made on 3D printers, and 20 such devices have been produced. In addition, "ear guards” have been produced for all health personnel requesting them, with 1200 units produced to date.
Free information on the 3D printing and laser cutting of face shields, as well as 3D printing of both surgical headlight accessories and clips is included as supplementary material and is also accessible on the web.10,11
Tube-mask connectors for non-invasive ventilation systemsThe COVID-19 pandemic may generate the need for non-invasive ventilation in infected patients with respiratory failure.12,13 The use of Decathlon© scuba masks has been described in this context, using an adaptor to connect the mask to the tubing through the filter and preventing leaks between the mask and the ventilator system. SLA or SLS technology was used for this purpose because it delivers high quality at critical points and its layer-by-layer technology prevents leakage and viral contamination (Fig. 1).
Swabs for nasopharyngeal and oropharyngeal samplingNasopharyngeal and oropharyngeal swabs are made from a flexible polymer. In the first phase prototypes are printed to check the combination of maximum tensile and compression strength. These factors have also been studied by the Cantabria Technological Centre. In a second phase, the effectiveness of the swab for collecting samples for the diagnosis of infection by the microbiology service of the Valdecilla hospital was evaluated. All 14 swabs in a pilot sample collected enough material for the test and 5 cases were positive for COVID-19 infection (unpublished internal data). The final design is shown in Fig. 1, and the details of the geometry of the shaft, the insertion of the base and the root of the collector, the mesh on the swab collector, the types of resin used, as well as the results of strength and flexibility tests can be consulted openly as supplementary material to this article and on the web.10,14 At the date of submission, the production of more than 7500 swabs has supplemented existing stocks of protective equipment and helped confirm or rule out COVID infection and guide the diagnosis and treatment of patients in the surgical and other units.
Discussion3D printing for the protection of hospital staff against COVID-19Three-D printing in a health service has provided staff members on the front line of treating coronavirus patients in surgical and critical areas with personal protection equipment that is in short supply during the COVID-19 pandemic. It can be an option for producing face shields, surgical headlight accessories, mask clips, ventilation system connectors, and sampling swabs when global supplies have been disrupted.
The ventilation system connectors attach the tubing of CPAP mode home ventilators (12−15 cm H20) or CPAP flow generators to the mask. These devices are used when non-invasive ventilators and face masks are not available to ventilate a patient continuously in the emergency situation.12 However, these solutions are not intended to be used continuously because they are often unable to deliver the high flow rates needed by these patients. Therefore, they are only available for compassionate use with the prior consent of the patient.13
The participation of an anaesthesiology service in coordination with all the agents involved in the design, evaluation, production and distribution process made it possible to develop products that can be implemented effectively and safely in clinical practice. In order to allow ideas put forward by clinicians on the front line of treatment to reach the market, it is essential to consider all the steps involved in the process and achieve effective coordination between institutions and public and private companies.
Other uses of 3D printing during the pandemicThis approach has previously been reported by other authors15,16 who have described the production of other materials, such as reusable face masks with a replaceable filter or ventilation valves to supply oxygen at fixed concentrations for patients with acute respiratory distress.17 Ventilator connectors, such as circuit splitters18 and one-way valves,19 have also been printed. However, there are no descriptions of how to approach this process in a comprehensive way and transform ideas into solutions for direct clinical use.
Selection of materialsThe range of materials available for additive manufacturing (3D printing) is very wide. The choice of material will depend on the manufacturing technology to be used and the required characteristics of the final product (such as flexibility, transparency, strength, biocompatibility, rapid prototyping or precision).
Current regulations that set the legal standards for material for sanitary use are found in Royal Decree 1591/2009 of October 16.20 The ISO 13485 standard also offers a comprehensive framework for medical devices that ensures product quality and regulatory compliance. It is important to minimise environmental waste as much as possible. Approval of the use of a particular plastic is based on migration testing that establishes certain values (mg/kg) that cannot be exceeded under legislation enacted in Europe and other countries (Council Directive of 18 October 1982, 82/711/EC). Some materials for additive manufacturing can be recycled, and there are currently companies that recycle plastic filaments for re-use in 3D printing.
The most frequently used technologies are fused deposition modelling (FDM), stereolithography (SLA), selective laser sintering (SLS) or direct metal laser sintering (DMLS). The most common technology (FDM) can be used with polylactic acid (PLA) and acrylonitrile butadiene styrene (ABS) thermoplastics. Printing with PLA has become widely accepted because it is made from renewable resources such as corn starch, tapioca root or sugar cane. It is one of the most widely used plastics for preliminary additive manufacturing and proof of concept testing before moving on to prototyping, due to its low cost and ease of printing. However, it gives a low quality finish, does not employ certified biocompatible consumables, and cannot be used to manufacture parts of medium complexity.
Other flexible materials also used with FDM are thermoplastic elastomers (TPE), nylon, polyethylene terephthalate (PET-PETG), polyvinyl acetate (PVA), high impact polystyrene (HIPS), materials with traces of carbon, wood, ceramic, etc., while technologies for more specific uses, such as SLA or SLS, make it possible to print using resins and polyamide powder particles that have a wide range of properties.21 Current studies into the antimicrobial behaviour of new polymers may show that these materials can provide an alternative for prototyping critical medical devices during a pandemic.6
In our case, SLA and FDM printing technology is used. The materials chosen for the project and their specific characteristics are shown in Table 2. Transparent, lightweight materials with high optical clarity such as polycarbonate and polyester, polyvinyl chloride and other synthetic polymers are used to manufacture 3D printed face shields. For non-invasive ventilation system connectors, material with an elongation at break of at least 30% is used to prevent the part from suffering unwanted stresses during assembly that could cause the connector to break. The documents related to the connector are available on the HvV website.10
Considerable progress has been made in the field of medical 3D printing, and the technology is also used for patient-specific implants and prostheses, scaffolds for the regeneration of biosynthetic tissues and organs, customized drug delivery systems, and anatomical modelling for perioperative simulation, although these uses are beyond the scope of this article. The material used for these indications (metals, ceramics, composites, methacrylate, etc.) should be biocompatible, mechanically durable, and easily moldable.22
Practical implicationsOur study has several practical implications. On the one hand, it provides a number of practical examples of the use of this technology during a pandemic. On the other, it describes the specific technical aspects of 3D printing, and also highlights the need for anaesthesiologists to be involved at all stages of the process, since they are involved in the first line of care for COVID-19 patients and their contributions to the prototyping, evaluation and final design are decisive. Collaboration with other surgical services most exposed to infection, such as nursing and ENT, is also essential. We also show the need to approach the process from a global production perspective with the aim of developing an end product that can immediately be introduced into clinical practice, instead of describing, as is common in the literature, the manufacture and use of individual materials. Similarly, our experiment has been made possible by the local clinical and administrative team leaders, and the existence of a high-performance medical training and innovation centre such as the HvV also contributed to the success of the project. Furthermore, the collaboration between hospital management and clinical team leaders has maximised response to the specific needs of professionals and the healthcare organization by combining all available resources. Finally, the participation of public hospitals and private companies in the process of piloting and evaluating the usability of the printed materials before their final production is especially significant.
Limitations and future researchThe main challenges faced by hospitals during the pandemic are the shortage of material for 3D printing and the lack of sufficient time to analyse the results after their implementation in clinical practice. Studies are needed to investigate these problems, and also the possibility of applying this technology in other areas of anaesthesiology and other medical fields
ConclusionIn conclusion, the use of 3D printing in a coordinated, collaborative project involving clinical departments, hospital management and regional authorities and private sector companies facilitates the production of materials that are in short supply during the pandemic. A global approach to the production process can lead to the development of materials than can be immediately introduced into clinical practice.
FundingNo funding.
Conflicts of interestThe authors declare that they have no financial relationship with any commercial company marketing products or services related to the simulation. The Valdecilla Virtual Hospital is affiliated with the Center for Medical Simulation, Boston, USA, Both are non-profit educational institutions that offer tuition-based training programs.
We would like to thank all the health professionals who faced the challenges arising from the COVID-19 outbreak in Cantabria, the heads of clinical services, care units and organisations in the autonomous community for reorganizing the resources available to address the pandemic, and both individuals and companies for their disinterested collaboration.
Please cite this article as: Pedraja J, Maestre JM, Rabanal JM, Morales C, Aparicio J, del Moral I. Papel de la impresión 3D para la protección de los profesionales del área quirúrgica y críticos en la pandemia de COVID-19. Rev Esp Anestesiol Reanim. 2020;67:417–424.