We present the results of the prospective follow up of a sample of large head metal-metal total hip arthroplasty obtained after the safety alert regarding a higher incidence of revision of these implants.
Material and methodsAll patients implanted with the Recap-M2a-Magnum cup between 2008 and 2011 were included. They were prospectively reviewed recording Harris Hip Score, clinical symptoms of chromium or cobalt intoxication. Serum levels of these ions were requested as well as X-rays and ultrasonography. An MRI was performed in the cases of positive ultrasonography.
ResultsTwenty-six males with a mean age of 48.54 years [32–62, SD: 7.18] were included. An anterolateral approach and Bi-Metric (7) and F-40 (19) stems were used. Cephalic diameters ranged 42–52 (mode: 46) and the mean cup inclination was 39.35° [21–59°, SD: 9.78]. During follow-up (7.3 years [5.9–9.4; SD: .78]), 3 patients (11.5%) underwent revision (2 cases aseptic loosening, 1 pseudotumour). Mean time until revision was 5.4 years [3.1–8.0; SD: 2.48]. The accumulated survival probability was 88.5% (95% CI 76.3–100%). Harris Hip Score was 94.47 [66.5–100; SD: 8.94] and the patients showed no metallic intoxication symptoms. The levels of chromium were 1.88μg/dl [0.6–3.9] and cobalt 1,74μg/dl [0.5–5,6]. One pseudotumour was found in an asymptomatic patient, and small amounts of periarticular liquid were found in 5 patients (19.2%)
Discussion and conclusionsHigh revision rates are still found when follow up is extended due to aseptic loosening and pseudotumour formation. MRI might not be the most adequate test to study the complications of these prostheses.
A raíz de las alertas sanitarias surgidas por la alta incidencia de recambios en la artroplastia de cadera metal-metal, se presentan los resultados obtenidos del seguimiento prospectivo de la serie de nuestro centro con cabezas de gran tamaño.
Material y métodosSe incluyeron todos los pacientes tratados con el cotilo Recap-M2a-Magnum, Biomet de 2008 a 2011. Se revisaron prospectivamente todos los pacientes registrando Harris Hip Score y síntomas de intoxicación por cromo-cobalto y se solicitaron niveles séricos de estos iones, radiografía y ecografía. Se solicitó resonancia magnética en caso de ecografía positiva.
ResultadosSe incluyeron 26 varones de 48,54 años de edad media [32-62, DE: 7,18]. Se utilizó un abordaje anterolateral y vástagos Bimetric (7) o F40 (19). La moda de los diámetros cefálicos fue 46 [42-52]. La inclinación media del cotilo fue 39,35° [21-59°, DE: 9,78]. Durante el seguimiento (7,3 años [5,9-9,4 años, DE: 0,78]), 3 pacientes (11,5%) precisaron revisión (2 por movilización aséptica, un pseudotumor). El tiempo medio hasta la revisión fue 5,4 años [3,1-8,0, DE: 2,48]. La probabilidad acumulada de supervivencia fue del 88,5% (IC95% 76,3-100%). El Harris Hip Score fue de 94,47 [66,5-100, DE: 8,94] y los pacientes no mostraron ningún síntoma de intoxicación metálica, con niveles de cromo 1,88 mcg/dl [0,6-3,9] y cobalto 1,74 mcg/dl [0,5-5,6]. Se encontró un pseudotumor en un paciente asintomático y pequeñas cantidades de líquido periprotésico en 5 pacientes (19,2%).
Discusión y conclusionesSeguimos encontrando altas tasas de revisión al extender el seguimiento de los pacientes debido a la movilización aséptica y la formación de pseudotumores. La resonancia nuclear magnética no parece la prueba más adecuada para el estudio de las complicaciones de este tipo de prótesis.
Although they are currently one of the most successful operations, orthopaedic surgeons still face challenges when performing total hip arthroplasties and choosing the implant to achieve appropriate stability with the highest resistance to wear. Among other factors, the risk of dislocation is increased with the posterior surgical approach and excessive cup inclination. Furthermore, increased femoral head diameter, reduces the dislocation rate.1 This is explained by a double mechanism: large heads enable longer necks to be used, and the range of motion that the prosthetic joint is capable of before impingement is greater the more the cephalic diameter is increased.2,3 However, the largest head diameters generate the most wear to components.4 Highly cross-linked polyethylene implants enable larger diameters, at the cost of thinning of the insert, which might cause breaks. The metal-on-metal frictional torque will enable thin inserts to be used as well as less volumetric wear.5 This is how total hip prostheses with large heads came into existence, with excellent outcomes in the first published papers with short-term follow-ups, and initially recommended for young and active patients.6 As the follow-up periods became longer,7,8 complications due to this frictional torque started to appear, such as systemic toxicity due to elevated blood levels of cobalt and chromium ions, or pseudotumours (defined as aseptic lymphocyte-dominated vasculitis-associated lesions),9 the reported revision rate due to loosening or any of these complications reached 44.07% at 10 years for the Corail/ASR prostheses (DePuy Orthopaedics, Johnson & Johnson, Warsaw, USA).10 The same British records for 2017 published an accumulated revision rate per 1000 patients-years of 9.55 for cementless prostheses with metal-on-metal stem as a whole, and of 3.80 for surface prostheses as a whole. This resulted in the creation of the Medical Device Alert,11 and one of the manufacturers even received a multi-million fine.12
For all of the above reasons, the Spanish Hip Surgery Society and the European Federation of National Associations of Orthopaedics and Trauma published a series of consensus documents13–15 which, in addition to including the most relevant scientific evidence at the time of publication, showed the most appropriate follow-up guidelines for patients undergoing large head total hip arthroplasty.
This check should be annual and accompanied by blood tests for chromium and cobalt and plain X-rays for all the patients, adding complementary imaging tests such as ultrasound, computerised axial tomography (CAT) or nuclear magnetic resonance (MRI) with a metal artifact reduction sequence for those with clinical, analytical or radiographic signs not within the normal range. The aim of our study was to examine the mid-term complications from metal-on-metal torque in prostheses with stems.
Material and methodsWe undertook an ambispective review of 26 consecutive arthroscopies performed in our centre between January 2008 and February 2011. The data relating to diagnosis, surgery performed and initial follow-up were gathered from the hospital's electronic clinical histories. We then performed a prospective follow-up and data collection from all of the patients.
All the arthroplasties were performed by three orthopaedic surgeons with experience in arthroplasty, after administering 2g cefazolin under spinal anaesthesia. A modified anterolateral Watson-Jones approach was used. The ReCap M2a-Magnum® was used in all cases, together with Bi-Metric® and F40® (Biomet, Warsaw, USA) stems. The routine protocol comprised laboratory tests and radiography at 24h after the intervention, and walking started after approximately 48–72h. Initial follow-up was at 1, 3, 6 and 12 months after the operation. All the patients were recruited again for follow-up and study in February 2016, and a clinical history was taken to detect signs or symptoms of chromium or cobalt toxicity (hearing loss, dizziness, fear, depression or neurological problems) and a functional evaluation using the Harris Hip Score (HHS). A blood test to include serum chromium and cobalt ion levels, an anteroposterior X-ray of the pelvis and axial X-ray of the operated hip and an ultrasound of the hip were taken. Serum chromium and cobalt levels were measured by mass spectrometry; levels of under 15μg/litre) of chromium and under 10μg/l of cobalt in exposed individuals were considered to be within normal ranges. Plain X-ray studied the position of the components, and for signs of acetabular osteolysis according to the DeLee and femoral zones (Gruen zones) or signs of loosening. Ultrasounds that showed the presence of a mass occupying the space or free fluid were considered positive.15,16 A conventional MRI scan was requested as a complementary test for the cases with a positive ultrasound. We followed the protocol proposed by the European Federation of National Associations of Orthopaedics and Traumatology,14 which indicates strict monitoring of patients with blood levels of cobalt between 2 and 7μg/l and replacement for cases of loosening, pseudotumour, movement, chromium or cobalt toxicity or serum cobalt levels above 20μg/l. In the cases where there was movement of the acetabular cup, the patients were offered a replacement with a Regenerex® porous titanium cup (Biomet, Warsaw, USA) with a ceramic-polyethylene frictional torque. The cases of replacement with component integration were offered a replacement of mobile components using an Avantage® double mobility system (Biomet, Warsaw, USA) with a ceramic and polyethylene head enabling the first cup to be maintained.
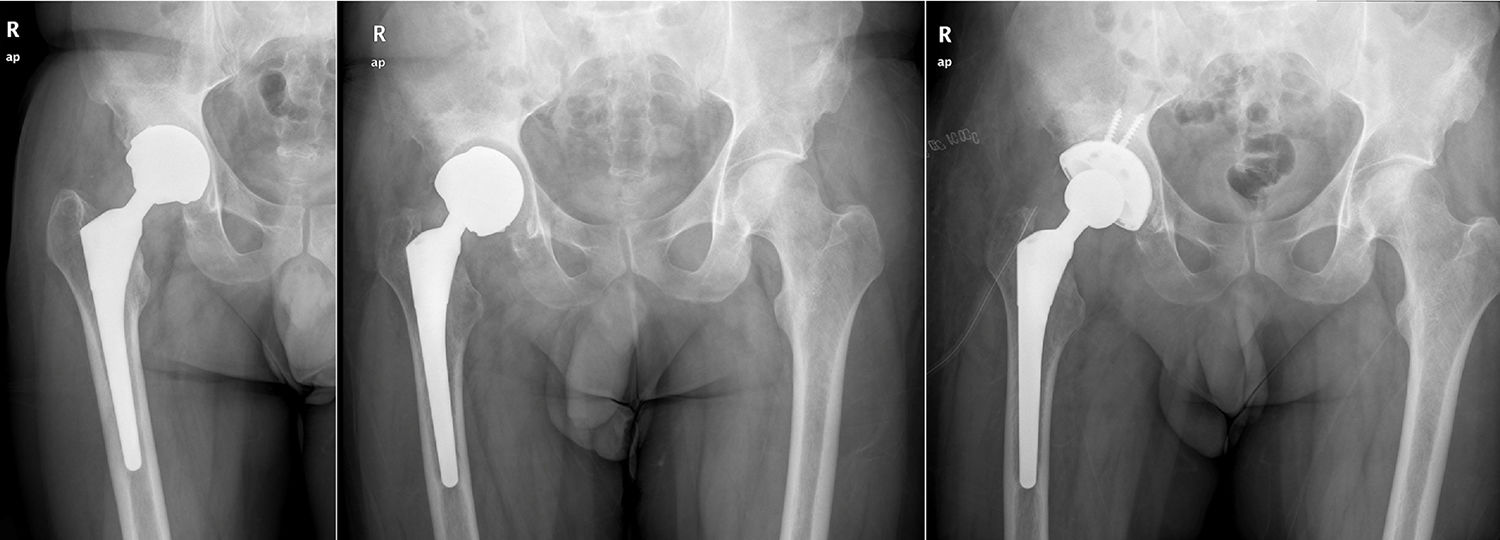
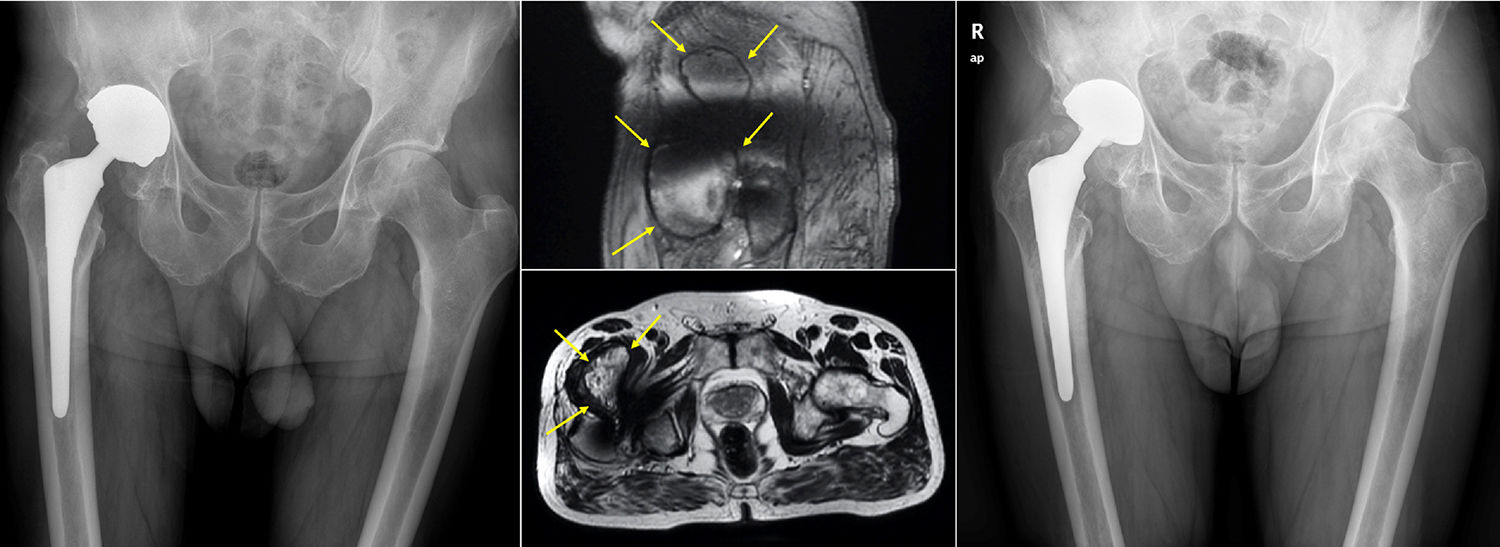
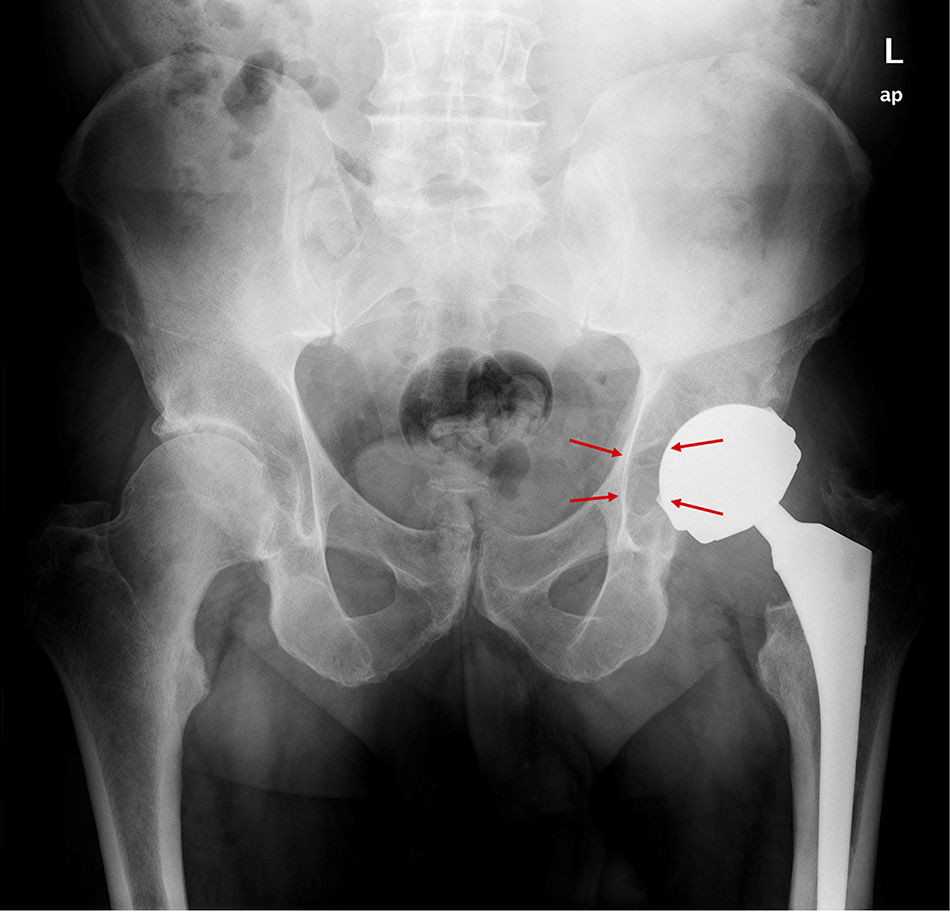
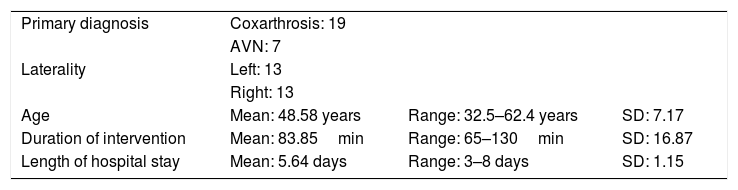
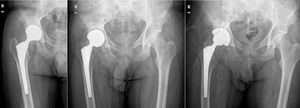
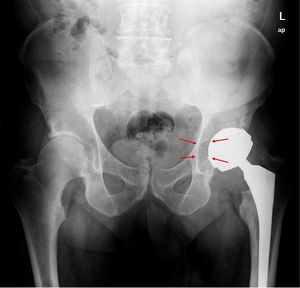
ResultsA total of 26 hips belonging to 23 patients were operated, all of whom were recruited for the study. These were 23 males with a mean age of 48.54 years [range: 32–62 years, SD: 7.18]. The demographic characteristics and those of the surgical intervention, as well as the implants used are shown in Tables 1 and 2. The mean HHS was 94.46 points [range: 66.5–100, SD: 8.94]. None of the patients reported signs or symptoms of chromium or cobalt intoxication. The mean follow-up time was 7.3 years [range: 5.9–9.4 years; SD: 0.78]. Three prostheses (11.54%) had been revised; in 2 cases due to aseptic loosening, and one case due to the onset of a pseudotumour (Figs. 1 and 2 and Table 3). The mean time until revision was 5.4 years [3.1–8.0; SD: 2.48]. One patient had periacetabular osteolysis in DeLee zone 3 (Fig. 3), with no loosening of the prosthesis. None of the patients showed signs of femoral osteolysis or prosthetic movement.
Features of the patients and the primary surgical intervention.
| Primary diagnosis | Coxarthrosis: 19 | ||
| AVN: 7 | |||
| Laterality | Left: 13 | ||
| Right: 13 | |||
| Age | Mean: 48.58 years | Range: 32.5–62.4 years | SD: 7.17 |
| Duration of intervention | Mean: 83.85min | Range: 65–130min | SD: 16.87 |
| Length of hospital stay | Mean: 5.64 days | Range: 3–8 days | SD: 1.15 |
SD: standard deviation; AVN: avascular necrosis.
Characteristics of the implants used.
| Stem | Bi-Metric (7) | |
| • Without collar (7) | ||
| F-40 (19) | ||
| • Without collar (13) | ||
| • With collar (6) | ||
| Cup | ReCap (26) | Size |
| • Mean: 52.85mm | ||
| • Mode: 52mm | ||
| • Range: 48–58mm | ||
| • SD: 2.60 | ||
| Inclination | ||
| • Mean: 39.35° | ||
| • Range: 21°–59° | ||
| • SD: 9.78 | ||
| Head | Modular M2a-Magnum (26) | Diameter |
| • Mean: 46.85mm | ||
| • Mode: 46mm | ||
| • Range: 42–52mm | ||
| • SD: 2.60 | ||
| Neck length | ||
| • Mean: −0.34mm | ||
| • Mode: −2mm | ||
| • Range: −6–8mm | ||
| • SD: 3.30 |
SD: standard deviation; n=26.
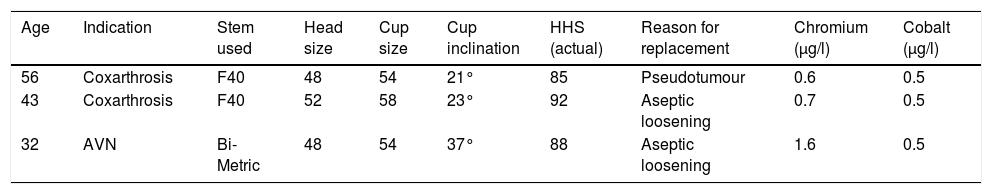
Demographic characteristics of the patients who required prosthetic replacement.
| Age | Indication | Stem used | Head size | Cup size | Cup inclination | HHS (actual) | Reason for replacement | Chromium (μg/l) | Cobalt (μg/l) |
|---|---|---|---|---|---|---|---|---|---|
| 56 | Coxarthrosis | F40 | 48 | 54 | 21° | 85 | Pseudotumour | 0.6 | 0.5 |
| 43 | Coxarthrosis | F40 | 52 | 58 | 23° | 92 | Aseptic loosening | 0.7 | 0.5 |
| 32 | AVN | Bi-Metric | 48 | 54 | 37° | 88 | Aseptic loosening | 1.6 | 0.5 |
HHS: Harris Hip Score; AVN: avascular necrosis.
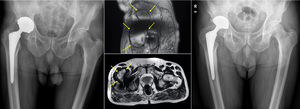
The ultrasounds were positive in 6 cases (23.02%), due to the presence of periprosthetic fluid in 5 cases, and the appearance of a mass occupying the space, which was a suspected pseudotumour. The accumulated incidence of pseudotumours was 7.69% (2 cases). An MRI was requested for these patients, which was only partially useful on two occasions, confirming the presence of a pseudotumour in one case (Fig. 2), and periprosthetic fluid in the other. On the remaining occasions (71.42%), the test could not be assessed due to the presence of artifacts.
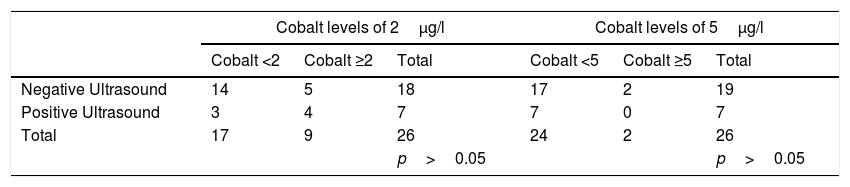
The serum levels of chromium (mean: 5.05μg/l, range: 0.60–55.90μg/l, SD: 11.08) and cobalt (mean: 3.81μg/l, range: 0.5–44.1μg/l, SD: 8.63) were above the recommended range in two of the patients. In line with the protocol, a new test was requested on both occasions, and normalised levels were found in both cases, obtaining the following new values: chromium (mean: 1.88μg/l, range: 0.6–3.9μg/l, SD: 1.02) and cobalt (mean: 1.74μg/l, range: 0.5–5.6μg/l, SD: 1.43). The altered levels in these two patients were therefore considered false positives. We then studied whether there was a clinical or statistical relationship between elevated serum cobalt levels with ultrasound positivity. Because there is controversy as to the serum ion levels which should indicate strict revisions, we undertook an analysis of the patients with levels above 2μg/l, 5μg/l and 7μg/l. These results were not clinically or statistically significant and are shown in Table 4. For the level of 7μg/l we could not perform a statistical analysis since this was higher than the maximum level obtained in our series.
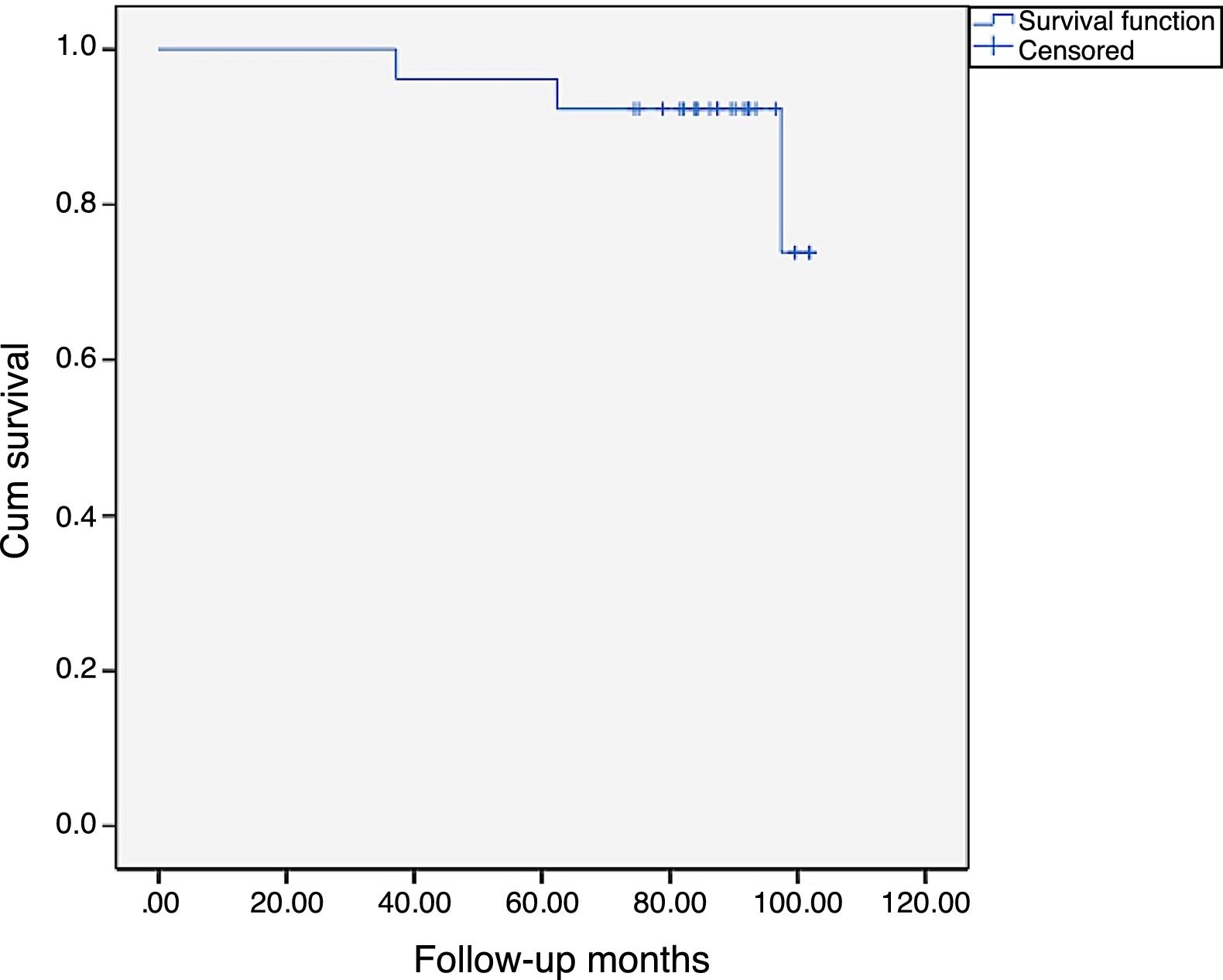
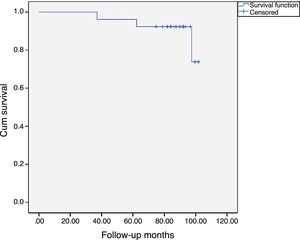
At the end of the study, we performed a survival function using the Kaplan-Meier method, considering the time of replacement as censored data which is shown in Fig. 4, the accumulated survival being 88.5% (95% CI 76.3–100%).
DiscussionThe results of the study reinforce the poor short and mid-term results of these types of implants. The strengths of the study come from its careful ambispective design that enabled a prospective review of 100% of the patients included in the study, from a clinical, analytical and radiological perspective. The data should be examined bearing in mind the possible limitations of the small sample size.
The use of the metal-on-metal torque is decreasing, essentially because orthopaedic surgeons are being cautious after the health alerts.17,18 In Spain, 80.8% of surgeons had abandoned the use of metal-on-metal torque by 2013; a lower figure is currently estimated.19
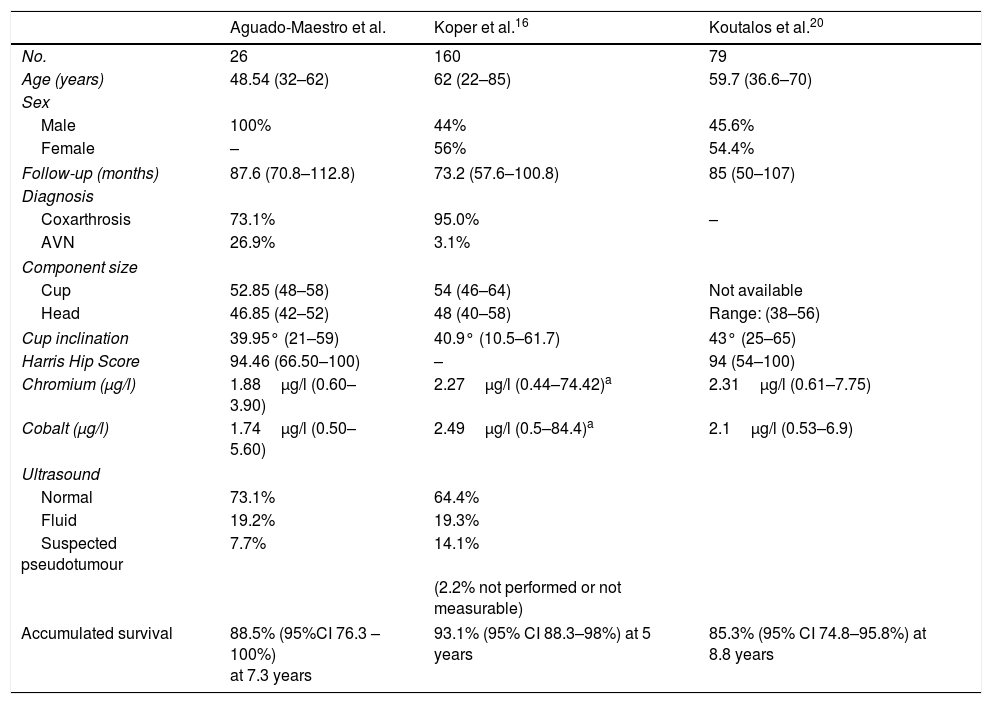
The results obtained in this paper were compared with those of the series by Koper16 and Koutalos,20 with sample sizes of 160 and 79 arthroplasties respectively, and can be found summarised in Table 5. In general, accumulated survival is similar in the three series, bearing mind that Koper's higher survival might be down to a shorter follow-up time. The survival at the end of follow-up of our series (7.3 years) was 88.46%, while we found accumulated survivals described in the literature of 93.1% at 5 years, 91.9% at 6.1 years, 91.1% at 7.1 years, and 85.3% at 8.8 years, for arthroplasties performed on patients of both sexes with mean ages of 62 and 59.7 years, higher in both cases than that of our series (48.54), which explains the approximate 7% losses to follow-up in these studies due to the death of one of the patients.
Comparison of the series obtained in our study with that described in the recent literature. The mean values are shown and the ranges in brackets.
| Aguado-Maestro et al. | Koper et al.16 | Koutalos et al.20 | |
|---|---|---|---|
| No. | 26 | 160 | 79 |
| Age (years) | 48.54 (32–62) | 62 (22–85) | 59.7 (36.6–70) |
| Sex | |||
| Male | 100% | 44% | 45.6% |
| Female | – | 56% | 54.4% |
| Follow-up (months) | 87.6 (70.8–112.8) | 73.2 (57.6–100.8) | 85 (50–107) |
| Diagnosis | |||
| Coxarthrosis | 73.1% | 95.0% | – |
| AVN | 26.9% | 3.1% | |
| Component size | |||
| Cup | 52.85 (48–58) | 54 (46–64) | Not available |
| Head | 46.85 (42–52) | 48 (40–58) | Range: (38–56) |
| Cup inclination | 39.95° (21–59) | 40.9° (10.5–61.7) | 43° (25–65) |
| Harris Hip Score | 94.46 (66.50–100) | – | 94 (54–100) |
| Chromium (μg/l) | 1.88μg/l (0.60–3.90) | 2.27μg/l (0.44–74.42)a | 2.31μg/l (0.61–7.75) |
| Cobalt (μg/l) | 1.74μg/l (0.50–5.60) | 2.49μg/l (0.5–84.4)a | 2.1μg/l (0.53–6.9) |
| Ultrasound | |||
| Normal | 73.1% | 64.4% | |
| Fluid | 19.2% | 19.3% | |
| Suspected pseudotumour | 7.7% | 14.1% | |
| (2.2% not performed or not measurable) | |||
| Accumulated survival | 88.5% (95%CI 76.3 – 100%) at 7.3 years | 93.1% (95% CI 88.3–98%) at 5 years | 85.3% (95% CI 74.8–95.8%) at 8.8 years |
L: litre; AVN: avascular necrosis; μg: microgram.
The paper by Koutalos20 also examines the functionality of the hips of patients who underwent arthroplasty and obtained a mean HHS of 94, practically identical to that found in our sample (94.46).
The serum chromium and cobalt levels found in our series were slightly lower than those found in the others. The measurement system of the external laboratory used by our centre is serum mass spectrometry; the type of measurement used in the studies by Koper and Koutalos is not specified. We obtained two false positives in the sample. The potential use of elevated serum chromium and cobalt levels as an analytical parameter indicative of the presence of pseudotumours is controversial. The study published by Bosker et al. describes an incidence of pseudotumours 4 times higher in patients with cobalt levels higher than 5μg/l. However, as with the data of our study, other authors in the literature consulted21,22 have failed to demonstrate any correlation between blood ion elevation and the presence of greater wear or positive ultrasound results. Nevertheless, patients with elevated serum levels of these ions might indeed have greater pain and score lower on the functional evaluation scales.23 Elevated chromium and cobalt levels have been related to the prosthetic design (modularity and coverage,) and the position of the components (essentially the cup inclination).22,24–26 These same factors are among those that relate to wear and the survival of these types of implants. There are few false positives associated with the methodology of the analysis. When they do occur, it is usually only in one of the ions tested. The results can be abnormally high when little time has passed between implantation of the prosthesis and the test (up to the first 18 months), if cyanocobalamin vitamin (vitamin B12) or multivitamin compounds have been taken, if contrast mediums such as gadolinium, iodine or barium have been used, or in the case of metal industry workers. With regard to the result of our studies, the most plausible option appears to be that the sample might have become contaminated. The laboratory suggested that patients with abnormally elevated serum chromium and cobalt levels should undergo testing for ions in urine.
The results of the imaging tests performed in our study were affected by the poor availability of MRI scans with metal artifact reduction software in our country. A total of 7 conventional magnetic resonance scans were undertaken after a positive ultrasound in 6 patients (one patient had been diagnosed by CAT, and had undergone a replacement due to a pseudotumour prior to the start of the study). The fact that only two of these resonance scans showed measurable results (28%) reinforces the superfluity of conventional MRI for these patients compared to ultrasound or CAT; ultrasound having the advantage over the latter as they are harmless for the patient.
Although the need remains high for revision of the M2a-Magnum prosthesis compared to other frictional torques such as the ceramic-on-polyethylene (around 1.77–2.59% at 7 years10), this has proved lower than other types of large head metal-on-metal implants,27,28 which might relate to the influence of the prosthetic design on the survival of the implant. The cup under study has a coverage arc that ranges from 154.6° to 163.6°, and is therefore higher than that provided by other implants such as the ASR prosthesis (DePuy, Johnson & Johnson Warsaw, USA), thus reducing excessive load at the edges.20 Therefore, manufacturing the connector component that joins the metal head to the femoral stem in titanium rather than chromium-cobalt, as in other models, might contribute towards less ion production at the level of the so-called trunnion, the union of the prosthetic head with the femoral stem.25 This might potentially result in the production of fewer particles, and therefore a lower incidence of pseudotumours and aseptic loosening.
In light of the data presented, our team cannot continue to recommend the use of large head metal-metal head hip arthroplasty due to the high reoperation rate.
Level of evidence IVLevel of evidence IV.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Aguado-Maestro I, Cebrián Rodríguez E, Paredes Herrero E, Brunie Vegas F, Oñate Miranda M, Fernández García N, et al. Resultados a medio plazo de la artroplastia total de cadera metal-metal con cabeza de gran tamaño Magnum. Rev Esp Cir Ortop Traumatol. 2018;62:310–317.