Osteoarticular tuberculosis, caused by a member of the Mycobacterium genus, represents approximately 10% of the total extrapulmonary tuberculosis in pediatric patients. Its low prevalence and nonspecific clinical presentation lead to a late diagnosis and elevated risk of sequelae.
Patients and methodsThis retrospective study included seven pediatric patients with non-vertebral osteoarticular tuberculosis diagnosed between 2006 and 2019. The patients were classified in accordance with the radiographic criteria of Kerri and Martini.
ResultsThe mean patient age was 7,4 years (median, 5 years; range, 2–16 years). The mean follow-up time was 18,5 months (range, 10–32 months). The mean diagnostic delay was 4,7 months (range, 1–8 months). The locations were femoral head osteoarthritis (two patients) and proximal humerus osteomyelitis, talus dome osteoarthritis, distal clavicle osteoarthritis, proximal ulna epiphysis osteoarthritis, and tibiotalar arthritis along with subtalar gland (one patient each). The clinical findings were lameness (four patients), localized pain (two patients), functional impotence, constitutional syndrome (asthenia, anorexia, and involuntary loss of >5% of total body weight) (two patients), local inflammatory signs (one patient), and fever (one patient). One patient was asymptomatic and received a diagnosis during pulmonary radiological analysis. Medical treatment with four drugs was performed in all patients; five patients required surgical treatment for abscess drainage, three of them open drainage, and two with laparoscopic drainage.
ConclusionsThe final results were satisfactory, such that 71% of patients recovered joint balance but with radiological sequelae in 57,1% patients. Good prognosis, according to our results, depends on younger age and early diagnosis with early medical or surgical treatments.
La tuberculosis (TBC) osteoarticular, causada por una bacteria del género Mycobacterium, representa alrededor de un 10% del total de TBC extrapulmonares en la edad pediátrica. Su baja prevalencia y su presentación clínica inespecífica conducen a un diagnóstico tardío y, con esto, a un mayor riesgo de secuelas.
Pacientes y métodoPresentamos una serie de siete casos pediátricos de TBC osteoarticular no vertebral diagnosticados entre el 2006 y el 2019, con una media de edad de 7,4 años y unamediana de cinco (rango de dos a 16 años).
El tiempo medio de seguimiento fue de 18,5 meses (rango 10 a 32 meses).
Los pacientes se clasificaron según los criterios radiográficos de Kerri y Martini.
ResultadosEl retraso diagnóstico fue constante, con una media de 4,7 meses (rango de uno a ocho meses).
La localización fue: cabeza femoral (dos casos), húmero proximal, cúpula astragalina, osteoartritis de clavícula distal, epífisis proximal de cúbito, y articulaciones tibioastragalina junto con subastragalina. Los hallazgos clínicos fueron cojera (cuatro casos), dolor localizado (dos), impotencia funcional (dos), síndrome constitucional (astenia, anorexia y pérdida invo-luntaria de más del 5% del peso corporal total) (dos), signos inflamatorios locales (uno), y fiebre (uno). Un paciente fue asintomático, realizando el diagnóstico osteoarticular de manera casual en el estudio radiológico pulmonar.
Se administró un tratamiento médico de inducción con tres o cuatro fármacos en todos los casos. En tres de ellos, fue necesario el desbridamiento quirúrgico, y, en dos se realizó un lavado vía artroscópica.
ConclusionesLos resultados finales fueron aceptables, con recuperación del balance articular en el 71% de los pacientes, pero con cambios radiográficos residuales en cuatro de los siete casos (57,1%). El mejor pronóstico parece correlacionarse con la menor edad, así como con el diagnóstico y el tratamiento médico-quirúrgico precoces.
Osteoarticular tuberculosis (TBC) is a disease that was recognised as such in ancient times and which has been detected in Egyptian mummies.1–3 Diagnosis is usually delayed due to low suspicion because of its low prevalence and non specific medical presentation.
At present TBC poses a major public health problem throughout the world. Its prevalence is associated with greater poverty, social disorganisation and infection by the human immunodeficiency virus (HIV). The World Health Organisation (WHO) has pinpointed the three countries where the incidence rate is highest: India, Indonesia and Bangladesh.4 In paediatric age, the regions with the highest number of cases are these three, followed by the Sub-Saharan countries.5,6
In Spain, in the year 2015, the incidence of TBC was 10.6 cases per 100,000 inhabitants. Out of a total of 4916 cases, 3946 corresponded to pulmonary TBC (80.3%), 60 to meningitis TBC (1.2%) and 910 to TBC in other localities (18.5%). According to WHO data from 2015, the mortality of this pathology worldwide was 17%.
A child infected with TBC runs a higher risk of developing the disease than adults and it is more commonly extrapulmonary or disseminated, particularly in the under fives. In Spain where the rate of TBC is low, the contact studies for diagnosis of a bacilliferous adult mean that the latent infections may be treated or the pulmonary forms of the disease may be identified early.
Of all the extrapulmonary TBC, 5.9% occurred in patients under or up to 15 years of age, with a distribution somewhat higher in the group aged from five to 15 than that aged from zero to four years of age. In cases of pulmonary TBC, distribution was similar to the two age groups.
TBC is an infectious disease caused by the Mycobacterium tuberculosis complex. Some of the subspecies, such as Mycobacterium bovis, Mycobacterium africanum, Mycobacterium canetti and Mycobacterium microti, cause this infection and do so generally in patients with associated pathologies.
M. tuberculosis is the most common origin of osteomyelitis by micobacteria.7 Osteoarticular TBC represents from 3% to 5% of all cases of extrapulmonary TBC.8 Due to its insidious medical symptoms and atypical location, osteoarticular TBC is a pathology which may frequently go unnoticed.9,10
The pathogenesis of osteoarticular TBC is haematogen dissemination. The bacillus is present in the bloodstream, may colonise the bone when the circulation slows down in the metaphyseal blood vessels. Bone lesion by TBC in children are usually located in the metaphysis. Occasionally, in infants under two years of age, where the transphyseal vessels are open,11 the lesion may cross over the physeal area and affect the epiphysis. More infrequently, this may be primary osteomyelitis of the epiphysis10 through the synovial vascularisation or the epiphyseal haematogen. In other words, the disease may start in the bone (epiphysis or metaphysic) or in the synovial membrane, and may quickly spread from one to the other.5
The most commonly affected locations are the spine (51%), osteoarthritis of the hip and the femur (10%), osteoarthritis of the knee and the tibia (10%), ribs (7%) and arthritis of the elbow (5%).12,13 Lower recurrence may be seen to affect small joints such as the acromioclavicular joint or the tarsometatarsal joint.14 Clinical symptoms are usually sub-acute, with insidious pain and swelling of soft tissues. General signs and symptoms are uncommon.14,15
Evolution is usually gradual, leading to delayed diagnosis, and possible irreversible osteoarticular destruction. As with any osteomyelitis, in the cases where response to medical treatment is partial, it will be necessary to rule out the presence of an osseous, articular or muscle abscess which requires draining.14
In pulmonary TBC, although leukocytosis is common and elevation of reactive C protein (RCP) in diagnosis, procalcitonin usually remains within normal limits.
Suspected diagnosis includes suggestive clinical presentation, a background of contact with a bacillipherous patient or staying in an endemic region, a immunodiagnostic test (cutaneous test of the purified protein derivative [PPD] or trial of release from positive interferon gamma [IGRA]) and findings which are compatible with the imaging techniques: radiography (Rx), computerised tomography (CT) or magnetic resonance (MR).
MR is the test of choice for suspected osseous or muscular abscess or when response to treatment is partial.16 Differential diagnosis must be made with sub-acute osteomyelitis, chronic bacterial osteomyelitis, simple cyst, aneurismal bone cyst, cartilaginous tumours, granulomatous legions, haematological diseases and some malignant tumours.17 If there are doubts, prior percutanous biopsy to possible surgery is obligatory.
Definitive diagnosis of extrapulmonary TBC requires microbiological confirmation by culture or by molecular techniques of the biopsy of affected tissue (granulomas, synovial, bone, lymph nodes, ulcer margins or fistulas) or other samples, usually respiratory (sputum and gastric juices).5
Osteoarticular TBC requires longer treatment than pulmonary TBC.18 In the majority of patients, this consists of an induction phase of two months which combines three to four first line oral antituberculosis drugs (antiTBC) (isoniazide [H], rifampicin [R], pyrazinamide [Z] and ethambutol [E]) and a maintenance phase with H plus usually R for a minimum of seven more months. This regime is successful in 95% of children with this pathology.5,19
The good prognosis of these lesions depends on the age of the patient, and is better in early ages and in early medical-surgical diagnosis and treatment.17
We present and discuss a case series of skeletal non axial osteoarticular TBC in children and teenagers.
Clinical casesIn this study we present a series of seven paediatric osteoarticular TBC cases of the locomotor system (Table 1) attended between 2006 and 2019 in two hospitals, a monographic paediatric tertiary centre and a pathology unit for children in a regional hospital centre.
Series of seven paediatric cases of osteoarticular TBC of the locomotive system.
| Age (years) | Sex | Side | Pulmonary TBC | Main clinical findings | Location and type of lesion | Duration of symptoms prior to diagnosis | Other lesions | Kerri y Martini classification (Rx), and other tests | Laboratory findings (ESR, CRP, PCT) at onset | Microbiological confirmation | Follow-up (months) | Normal IV and oral antibiotic treatment | Surgery | Residual pain | Final joint balance | Joint deformity | Sequelae in Xr | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Induction | Maintenance | ||||||||||||||||||
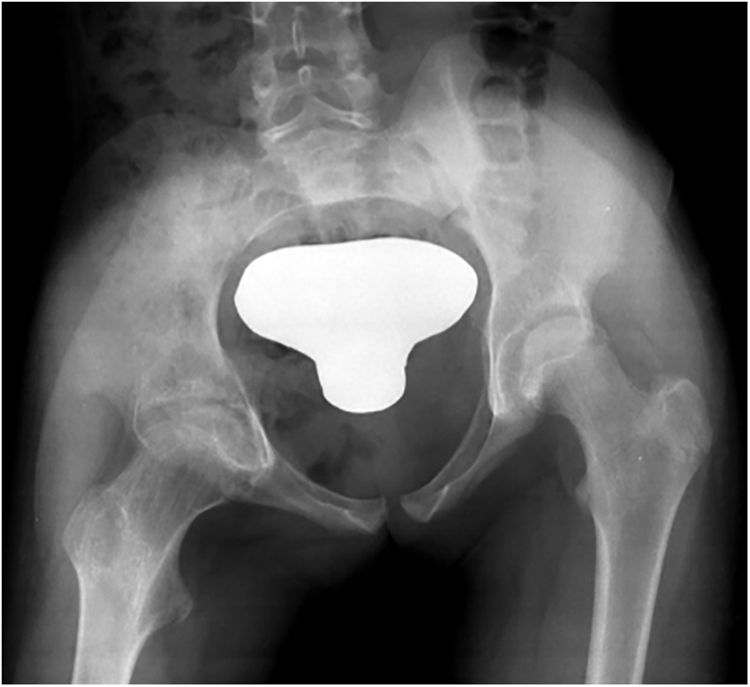
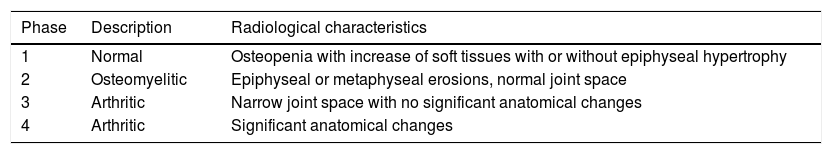
| 1 | 10 | M | R | Yes | Limp, asthenia and anorexia | Proximal femoral hip (epiphyseal involvement) | 8 months | Right Sacroilleitis associated with iliac involvement | Xr, CT and MR | Data unavailable due to onset in foreign hospital | Yes | 10 | INH+RFP+PRZ+ETH | INH+RFP | 2 surgical debridements | Yes | 40° hip flexion, dysmetria | Hip (proximal epiphyseal femoral involvement) | Joint surface irregularity |
| Grade 1 (Fig. 1) | 3 months | 10 months | Culture + | Limp | |||||||||||||||
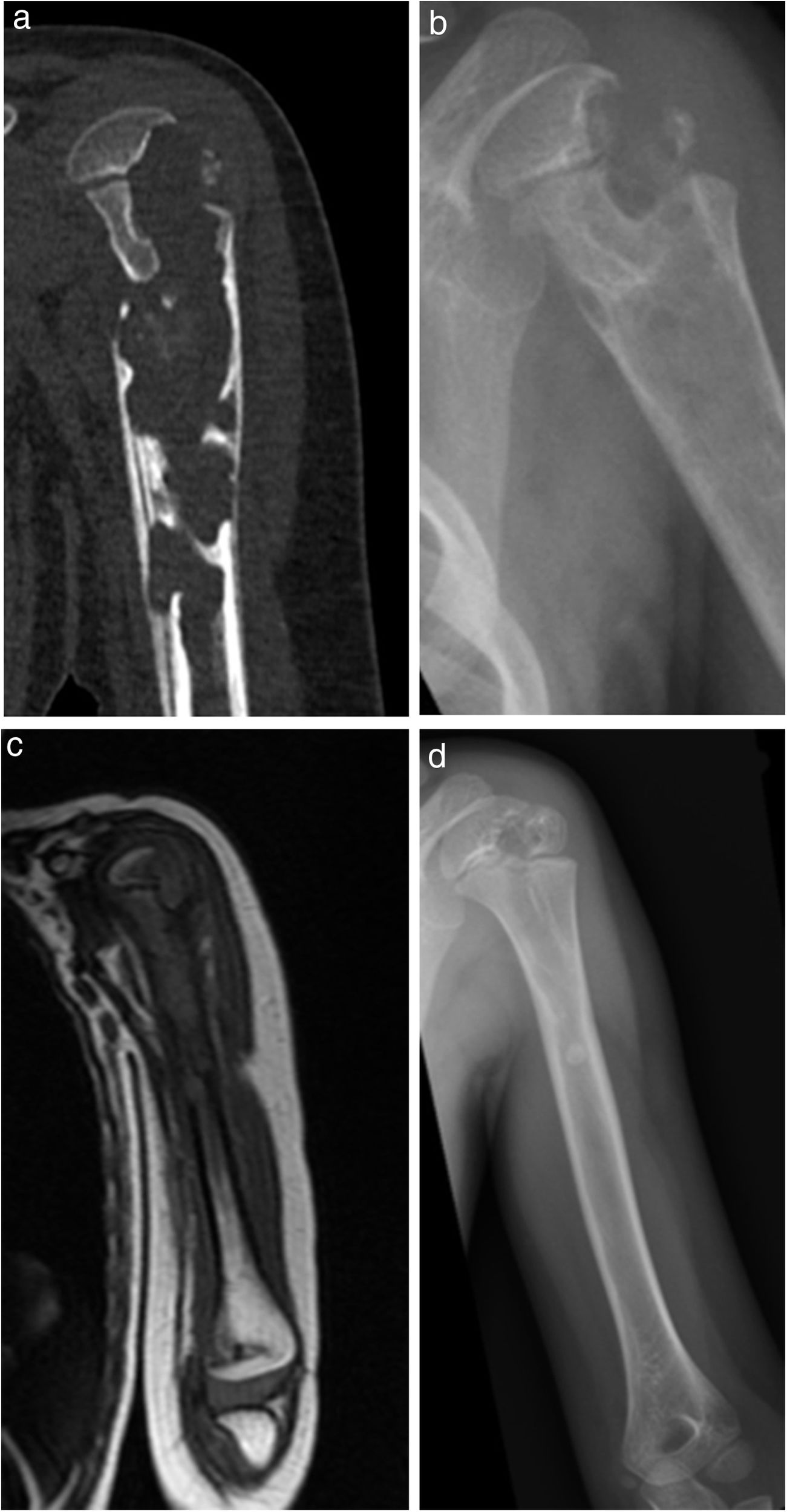
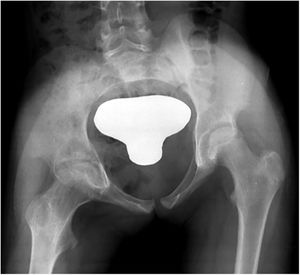
| 2 | 3 | F | L | Yes. Residual pulmonary infiltration | Casual finding in CT of lung | Proximal humerus (osteoarthritis of the shoulder with proximal epiphyseal involvement humerus) | 8 months | No | Rx, MR y TC | Haemogram, normal ESR and CRP PCT not requested | Yes | 32 | INH+RFP+PRZ+ETH | INH+RFP | 2 surgical debridements | No | Complete | No | Slight epiphyseal defect; correct joint surface |
| Grade 2 (Fig. 2) | 6,5 months | 5,5 months | Culture + | ||||||||||||||||
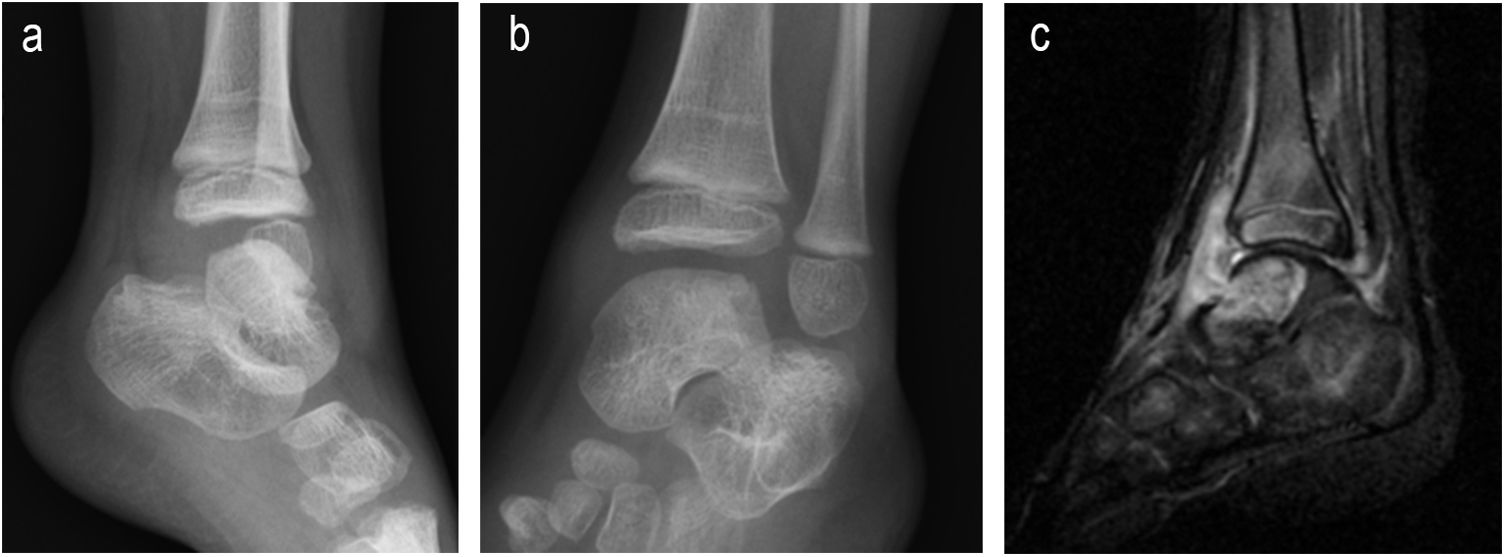
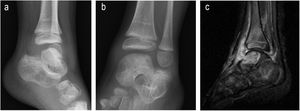
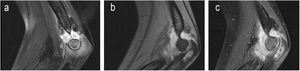
| 3 | 2 | F | L | No | Limp | Astragalar dome, osteoarthritis | 1 month | No | Xr and 2 MR of ankle | Normal Haemogram, ESR and CRP | Yes | 13 | INH+RFP+PRZ+ETH | INH+RFP | 2 arthroscopies for obtainment of synovial and bone material, together with lavage | No | Complete | No | No |
| Grade 1 (Fig. 3) | PCT not requested | 3,5 months | 9,5 months | ||||||||||||||||
| 4 | 2 | M | L | No | Limp | Coxarthritis | 5 months | No | Xr and hip ultrasound | Leukocytes 15,500, lymphocytosis 8800 Mil/mmcc, ESR 34mm, CRP 21mg/L (almost N) | Yes | 27 | INH+RFP+PRZ+ETH | INH+RFP | Arthroscopic lavage and bone biopsy | No | Complete | Minimum flattening | Slight widening of neck and flattening of femoral head. Minimum changes |
| Grade 1 | 2 months | 7 months | |||||||||||||||||
| 5 | 16 | F | L | Yes | Shoulder pain | Distal acromioclavicular third | 2 months | Pulmonary involvement | Rx. Grade 2 | Normal leukocytes | Yes | 26 | RFP+PRZ+ETH, resistance to INH | RFP+ETH | Surgical debridement of abscesses and distal clavicle curettage | No | Complete | No | Distal extremity resection |
| Clavicular (osteoarthritis with distal clavicular epiphyseal involvement) | CRP 27mg/L | 6 months | 6 months | Culture + | Clavicle | ||||||||||||||
| ESR 64mm | |||||||||||||||||||
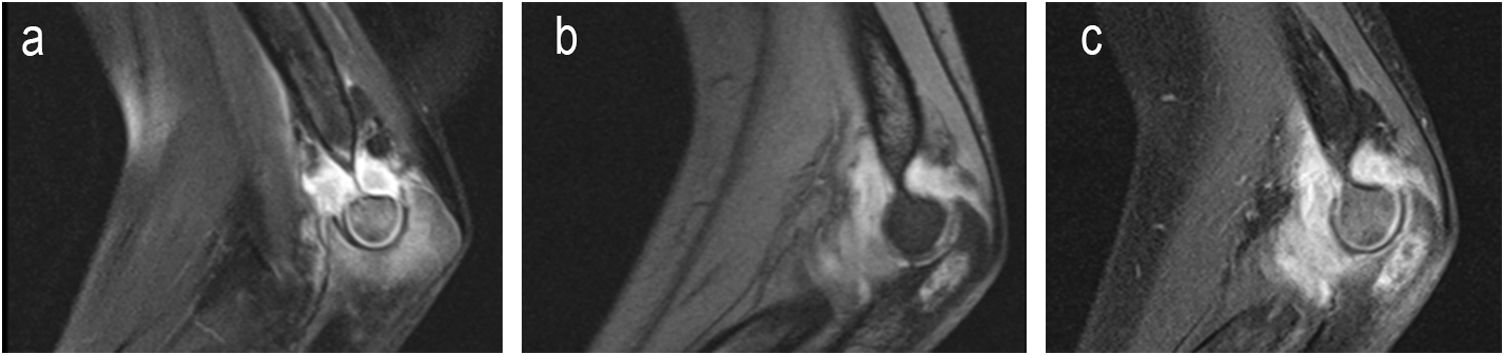
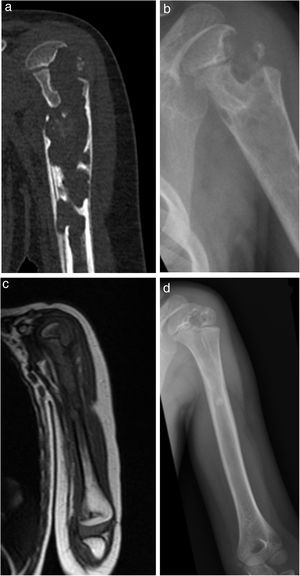
| 6 | 14 | F | L | Yes | Elbow pain and impaired mobility | Elbow (osteoarthritis with epiphyseal ulna abscess) | 3 months | Pulmonary involvement | Grade 1 (Fig. 4) | Normal leukocytes. | Yes | Loss of follow-up at 10 months | RFP+INH+PRZ+ETH | INH+RFP | Open synovial and bone biopsy | No | Limited joint balance: flexion 90°, extension −70°. | Loss of follow-up | Loss of follow-up |
| Prior trauma | Normal CRP | 2,5 months | 6,5 months | Normal pronosupination | |||||||||||||||
| ESR 113 | Corticoids. | ||||||||||||||||||
| 7 | 5 | M | R | Yes | Limp, swelling and ankle pain | Ankle (arthritis) | 6 months | Pulmonary involvement | Grade 1 | ESR 66mm. | Yes | 12 | RFP+INH+PRZ+ETH | INH+RFP | Ultrasound-guided joint puncture. | No | Correct, functionality complete | No | No |
| Constitutional syndrome, inguinala adenopathy and fever | CRP 69,2mg/L | 2,5 months | 10 months | Culture − | |||||||||||||||
| PCT 0,3 | |||||||||||||||||||
| Normal leukocytes | |||||||||||||||||||
CRP: C-reactive protein; ESR: erythroctye sedimentation rate; ETH: ethambutol; INH: isoniazid; MR: magnetic resonance; PCT: procalcitonin; PRZ: pyrazinamide; RFP: rifampicin; TBC: tuberculosis; TC: computerised tomography; Xr: X-rays.
Patients were classified according to the Kerri and Martini20 radiographic criteria (Table 2). Epidemiological, clinical, microbiological and therapeutic variables were collected. Cases were described which required surgery and final patient assessment.
Kerri and Martini radiographic criteria.
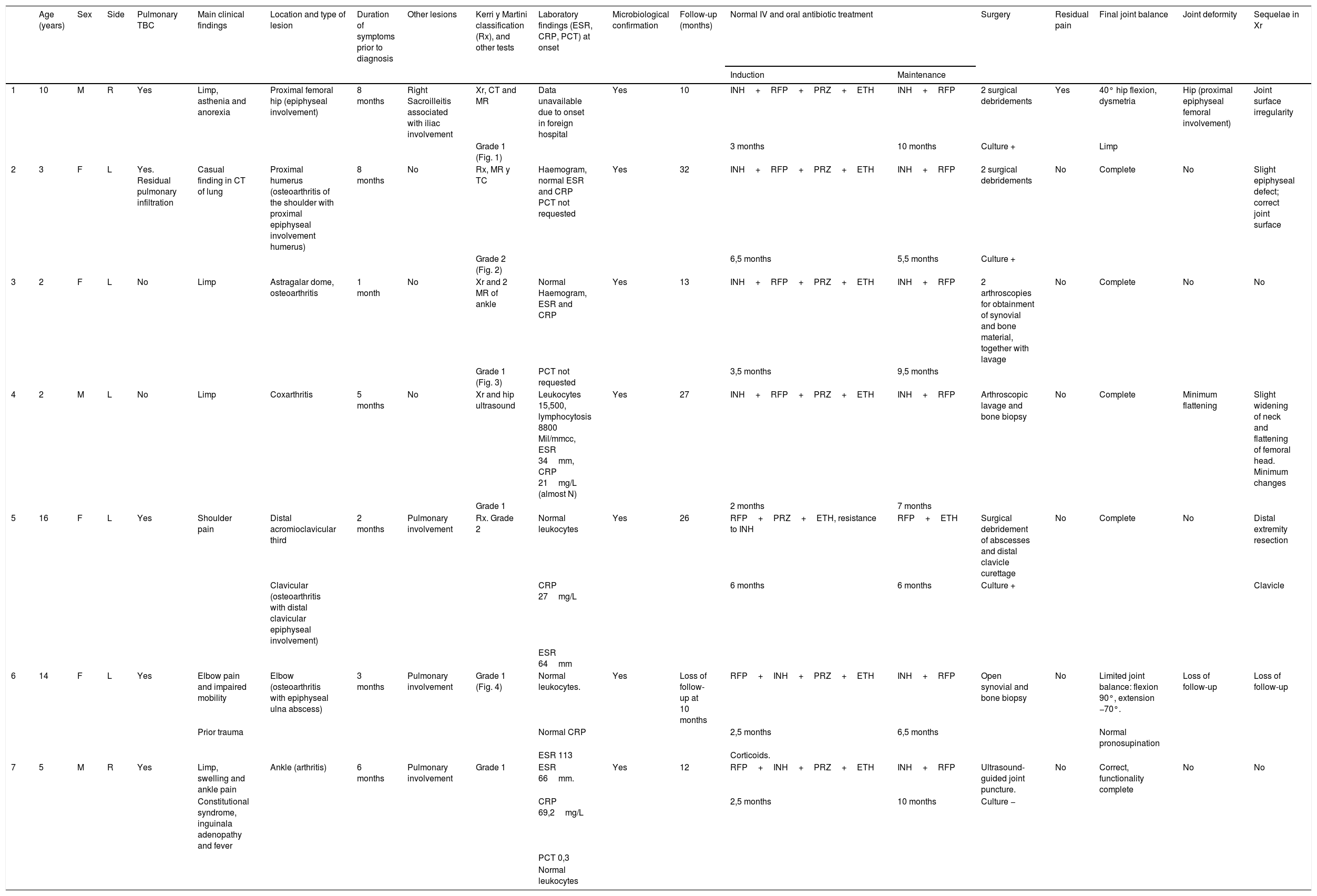
| Phase | Description | Radiological characteristics |
|---|---|---|
| 1 | Normal | Osteopenia with increase of soft tissues with or without epiphyseal hypertrophy |
| 2 | Osteomyelitic | Epiphyseal or metaphyseal erosions, normal joint space |
| 3 | Arthritic | Narrow joint space with no significant anatomical changes |
| 4 | Arthritic | Significant anatomical changes |
Source: Kerri and Martini.19
We present three men and four women. Their ages stretched from two to 16 years (mean 7.42 years). The affected side was the right side in two cases, and the left in five.
Diagnostic delays was constant, with a mean of 4.7 months (range of one to eight months).
Clinical findings were those of a limp (four cases), localized pain (two), functional impotence (two), constitutional syndrome (two), signs of local inflammation (one) and fever (one). We also present an asymptomatic patient whose diagnosis of osteoarticular compromise was made coincidentally in a pulmonary study (Figs. 1–4).
Radiographic studies were made with plain radiography, ultrasound scan and/or MR. These initially served for diagnostic orientation, and later for the evolution and evaluation of sequelae (Figs. 1–4).
All patients were treated with tuberculostatic agents for a minimum of nine months, three or four drugs (R, H, Z and/or E) during the induction phase and two during the maintenance phase.
For three of them (cases one, two and five) a surgical debridement was made due to the presence of many periarticular abscesses and/or severe bone compromise in the initial MR. With this, in our series, open surgery for debridement was necessary in 42% of patients (three out of seven). The obtainment of material and lavage was arthroscopically performed in two of the remaining four people who did not undergo surgery (cases three and four). The other two patients (cases six and seven) were studied using open biopsy (case sixe) and ultrasound-guided biopsy (case seven), without the need for open or arthroscopic surgical cleaning.
Open surgical review, due to slow clinical evolution in the days immediately after the first surgery, was necessary in two patients (cases one and two). And arthroscopic surgical review for the same reason was performed in one (case three). Thus, this type of review was performed in three people (cases one, two and three), i.e. in 42%.
Mean follow-up time was 18.5 months (range 10–32 months).
Results were globally satisfactory, with definitive resolution of infection and recuperation of joint balance in all patients except in cases one and six, with ankylosis of the hip (case one) and compromise of the elbow joint (case six, with no follow-up until discharge due to change of residence of the person to another country). There were residual radiographic variances in four out of seven patients (57.1%).
DiscussionThe most normal presentation, in five out of seven patients, was osteoarthritis with adjacent epiphyseal involvement. As reported by Agarwal et al.,17 cases of osteomyelitis from TBC could more frequently impact the epiphysis. This is due to the fact that the microbacteria cause synovial damage, from where they colonise the joint and epiphysis, although this may also be related to the haematogenous spread directly by the epiphyseal blood vessels.
Extrapulmonary infection from TBC in children is more common than in adults.21 Mostly, the first infection is respiratory and it is normal to find concomitant pulmonary disease, although this may not be symptomatic.22 In our series, five patients had this involvement. In absence of microbiological confirmation, pulmonary damage reinforces TBC diagnosis. In the event of a case of extra pulmonary TBC, it is always necessary to rule it out.
Damage to the joints is usually to a single joint and frequently occurs as a result of a metaphyseal osteomyelitis which crosses over the epiphysis until it reaches the joint.23 The five cases we present were patients with single joint involvement.
Septic arthritis of acromioclavicular location is exceptional. This is associated with patients with risk factors such as HIV-associated immunosuppression, diabetes or immunosuppressant therapy,24 abuse of intravenous substances, a background of prior trauma or acromioclavicular surgery, or local injections.25 Its principal germ involved is Staphylococcus aureus.26,27 In the case of osteoarthritis from TBC, this is a less exceptional location than the other infectious cases of arthritis.
Four of the seven cases were immigrants or families of immigrants, who had come from another country such as Morocco or Pakistan. This is characteristic of paediatric TBC in our environment and is a detail which could be useful in the early diagnosis of the patient with a slowly evolving osteoarticular infection.
The classification used in this study was that of Kerri and Martini.20 This was applied in the TBC of the knee, and later was used for the elbow by Dix et al.28 (Table 2). It is of note that there are certain limitations to this classification of different joints.
As indicated by the literature, in our series there was also a delay in the diagnosis with a mean of 18.8 weeks, higher than the 10 weeks reported by Dix et al.28 In two of the cases, symptomology was attributed initially to a trauma.
In all of them, tissue and a culture was taken, which led to the diagnosis of M. tuberculosis except in one associated with pulmonary TBC where this bacteria was not identified in the joint biopsy.
Analysing the possible correlation between the greater initial radiographic stage and the poorest final evolution, Dix et al.28 published their series of 10 patients in which there appears to be a relationship between the two. However, Shanmugasundaram was the first to hypothesize about radiological stages at the time of diagnosis with a final prognostic, which was good for Grades one and two (osteopenia with or without erosions), on having only one synovial compromise and bad for grades three and four (compromise of the joint space).29 However, on the contrary, in our series, there does not appear to be a relationship between the initial grade of Kerri and Martini and final clinic radiological evolution. The two patients with functional sequelae (cases one and six) presented with an initial grade one stage, whilst the two patients with grade two at the beginning (cases two and five) presented with complete mobility.
If we analyse time of onset to final clinical diagnosis we see that the only two patients with final functional limitation (cases one and six) presented with a long evolution time prior to diagnosis, 32 and 12 weeks, respectively. In contrast, the only two cases with a shorter time of symptom onset prior to diagnosis (cases three, four weeks and case five, eight weeks), presented with a perfect clinical course. Although it is not conclusive due to the limited number of patients in the series and to the heterogeneity of the sample in location and in age, the data seem to suggest there is a relationship between the higher period of evolution prior to the beginning of treatment and a greater risk of functional sequelae.
On assessment of patient age and final functional results, we observed that the four cases of up to five years of age presented with final complete joint mobility and with no or minimal final radiological compromise. Only one patent who was older than this presented with very good final functionality but had undergone distal clavicular resection.
There are very few publications of TBC osteoarticular infection cases. Aggarwal presented his series of 47 cases, a large patient number but not paediatric in age. They were mostly adults up to 30 years of age, with48 people presented with arthritis of the elbow from TBC.10 Mittal published two cases of acromioclavicular location.9 Filon presented a patient with miliary pulmonary compromise with gradual evolution and posterior acromioclavicular arthritis from the same germ.14 Dix worked in a paediatric series of elbow compromise with 10 people.28 This research study is therefore the second in number of paediatric patients.
Despite being a rare pathology, and even more so in our environment, osteoarticular infection from TBC is an existing condition and we should therefore include it when reaching a diagnosis. Suspected diagnosis will be essential for early treatment and therefore for a better functional result. Septic arthritis from M. tuberculosis should be included in the differential diagnosis in cases of joint pain with sub-acute evolution.
Due to all of the above, we may conclude:
- 1.
Osteoarticular TBC should be suspected in the event of any patient with clinic radiological compromise or a joint suggestive of infection, and especially when the evolution is sub-acute and/or the patient comes from countries with a high prevalence of TBC.
- 2.
Open surgical debridement is necessary when there are several bone or periarticular abscesses. In patients with lower initial radiological compromise, the arthroscopic technique for lavage and taking samples seems to lead to good results.
- 3.
Our results do not back up the findings from Kerri and Martini which relate the initial radiological stage with final clinical evolution. Future studies will be required to provide scientific evidence on this hypothesis.
- 4.
There seems to be a relationship between two factors, an early age (up to five years) and the longer time of evolution to diagnosis (12 weeks or more), with a higher risk of functional sequelae.
The authors have no conflict of interests to declare.
Level of evidenceLevel of evidence IV.
Please cite this article as: Pérez-López LM, Subirá-Álvarez T, Martínez-Ruíz A, Noguera-Julian A, Moreno-Romo D, Torner-Rubies F, et al. La tuberculosis osteoarticular no axial en la edad pediátrica. Rev Esp Cir Ortop Traumatol. 2021;65:186–194.