In recent years cross-linked polyethylenes have been developed in an attempt to reduce the wear, as has been demonstrated in knee simulators. The aim is to assess, by counting particles of polyethylene in synovial fluid, whether the reduction in wear is confirmed in patients with a highly crosslinked polyethylene prosthesis.
Material and methodsA prospective randomised study was designed. During the implantation of a knee prosthesis, one group of patients was assigned the use of a conventional polyethylene (group A), and the other group a highly crosslinked polyethylene (X3®, Stryker Orthopaedics) (group B). At 12 months after surgery a knee arthrocentesis was performed, and the number of polyethylene particles was counted in a scanning electron microscopy. Fourteen samples from each group were studied.
ResultsBoth groups were comparable in all study variables. We found no significant differences in the concentration of polyethylene particles/ml (1.49±0.85 million in group A vs 1.42±0.91 million in group B, P=0.60) or the total number of isolated particles. We found no differences either in size or morphology of particles between both groups.
Discussion and conclusionsAlthough several in vitro studies in vitro using different types of highly crosslinked polyethylene found a significant reduction, we did not find that that wear was reduced in the knees of these patients. The great variability in the number of particles between individuals suggests that polyethylene wear in vivo depends on many factors, so perhaps the type of polyethylene is not the most significant factor.
En los últimos años han aparecido polietilenos altamente entrecruzados para intentar disminuir el desgaste, tal como se ha demostrado en simuladores de rodilla. El objetivo es evaluar mediante el recuento de partículas de polietileno en líquido sinovial, si se confirma un menor desgaste en pacientes con prótesis de polietileno altamente entrecruzado.
Material y métodoEstudio prospectivo aleatorizado, en el que durante la implantación de una prótesis de rodilla se asignó a un grupo de pacientes la colocación de un polietileno convencional (grupo A), y a otro grupo un polietileno altamente entrecruzado (X3® de Stryker Orthopaedics) (grupo B). A los 12 meses tras la cirugía se practicó una artrocentesis de rodilla y se hizo un recuento de partículas de polietileno en el líquido sinovial mediante microscopio electrónico de barrido. Se han analizado 14 muestras en cada grupo.
ResultadosAmbos grupos son comparables en todas las variables estudiadas. No hemos hallado diferencias significativas en la concentración de partículas de polietileno/ml (1,49±0,85 millones grupo A vs. 1,42±0,91 millones grupo B; p=0,60) ni en el número total de partículas aisladas entre ambos grupos. Tampoco hemos hallado diferencias en el tamaño ni la morfología de partículas entre ambos grupos.
Discusión y conclusionesAunque diversos trabajos in vitro han hallado una reducción muy significativa del desgaste del polietileno altamente entrecruzado, no se ha hallado que en las prótesis de rodilla implantadas en pacientes este desgaste se vea reducido. La gran variabilidad del número de partículas entre individuos sugiere que el desgaste de polietileno in vivo depende de muchos factores y probablemente el tipo de polietileno no sea el más determinante.
Osteolysis is one of the main causes of failure in total knee arthroplasty. Consequently, osteolysis secondary to polyethylene wear and aseptic loosening is the principle cause of revision starting from 5 years after the index operation.1,2
In knee arthroplasty, the chief wear mechanism is fatigue. It produces larger particles than those produced in hip arthroplasty, in which wear occurs from abrasion and adhesion.3 The size of the particles is important, given that the smaller ones have greater biological activity and producer greater osteolysis.3 Particles >10μm are not very pro-inflammatory because they are not susceptible to phagocytosis.4
Osteolysis is the result of a foreign-body reaction, induced by the particles generated by polyethylene wear.5 These particles are phagocytosed by macrophages and giant cells, which are activated and liberate cytokines. Interleukins IL-1β, IL-6 and tumour necrosis factor alpha produce inflammation and stimulate the osteoclast; the activated osteoclast is the cell responsible for the osteolysis6–8; however, the cytokines also inhibit bone formation by the osteoblasts.9
To decrease polyethylene particle release, the industry has introduced different improvements over the last decades: improving the polyethylene sterilisation processes, using gamma radiation in an oxygen-free medium,10 introducing new friction pairs and developing highly cross-linked polyethylene (XLPE).11,12 Among these XLPE is the X3® (Stryker Orthopaedics, Mahwah, NJ).
The XLPE have been used for over 10 years in total hip arthroplasty, where they have shown better in vivo wear results than conventional polyethylene.13–15 However, the studies carried on knee arthroplasty, and in those based on current use in this joint, are in vitro studies on a simulator.16,17 In one study, Wang6 found a reduction in polyethylene wear of 68% and 64% in standard arthroplasties with posterior cruciate ligament retention (CR) and posterior-stabilised (PS) total knee prosthesis (TKP) respectively, comparing polyethylene X3® to conventional polyethylene.1 However, we know that simulator and in vitro studies do not always faithfully reproduce the conditions found in clinical practice.
The hypothesis of this study was that using XLPE X3® would reduce the number of polyethylene particles from wear, compared to conventional polyethylene conventional in an in vivo context. The main study objective was to compare the number of polyethylene particles isolated in synovial fluid following primary total knee arthroplasty, as based on using a conventional or XLPE polyethylene. The secondary objective was comparing particle size and morphology in both groups.
Material and methodsWe designed a random, prospective study. The study protocol was approved by the local Ethics Committee. Patients gave informed consent for their inclusion in the study, which covered synovial fluid extraction by arthrocentesis at 12 months.
In all of the cases, the implant was the Triathlon® TKP posterior stabilised (PS) bone cement type (Stryker Orthopaedics, Kalamazoo, MI, USA), with the same surgeon performing all of the operations.
The patients were randomised by intraoperative closed-enveloped assignment, putting 50% of the patients in group A (conventional polyethylene) and 50% in group B (XLPE, or X3®). The conventional polyethylene was high molecular weight polyethylene (GUR 1020) irradiated at 30kGy (gamma irradiation) in nitrogen. The X3® polyethylene was high molecular weight XLPE, submitted to 3 irradiation cycles at 30kGy in air, followed by air annelation at 130°C for 8hours. After the 3 cycles, it was sterilised with gas-plasma.
The criteria for inclusion were the implantation of a primary PS TKP, in patients aged between 65 and 85 years.
The criteria for exclusion were a diagnosis other than primary gonarthrosis (post-traumatic, inflammatory arthropathy), the presence of severe preoperative deformity (>12° of varus or valgus in the tibiofemoral axis), the presence of postoperative varus or valgus alignment >4°, voluntary withdrawal from the study and the impossibility of obtaining 3cc of synovial fluid at the 12-month control visit.
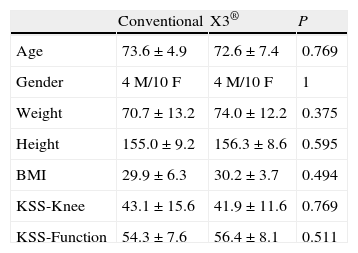
The variables considered in the preoperative assessment were age, sex, weight, height, body-mass index (BMI), tibiofemoral angle (TFA) measured in telemetric radiological projection, and the knee and function values using the Knee Society Score (KSS) scale.18
The mean patient age was 73.1±6.2 years; there were 8 males and 20 females. The preoperative KSS scale score was 42.6±13.6 points in the knee subscale and 55.4±7.8 for function.
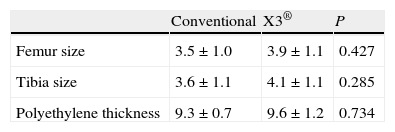
The implant variables considered were size of the femoral and tibial components and polyethylene thickness. The most commonly used femoral and tibial sizes used were 3 and 4. The polyethylene used was the thinnest, 9mm thick in 82% of the cases.
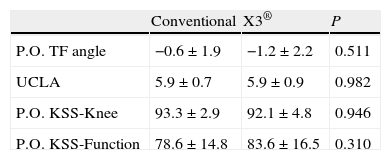
The patients had the normal follow-ups in the postoperative period. In the assessment at 12 months after the operation, the following variables were considered: KSS scale knee and function values, postoperative TFA measured in telemetry radiology projection and the result of the physical activity questionnaire according to the UCLA scale.19 The 12-month KSS scale values were 92.4±3.8 points on the knee subscale and 80.9±16.3 for function. The mean postoperative TFA was 0.9±1.9°. In the UCLA scale, 57% of the patients were in Category 6, 21% in Category 7, 14% in Category in Category 5 and 7% in Category 4.
All of the patients received an arthrocentesis under sterile conditions at the 12-month follow-up, with the same technician having evacuated the maximum amount of synovial fluid. In all cases, the minimum volume was 3ml; the mean volume obtained was 12.4±6.9ml.
Once the synovial fluid samples were identified, they were stored in a sterile tube in a freezer at a −20°C until they were processed in the following weeks. The samples were then processed according to the technique described by Minoda et al.,20 which basically consisted of digesting the sample by sodium hydroxide at 65°C for 12hours to destroy the tissue residues. Next, a continuous sucrose slope was applied in a 14-ml tube and ultracentrifuged at 28,000rpm for 3hours at 4°C. The top layer of the tube was immediately taken and an isopropanol–water slope was applied in a 40-ml tube likewise ultracentrifuged at 28,000rpm for an hour at room temperature to group the particles. Finally, the inter-stage line that was filtered through a 0.1-μm polycarbonate filter was taken, which was where the polyethylene particles were trapped, placing the filter in a Petri dish.
In the scanning electron microscopy service, the filter was mounted on a brass stub with conducting bioadhesive tabs and colloidal silver; this was then covered with a 40-nm gold stub and analysed with the microscope. The microscope used was a Quanta 200 field emission gun (FEG) environmental scanning electron microscope (ESEM) (FEI, Co, USA). Using this instrument, each sample was analysed in 10 random fields at 10,000× by a specialised technician. In cases of doubt as to the particle composition, the sample was analysed with an energy-dispersive microanalysis system (EDAX Genesis and Polaron E5000 cathode sputter diode); this made it possible for us to know whether what we had was really a polyethylene particle or a contaminant particle.
In each high magnification field, the number of particles, their means and their morphology were analysed.
Statistical analysisWe used the statistical package SPSS 15.0 for Windows for the statistical analysis. Quantitative variables were compared with the Mann–Whitney U-test and categorical variables were analysed with the Chi square or Fisher exact test, as applicable. Statistical significance was set at P<0.05.
ResultsBoth groups were comparable in all the preoperative variables, including the anthropometric and radiological variables and the KSS score (Table 1). Likewise, there were no significant differences in implant size or polyethylene thickness (Table 2). The 2 groups were also comparable in the postoperative variables: TFA, UCLA scale and KSS scale score (Table 3).
The volume of the arthrocentesis-obtained synovial fluid was 11.7±7.2ml in group A (conventional polyethylene) and 13.1±5.7ml in group B (X3®polyethylene) (P=0.635).
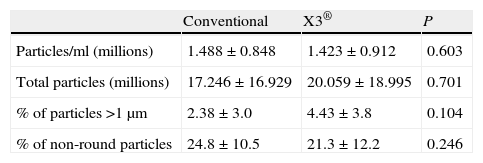
The mean polyethylene particle concentration/ml was 1.46×106±0.88×106. The total number of polyethylene particles per sample was 18.63×106±17.93×106. We found no differences in polyethylene particle concentration/ml or in the total number of particles isolated between the samples in groups A and B (Table 4).
Results of the electronic microscope study on synovial fluid samples.
| Conventional | X3® | P | |
| Particles/ml (millions) | 1.488±0.848 | 1.423±0.912 | 0.603 |
| Total particles (millions) | 17.246±16.929 | 20.059±18.995 | 0.701 |
| % of particles >1μm | 2.38±3.0 | 4.43±3.8 | 0.104 |
| % of non-round particles | 24.8±10.5 | 21.3±12.2 | 0.246 |
The polyethylene particles isolated varied greatly in size, from 0.1μm to 30μm, but 96.57% were equal to or less than 1μm in diameter. Comparing the percent of particles larger than 1μm between both groups revealed a tendency towards a greater percentage of larger particles in group B, although the difference (P=0.104) was not statistically significant (Table 4).
With respect to particle morphology, we found 76.5% of them to be round. There was no difference in the percentage of non-round particles between the 2 groups (Table 4).
DiscussionThe main study finding was the lack of difference in the number of polyethylene particles in synovial fluid between the group with the knee prosthesis made of conventional polyethylene and the group having ones made of XLPE. There are few articles in the literature on the study of polyethylene particles in synovial fluid in patients and, as far as we know, only one compared 2 groups with different types of polyethylene. However, that was a retrospective study, analysing few cases.21
The number of particles isolated in that study (some 18.7 million per sample) compared positively with other series using metal–polyethylene TKP (57 million). However, the number compared negatively with respect to TKP with frictional torque ceramic–polyethylene (7.1 million).22
Various studies have compared polyethylene wear in vitro previously between an XLPE polyethylene and a conventional one in a knee simulator subjected to several million cycles. Poppola23 found a 72–85% wear reduction with NexGen® XLPE (Zimmer, Warsaw, Ind, USA). Other in vitro studies using Stryker's X3® XLPE also found reductions in wear above 60%.16,24
There are hardly any studies analysing XLPE wear in vivo. In one TKP study, the authors found reduced wear analysing the particles in synovial fluid, but these results were based on only 7 cases (3 in 2 group and 4 in the other).21 In contrast, in a study on hip prostheses revised, the XLPE implants extracted showed wear similar to that of the conventional polyethylene.25
In our study, we found a reduction of only 4.37% in particles/ml of synovial fluid, which is not statistically significant. In addition, there was a great variability among individuals, suggesting that in vivo, apart from the type of polyethylene, many other factors affect wear: patient weight and activity, the three-dimensional alignment of the components, the size of the prosthetic components (which determines its surface), ligament tension and many others.
The adverse biological reactions that provoke osteolysis do not depend on only wear factors; they also depend on particle size and concentration.26 In reference to particle size, the first generation of XLPE generated larger particles, which has been associated with increased capability to generate osteolysis.27 Nevertheless, other studies using contemporary XLPE revealed no differences in particle size in comparison to conventional polyethylene,28,29 even if the friction surface was ceramic instead of metal.30 In this study, we did not find any significant differences in particle size between the 2 groups either. However, we did observe that the percentage of particles larger than 1μm tended to be greater in the XLPE group.
Likewise, although there is evidence that elongated particles are more pro-inflammatory than round ones,4 we did not find significant differences in the polyethylene particle morphology between the 2 groups.
There has been speculation that the post in the SP component might be more fragile.31 However, from a clinical point of view, a few studies have shown that using XLPE in knee prostheses is safe in the short and medium term, but that it does not provide any advantages with respect to clinical or radiological results.32,33
Our study has a few limitations, such as the small number of cases and how difficult it is to control some variables that can affect polyethylene wear as much as or more than the type itself (for example, ligament tension, which is impossible to measure in vivo). There is also the fact that we used 0.1-μm pore filters as in the technique described by Minoda20 to process the synovial fluid processing; consequently, the particles smaller than this were not trapped in the filter. However, previous studies have shown that most of the TKP wear particles fall in the range between 0.1 and 1μm34,35 and the particles that provoke the greatest osteolysis are those that measure from 0.3 to 1μm.36 Therefore, the particles not retained by the filter that we used would hardly be causes of osteolysis.
Another limitation in the study is the follow-up length, limited to 12 months. Even though we chose the same figure used in other previous studies that compared different TKP,22,37 we cannot rule out that the lack of differences would be modified as the polyethylene aged, as some in vitro studies have demonstrated.31,38
In conclusion, we can state that no in vivo reduction in the number of polyethylene particles was found at 12 months using XLPE. The great variability observed between individuals suggests that polyethylene wear is multifactorial and that the type of polyethylene is probably not the main factor that affects it.
Level of evidenceLevel of evidence I.
Ethical DisclosuresProtection of human and animal subjects. The authors will declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of Data. The authors declare that no patient data appears in this article.
Right to privacy and informed consent. The authors declare that no patient data appears in this article.
FundingThis study was funded by a fellowship awarded by the SECOT Foundation in the 2009 application process.
Conflict of interestsThe authors have no conflict of interests to declare.
Thanks are expressed to the SECOT Foundation for the 2009 fellowship for the preparation of this study, to Gabriel Gil at the Institut Municipal d’Investigació (IMIM) in Barcelona for his collaboration in the sample processing, and to Sergi Mojal (IMIM) for his collaboration in the statistical study.
Please cite this article as: Hinarejos P, et al. Partículas de polietileno en líquido sinovial tras artroplastia de rodilla con un polietileno convencional o uno altamente entrecruzado. Estudio preliminar. Rev Esp Cir Ortop Traumatol. 2012;56:210-15.