The main complication of percutaneous iliosacral screw fixation is implant malposition, which can lead to vascular and nerve damage. The anatomical variability of the sacrum can make screw insertion difficult under fluoroscopic guidance. Among the methods described to improve the accuracy of this technique, stands out the use of computed tomography (CT). The aim of this study is to compare the results of iliosacral screw insertion with fluoroscopy or CT navigation.
MethodologyRetrospective cohort study of 66 iliosacral screws in 56 patients during 11 years. The screws were inserted with fluoroscopy in the operating room or with CT in the radiodiagnosis area. We collected data on patient characteristics, lesions, treatment, and clinical and radiological results.
ResultsForty-seven screws were inserted with fluoroscopy and 19 with CT. A percentage of 18.2 of screws perforated the S1 osseous corridor. All of them were inserted with fluoroscopy guidance (0 vs. 34%; p<0.01). Those operated with CT accumulated more sacral dysmorphism criteria than those operated with fluoroscopy (2.2 vs. 1.6; p=0.02). The S1 corridor on the axial CT view was narrower in those in whom perforation had occurred (18.8 vs. 21.0mm; p=0.02). Two cases with perforation developed S1 radiculalgia. Two endopelvic screws had to be removed.
ConclusionWe advise the use of CT guidance for iliosacral screw insertion in patients with sacral dysmorphism or narrow S1 corridors in facilities where other navigation methods are not available.
La principal complicación de la osteosíntesis percutánea con tornillos iliosacros es la malposición del implante que puede ocasionar lesiones vasculares y nerviosas. La variabilidad anatómica del sacro llega a dificultar la inserción del tornillo bajo control fluoroscópico. De entre los métodos descritos para mejorar la precisión de esta técnica, destaca el uso de la tomografía computarizada (TC). El objetivo del estudio es comparar los resultados de la implantación de tornillos iliosacros con fluoroscopia y TC.
MetodologíaEstudio de cohortes retrospectivo sobre 66 tornillos iliosacros implantados en 56 pacientes durante 11 años. Estos fueron introducidos con fluoroscopia en el quirófano o con TC en el área de radiodiagnóstico. Recogimos datos sobre las características de los sujetos, sus lesiones, el tratamiento y los resultados clínicos y radiológicos.
ResultadosCuarenta y siete tornillos fueron implantados con fluoroscopia y 19 con TC. El 18,2% de los tornillos perforaba el corredor S1. Todos ellos se intervinieron con fluoroscopia (0 vs. 34%; p < 0,01). Pese a ello, los intervenidos en TC acumulaban más criterios de dismorfismo sacro que los intervenidos con fluoroscopia (2,2 vs. 1,6; p = 0,02). El corredor S1 en la TC axial era más estrecho en aquellos en que se había producido una perforación (18,8 vs. 21,0 mm; p = 0,02). Dos casos con perforación desarrollaron una radiculalgia S1. Fue necesario retirar 2 tornillos endopélvicos.
ConclusiónAconsejamos el uso de la guía por TC para la inserción de tornillos iliosacros en pacientes con sacros displásicos o corredores estrechos en S1 en instalaciones que no dispongan de otros métodos de navegación.
Percutaneous iliosacral screw fixation (ISS) plays an indispensable role in the osteosynthesis of unstable pelvic ring fractures. However, the variability in the morphology of the sacrum can impede interpretation of the radiological anatomy, leading to penetration of the implants into extraosseous anatomical spaces and damage to vascular and nerve structures.1–3
In recent years, various navigation-based technologies have emerged that allow better implantation of ISS in a radiation-free environment. However, these instruments are currently expensive, which hinders their widespread use.4–7
Implantation of CT-guided ISS in the radiodiagnostic area has emerged as an alternative to conventional fluoroscopy and has improved the accuracy of this procedure. It is an effective and safe method that can be used in most hospitals.8–14
The aim of the present study is to compare the results of ISS insertion using as an imaging guide conventional fluoroscopic guidance (FG) in the operating theatre with CT in the radiodiagnostic area. The secondary aim is to describe the technique of ISS fixation with CT.
MethodologyWe obtained the approval of our Clinical Research Ethics Committee – reference PR(ATR)369/2020 – prior to conducting the present retrospective cohort study, and its wording was adapted to the recommendations of the STROBE statement. All the patients underwent surgery at a single public, level III university hospital during the period from September 2010 to September 2021. We included all skeletally mature patients (age 18 years or older) with pelvic ring fractures whose subsequent injury had been stabilized with CT-guided ISS in S1 in the radiodiagnostic area or, alternatively, in the operating theatre with FG and who had undergone a postoperative control CT scan. The use or not of intraoperative CT guidance for ISS placement defined the 2 exposure groups. We excluded lumbopelvic dissociations and cases that had undergone open reduction of the posterior lesion had been performed. A postoperative follow-up period of 1 year was considered.
Data on the patients’ baseline characteristics, injuries, treatment, clinical and radiological findings were collected on paper forms and transcribed into an identity-coded database in Microsoft Excel format. We used RAIM Viewer software (Corporació Sanitària Parc Taulí, Spain) for radiological assessment. The images assessed were plain pelvic radiographs obtained urgently during the initial management of the patient; preoperative CT for ISS fixation, whether obtained during emergency care or at some point before surgery for planning purposes; intraoperative CT, if the ISS were inserted using this imaging method; and/or postoperative control CT, if the ISS were inserted under FG. Each image was evaluated once by one of the 3 designated investigators (CAPC, FBC, and EGA). The systems described by Tile,15 Denis et al.,16 and Day et al.17 were used to classify the lesions.
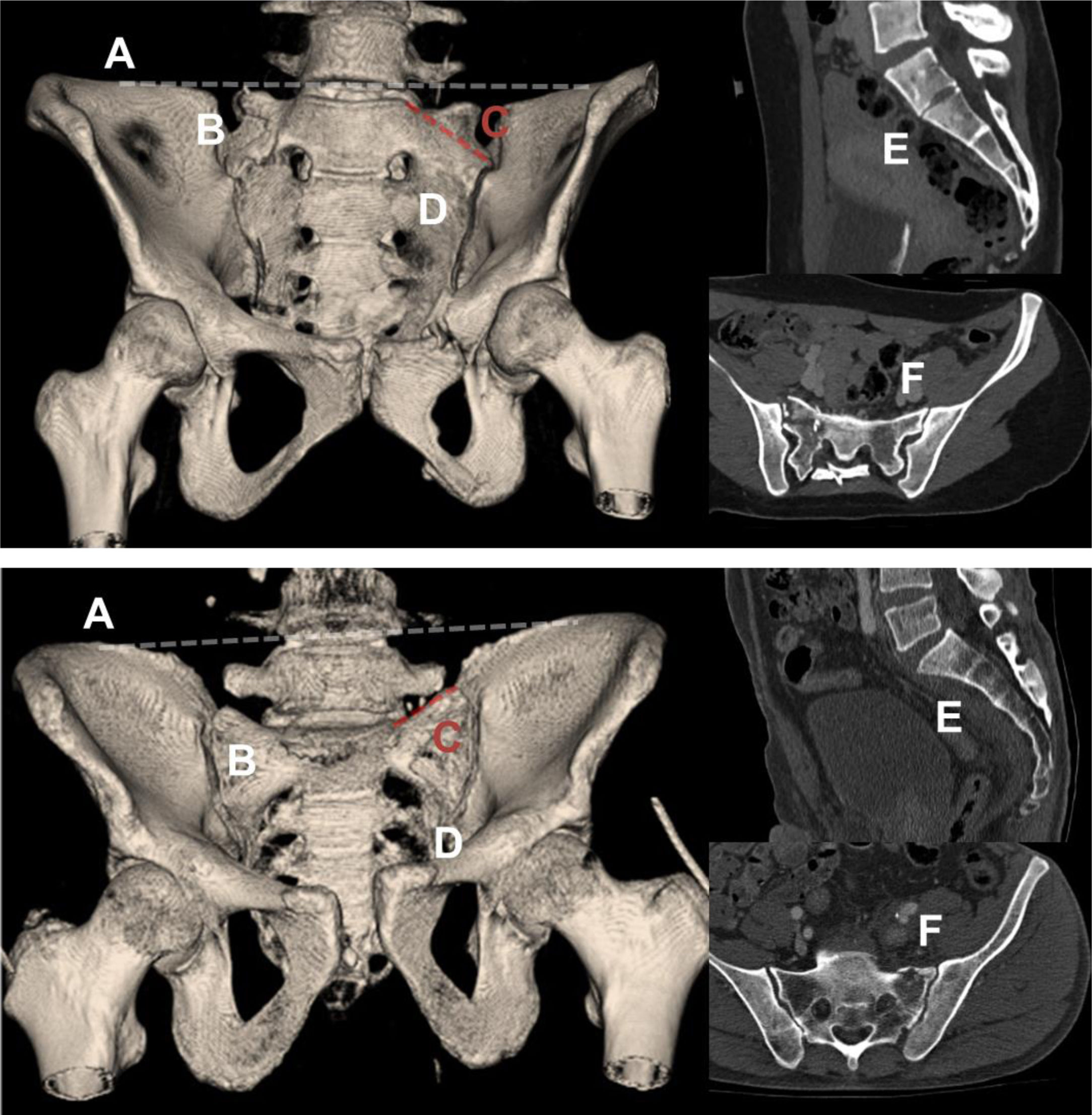
The outlet view on plain radiography and preoperative CT were used to determine the presence of the 6 criteria for sacral dysmorphism (SD)1 (Fig. 1). In addition, the maximum width in millimetres of the S1 corridor was assessed on coronal and axial slices of the preoperative CT scan. The definitive position of the implants was assessed on intraoperative CT or, alternatively, on postoperative CT. Penetration of the anatomical space was determined according to the criteria proposed by Smith et al.4: grade 0, no perforation; grade 1, perforation<2mm; grade 2, perforation 2–4mm; and grade 3, perforation>4mm.
Anatomical criteria for sacral dysmorphism. CT images of a pelvis that meets all the anatomical criteria for sacral dysmorphism (top) and one that meets none (bottom) are shown: (A) Collinearity of the S1 platform with the iliac crest. In the image below, the platform is clearly inferior to the iliac crests. (B) Mammillary tubercles. (C) Acute sacral slope. In the image below, the slope is reversed. (D) Non-spherical and irregular S1 foramen. (E) Persistence of residual intervertebral disc between S1 and S2. (F) Tongue-in-groove sacroiliac joints. In non-dysmorphic pelvis, the profile of the sacroiliac joints in the axial section of the CT scan is regular.
Data analysis was performed using Stata 14.2 software (StataCorp, USA). Continuous variables were expressed using mean and standard deviation. Counts and percentages were used for categorical variables. Because it was not possible to guarantee the normal distribution of quantitative variables, we used the nonparametric Wilcoxon–Mann–Whitney test for the comparison of means. To compare categorical variables, we used Pearson's nonparametric χ2 test. Any bilateral test with a p<.05 result was considered statistically significant.
All patients were attended in our emergency department according to the guidelines of the advanced trauma life support protocol. Both preperitoneal packing and angioembolisation were used for initial management of pelvic haemorrhage. Initial stabilization of the pelvic skeletal injury included the use of pelvic hammocks and external fixators. A bladder catheter was inserted urethrally or, alternatively, through a cystostomy in all patients. The treatment of open fractures included, according to the best evidence, early administration of antibiotics, surgical debridement and irrigation of the wounds, and adequate coverage. Once they had come round, depending on their therapeutic needs, the patients were transferred to an intensive care unit or to the inpatient ward. Surgery for definitive pelvic stabilization was performed when the patients were sufficiently stable, and the procedures were properly planned. In all cases, perioperative antibiotic prophylaxis was administered according to hospital guidelines. A team of surgeons specializing in pelvic trauma were in charge of the definitive treatment of all injuries. The decision to insert the ISS in the operating theatre under FG or in the CT suite was made by the senior co-authors of the paper (JTS and JTH) based on the SD criteria and the morphology of the corridor in S1 on the available imaging tests.
Patients in whom FG was used underwent surgery in a conventional operating theatre under general anaesthesia. Depending on the patient's volume and the fixation needs of the anterior pelvic elements, the patient was placed in supine (usually) or prone position; always on a radiolucent table. The fixation sequence was planned according to the instability pattern of each case. For ISS insertion, we used the technique originally described by Matta and Saucedo, in which visualization of the corridor is possible thanks to a combination of FG in AP, outlet, and inlet views.18 We added the FG view of the sacrum in profile to locate the entry point of the ISS in the iliacus.19
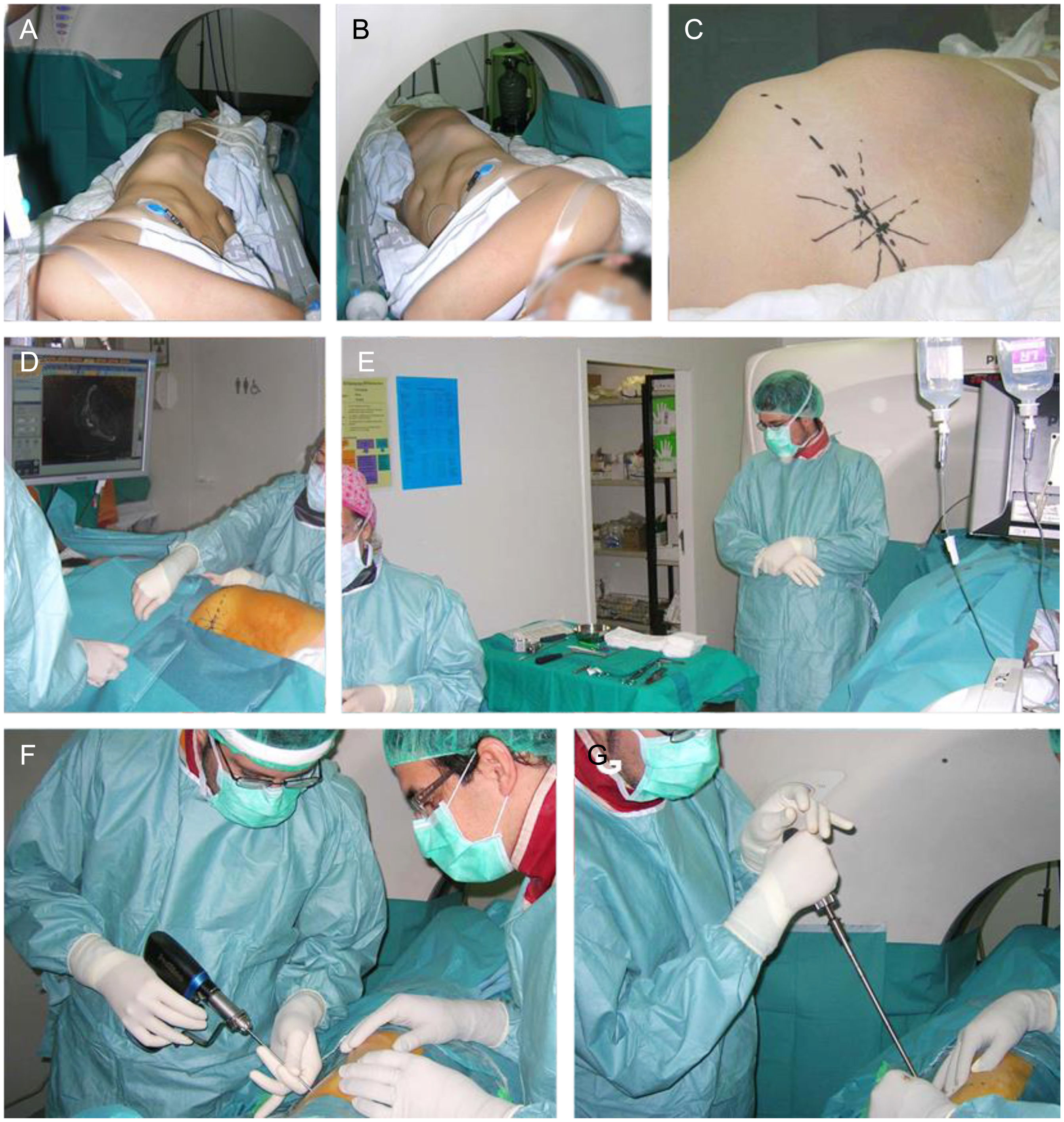
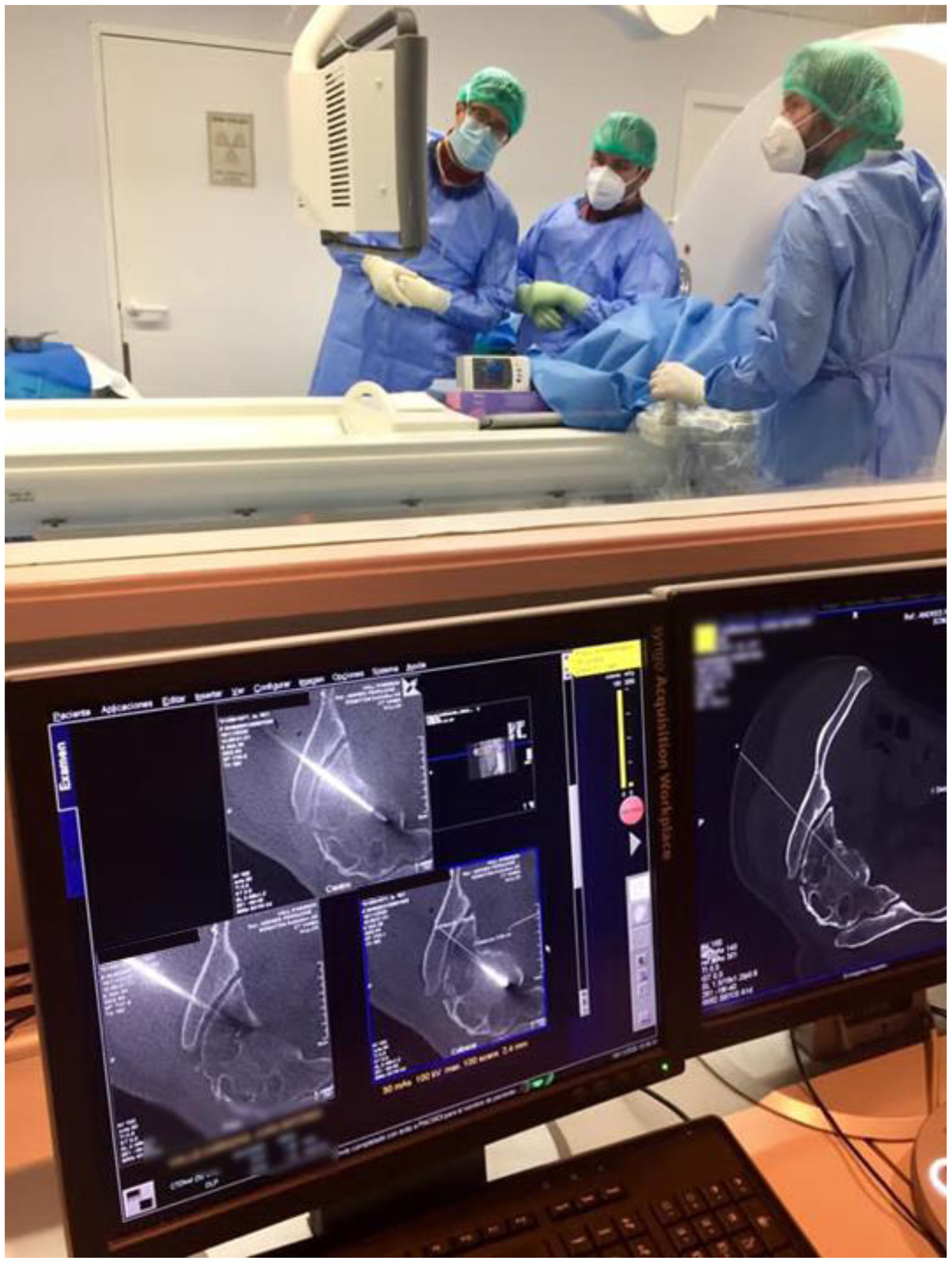
The patients in whom the ISS were implanted in the CT suite received general anaesthesia in the operating theatre and were transferred under orotracheal intubation and mechanical ventilation to the radiodiagnostic area, located on a lower floor and a 60m walk from the surgical area. The operating theatre used for anaesthesia was left unoccupied and prepared for potential conversion to open surgery. In cases where it was only necessary to insert an ISS, the patient was positioned in lateral decubitus with the injured side up on the CT scanning table. To maintain the desired position, supports were placed on both sides of the thorax and abdomen, which were fastened to the examination table with adhesive tapes (Fig. 2A and B). If bilateral posterior osteosynthesis was required, the patient was positioned prone, avoiding decubitus over the bony prominences. Once the patient was positioned, it was verified that no element interfered with the normal sliding of the examination table. For the preliminary location of the ISS entry point, the CT's control laser was used when it was projected on a slice that provided a safe corridor in all planes (Fig. 2C). Occasionally, a spinal needle was used to anticipate the position of the guide wire used for insertion of the ISS. Once the cut offering a safe corridor was determined, an attempt was made to keep the ISS wire parallel to the control laser on the sagittal plane, so that only the entry point and navigation of the wire on the axial plane had to be controlled. The notching included a rectangular area peripheral to the anticipated entry point (Fig. 2D). Both the scrub nurse and the lead surgeon were positioned at the patient's back (Fig. 2E). The position of the guide wire was checked frequently with the aid of CT, confirming that it passed through a safe corridor past the lesion area (Fig. 2F). Once the final length of the implant was determined with the help of gauges and the CT scan itself, drilling and insertion of the ISS was performed (Fig. 2G). At the end, a CT scan of the entire pelvis was acquired to certify the adequate position of the implants and the correct reduction of the injury. In the event that, once posterior stabilization had been completed, stabilization of the anterior elements was required, the patient was transferred back to the operating theatre the remainder of their pelvic injuries operated. All CT procedures were performed on a multidisciplinary basis, with the collaboration of radiologists and radiology technicians with extensive experience in musculoskeletal intervention, who operated the CT apparatus and collaborated in the interpretation of the images (Fig. 3).
Multidisciplinary collaboration in the insertion of iliosacral screws with CT. On the left, the 2 surgeons perform the procedure. On the right, the interventional musculoskeletal radiologist controls the CT scan. The radiology technician collaborates in the control of the apparatus and the generation of images of clinical interest during the procedure.
For osteosynthesis with ISS, 6.5 and 7.5mm cannulated screws with partial or complete thread were used, depending on the injury pattern; washers were used in all cases. Stabilization of the anterior ring of the pelvis included osteosynthesis with plates, the use of supraacetabular external fixations as definitive treatment for 6–8 weeks, and conservative treatment. If necessary, the same surgical session included stabilization of other skeletal injuries. The interventions were concluded with haemostasis, lavage, and closure in layers of the surgical wounds.
The patients were observed as inpatients until their injuries and medical conditions had sufficiently improved. Physiotherapy was started as early as possible. However, non-weightbearing of the injured sacroiliac joint or hemisacrum was maintained until 8–12 weeks after the procedure. Outpatient follow-up included periodic clinical and radiological examinations.
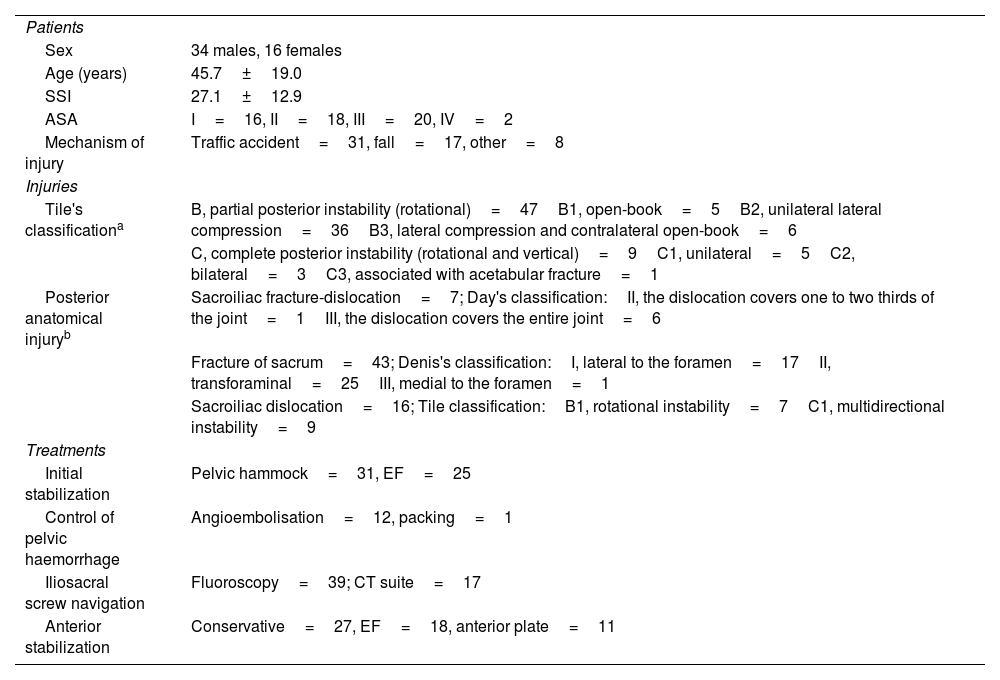
ResultsDuring the study period, 279 pelvic ring injuries were operated on in our hospital. We detected a total of 75 patients with ISS implanted in the CT suite or under FG with a postoperative control CT scan. We discarded 14 cases with insufficient follow-up and 5 who had undergone open reduction. In the end, we had 56 patients and 66 ISS for analysis (10 bilateral cases). These were 34 men and 22 women with a mean age of 45.7±19.0 years. Of the patients, 85.2% had an injury severity score≥16, which is a situation of polytrauma. Sacral fractures (76.8%) and, consequently, partial instability or Tile B patterns (83.9%) caused by traffic accidents (55.4%) or falls (30.4%) prevailed. There were 2 open fractures.
External fixation was used in the initial stabilization of 25 patients. Angioembolisation was used in the control of pelvic haemorrhage in 16 cases, while preperitoneal packing was used on only one occasion. The ISS were inserted in the CT suite in 17 patients (30.4%) and in the operating theatre under FG in 39 (69.6%), making a total of 19 and 47 ISS, respectively. All patients remained stable during the transfer from the surgical area to the radiodiagnostic area, and during the CT-guided procedure. The average number of days from trauma to ISS insertion was 10.0±9.7 days, which was not influenced by the technique used (9.6 days delay using FG vs. 11.1 with CT; p=.27). Hospitalisations were prolonged, with an average of 46.1±33.7 days. Table 1 shows detailed information on the characteristics of the patients, their injuries, and the treatment applied.
Characteristics of the patients, their injuries, and treatment.
| Patients | |
| Sex | 34 males, 16 females |
| Age (years) | 45.7±19.0 |
| SSI | 27.1±12.9 |
| ASA | I=16, II=18, III=20, IV=2 |
| Mechanism of injury | Traffic accident=31, fall=17, other=8 |
| Injuries | |
| Tile's classificationa | B, partial posterior instability (rotational)=47B1, open-book=5B2, unilateral lateral compression=36B3, lateral compression and contralateral open-book=6 |
| C, complete posterior instability (rotational and vertical)=9C1, unilateral=5C2, bilateral=3C3, associated with acetabular fracture=1 | |
| Posterior anatomical injuryb | Sacroiliac fracture-dislocation=7; Day's classification:II, the dislocation covers one to two thirds of the joint=1III, the dislocation covers the entire joint=6 |
| Fracture of sacrum=43; Denis's classification:I, lateral to the foramen=17II, transforaminal=25III, medial to the foramen=1 | |
| Sacroiliac dislocation=16; Tile classification:B1, rotational instability=7C1, multidirectional instability=9 | |
| Treatments | |
| Initial stabilization | Pelvic hammock=31, EF=25 |
| Control of pelvic haemorrhage | Angioembolisation=12, packing=1 |
| Iliosacral screw navigation | Fluoroscopy=39; CT suite=17 |
| Anterior stabilization | Conservative=27, EF=18, anterior plate=11 |
ASA: American Society of Anaesthesiologists’ physical status classification; CT: computed tomography; EF: external fixation; ISS: injury severity score.
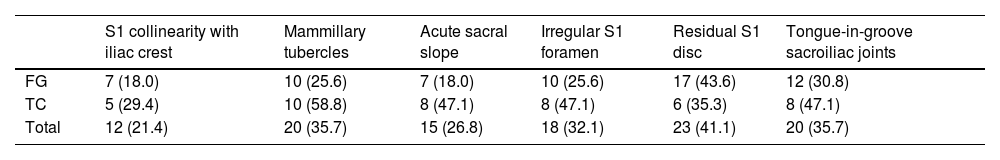
Twelve of the 66 ISS perforated the S1 corridor at some point (18.2%). The intervened pelvises accumulated an average of 1.9±1.7 SD criteria, which was more pronounced in patients intervened using CT with respect to FG (average 2.6 vs. 1.6; p=.02). All the morphological features of SD were more frequently present in the patients operated using CT, except for the presence of residual S1 disc, which was also the most common variant (Table 2). Nevertheless, all perforations occurred in cases where FG was employed (.0% vs. 34.0%; p<.01). In contrast, there were no differences in SD in patients with and without perforation intervened under FG (mean SD criteria 1.7 vs. 1.7; p=.81). The maximum width of the S1 corridor on axial CT slices was smaller in cases where perforation had occurred (18.8 vs. 21.0mm; p=.02), which was not confirmed for the coronal plane (20.0 vs. 19.6mm; p=.73). However, there were no differences between CT and FG in corridor width in the axial (20.0 vs. 20.8mm for CT and CF, respectively; p=.55) or coronal (19.6 vs. 20.0mm; p=.73) plane, and therefore this was not a determining variable to indicate one technique or the other. Neither did the fracture pattern have a significant influence on surgical indication or the risk of perforation of spaces.
Criteria for sacral dysmorphism based on iliosacral screw navigation under fluoroscopic guidance or computed tomography.
| S1 collinearity with iliac crest | Mammillary tubercles | Acute sacral slope | Irregular S1 foramen | Residual S1 disc | Tongue-in-groove sacroiliac joints | |
|---|---|---|---|---|---|---|
| FG | 7 (18.0) | 10 (25.6) | 7 (18.0) | 10 (25.6) | 17 (43.6) | 12 (30.8) |
| TC | 5 (29.4) | 10 (58.8) | 8 (47.1) | 8 (47.1) | 6 (35.3) | 8 (47.1) |
| Total | 12 (21.4) | 20 (35.7) | 15 (26.8) | 18 (32.1) | 23 (41.1) | 20 (35.7) |
CT: computed tomography; FG: fluoroscopic guidance.
The distribution of sacral dysmorphism criteria is represented as count (percentage).
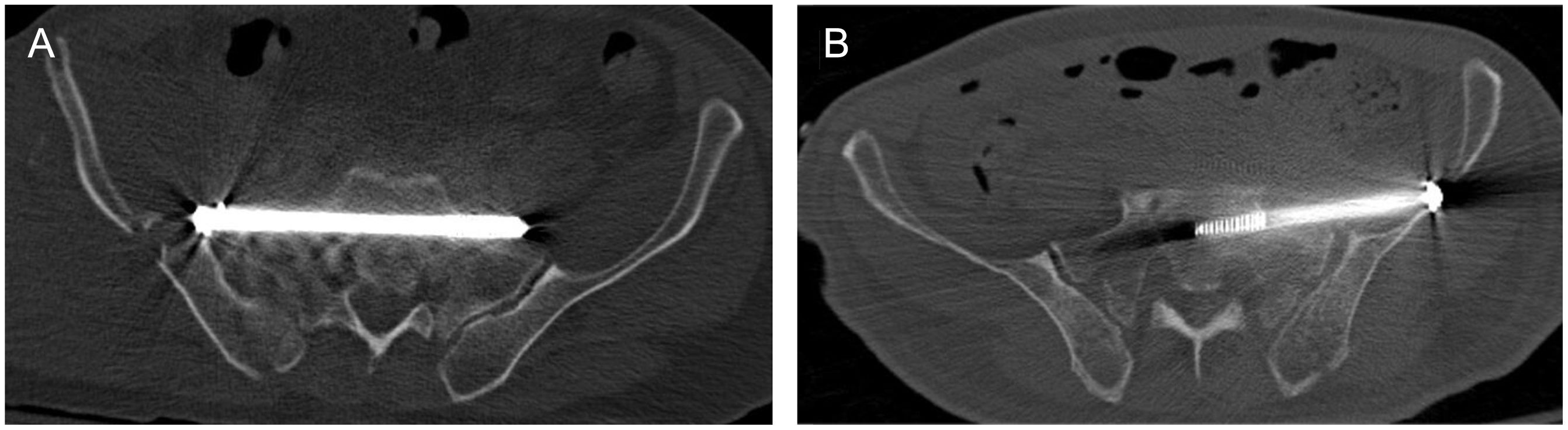
Eight of the perforations were grade III and 4 were grade IV. Seven cases involved the ipsilateral S1 foramen (inferior), 4 involved the endopelvis (anterior) and one involved the sacral medullary canal (posterior). In 2 of the ISS in which an anterior perforation had occurred, it was considered that its location put endopelvic structures at risk and impeded their correct mechanical functioning (Fig. 4). Because these were elderly patients with low demands and partial posterior instability, it was decided to simply remove the ISS, which did not interfere with consolidation. Two cases developed S1 radiculalgia after foramen perforation, but no motor deficit. The symptoms were tolerable and improved progressively with the administration of oral neuromodulators, and therefore the ISS were retained. There were no other cases of conflict with noble structures located in the perforated spaces. No other local complications or causes for reoperation related to stabilization with ISS were detected during the study period. All fractures consolidated and all dislocations were considered resolved in an average of 4.6±2.4 months, with no differences between FG and CT.
Cases with anterior iliosacral screw perforation in which early implant removal was performed. (A) The image corresponds to a 72-year-old patient who suffered a traffic accident resulting in a sacroiliac fracture-dislocation type III of Day's classification (Tile B2 and Young–Burgess LC-II). Insertion under fluoroscopic guidance resulted in anterior perforation of the iliosacral screw, which was removed as soon as it was discovered. The malposition had no repercussions on endopelvic structures. (B) An 81-year-old woman, victim of a traffic accident, suffering a similar injury to the above: Day 1, Tile B2, and Young–Burgess LC-II sacroiliac fracture-dislocation. After the fluoroscopic technique, the iliosacral screw was removed. There was no involvement of endopelvic structures.
The management of unstable pelvic fractures is a real challenge in the field of orthopaedic trauma. Correct osteosynthesis of the posterior pelvic elements is of paramount importance to achieve a stable ring. Open reduction, usually performed through posterior approaches to the sacroiliac joint, has high complication rates, especially infection.20 The description of percutaneous fixation techniques with ISS was a true revolution in the management of these fractures, as an indispensable tool in modern osteosynthesis of the pelvic ring.18,21
Percutaneous osteosynthesis with ISS involves a minimal risk of infection; the main complication is implant malposition, which is 6% globally and can reach up to 30%. Penetration of the screw into anatomical spaces may cause or worsen L5 and S1 nerve damage.22–24 In this case, it is advisable to remove or modify the position of the screw.22 Particularly frequent is abruption in the S1 foramen, where there is an average safety margin between the cortex and the implant of only 3mm.25 In the present work, we found that 18.2% of ISS perforated the S1 corridor. We interpret the figure to be attributable to the fact that only patients were included with available postoperative CT scans, which were probably requested in more complex fractures or in cases where there was a prior suspicion of penetration. However, the reduction manoeuvres themselves can cause deterioration of neurological status, and therefore, whenever possible (conscious and cooperative patient) the preoperative neurological examination should be documented.18
There are several risk factors for ISS malposition, most notably patient obesity, fracture malreduction, vertical instability patterns, corridor narrowing at S1, and SD.1,2,23,24 SD has been reported in the population with a prevalence ranging from 30% to 50%.1,2,26,27 The morphology of the dysplastic sacrum makes the use of transacral and transiliac screws impossible, and hinders the anatomical interpretation of the corridor, increasing the risk of penetration of extraosseous spaces and the consequent onset of vascular and nerve lesions.1–3
CT navigation is an effective method for improving the accuracy of ISS insertion with respect to FG. It allows better interpretation of the posterior pelvis anatomy in obese patients or patients with SD, contributing to better implant placement and reduction of complications. It facilitates correction in the position of the ISS and the identification of structures at risk in real time. Furthermore, it can be organized in most hospitals, as it does not require technical means beyond those usually present in any radiology department.8–14 The use of CT resulted in the absence of implant malposition despite a higher aggregation of SD criteria, which is consistent with previously reported data.8–14 Paradoxically, we found no difference in SD intensity in patients undergoing surgery with FG in whom space penetration had occurred with respect to those in whom it had not occurred. We interpret this finding as a limitation of FG over and above the difficulties posed by the radiological evaluation of the sacral anatomy variable. However, the use of CT raises a number of trade-offs.
CT cannot be performed in patients with extreme obesity who exceed the maximum weight supported by the examination table or the diameter of the tunnel.10 Another drawback is the need for multidisciplinary collaboration which, although enriching, could cause delays due to incompatibilities in the schedules of surgeons and radiologists. In our work, fortunately, this collaboration did not result in a significant increase in delays in performing the procedure. Potential vascular or nerve damage while performing the procedure in a radiodiagnostic area poses a dilemma regarding patient safety.4 The main concern with the use of CT is the accumulated ionizing radiation dose to both patient and staff, with its inevitable long-term health risks. Minimal dose adjustment and radiological protection of particularly sensitive body areas is always advisable.8,10,11 It is also necessary to stress the need for rational use of CT over FG in ISS insertion, as FG can offer good results in patients with an adequate S1 corridor.
In recent years, navigation-based tools have been developed that aim to improve the results of ISS insertion with FG while reducing CT radiation doses. These are techniques that are more accurate than FG, but not yet more accurate than CT. They also require the involvement of very expensive technical means, which limits their use in most settings.4–7
We acknowledge the limitations of the present study, which include its retrospective nature, the absence of randomization, and each radiological image being evaluated on a single occasion and by a single investigator, which precludes an analysis of concordance. Due to the usual concatenation of procedures in the same surgical session, it was not possible to collect the specific duration of ISS placement, nor do we have data on the radiation dose associated with the procedures. Nevertheless, we believe that the considerable sample size, the detailed description of this technique, and the enormous potential to apply it in diverse settings make the messages conveyed by this work of interest to the orthopaedic community.
In conclusion, ISS is a safe and effective method for stabilization of posterior pelvic ring injuries, implant malposition being the most common complication. Navigation under FG can be technically demanding, especially in patients with SD. We advise the use of CT guidance for ISS insertion in patients with dysplastic sacrum or narrow corridors in S1 in facilities where other navigation methods are not available.
Level of evidenceLevel of evidence iii.
Data availabilityData supporting the findings of this paper are available from the corresponding author, JVAP, upon reasonable request.
Ethical considerationsThis study was approved by our Clinical Research Ethics Committee (REC); reference number PR(ATR)369/2020. This is a retrospective study. Conducting the study did not involve any risk to patients and all data collected are devoid of any link to the identity of the participants. Consequently, the Ethics Committee approved a full waiver of Informed Consent.
FundingThe present study had no external source of funding.
AuthorshipAll the authors contributed to the conception and design of the study. Materials preparation, data collection, and analysis were undertaken by JVAP, CAPC, FBC, and EGA. The first draft of the manuscript was written by JVAP, and all the authors contributed to the creation of successive versions. All the authors read and approved the final version.
Conflict of interestsThe authors have conflicts of interest to declare with Smith & Nephew, Zimmer Biomet, Link Orthopaedics, Stryker, and MBA Surgical Empowerment.
Ethical disclosuresApproved by the Clinical Research Ethics Committee (CEIC) of Vall d’Hebron Institut de Recerca (VHIR); reference PR(ATR)369/2020.