Idiopathic avascular necrosis of the scaphoid or Preiser's disease is a condition about which few cases have been described. In the majority of cases, it is debatable whether it is actually a spontaneous osteonecrosis, or a pathological process secondary to a systematic disease, or a result of chemotherapy treatment, or associated with taking steroids.
We present the case of a 20-year-old woman, with no previous trauma, with pain in her right wrist, which progressed over a period of four years. It was wrongly diagnosed as a tendinitis, and was treated conservatively without any improvement. A simple X-ray was requested that showed an abnormality of the proximal pole of the scaphoid that was beginning to fragment, a fact which was confirmed later by performing a CT scan. This was followed by nuclear magnetic resonance spectroscopy (NMR), which showed signs of ischaemia, mainly in the proximal pole. There were signs of viability in the distal fragment in the NMR using paramagnetic contrast.
After the imaging tests, idiopathic avascular necrosis of the scaphoid was diagnosed. The surgical treatment consisted of the removal of necrotic fragments of the proximal pole and removal of the posterior interosseous-nerve.
Two years after the operation, the patient was asymptomatic and had a complete range of movement in the operated wrist.
La necrosis avascular idiopática del escafoides o enfermedad de Preiser es una entidad de la que se han descrito pocos casos. En algunas ocasiones es discutible si se trata de una osteonecrosis espontánea o representa un proceso patológico secundario a enfermedades sistémicas, tratamiento quimioterápico o está relacionada con el consumo de esteroides.
Presentamos el caso de una mujer de 20 años de edad, sin traumatismo previo, con dolor en la muñeca derecha de 4 años de evolución. Había sido erróneamente diagnosticada de una tendinitis y tratada de forma conservadora sin mejoría. Se solicitaron estudios de imagen mediante radiología simple. Los hallazgos radiológicos presentaban una anomalía del polo proximal del escafoides que originaba una fragmentación del mismo, hecho que se confirmó posteriormente con la realización de una TC. A continuación se realizó una RMN en la que se observaron criterios de isquemia, fundamentalmente en polo proximal. En la RMN con contraste paramagnético aparecieron signos de viabilidad en el fragmento distal y de osteonecrosis en el proximal.
Tras las pruebas de imagen se diagnosticó de necrosis avascular idiopática del escafoides. El tratamiento quirúrgico consistió en extirpar los fragmentos necróticos del polo proximal y practicar una neurectomía del nervio interóseo posterior.
A los 2 años de la intervención, la paciente se encuentra asintomática y con un balance articular completo de la muñeca intervenida.
Idiopathic avascular necrosis may affect several bones. In the carpus, semilunate necrosis (Kienböck's disease) is a very well-known entity; however, idiopathic avascular necrosis involving the scaphoid is much less common.1 The latter has been associated with the name of Preiser,2 the first author who described it, since its publication in 1910.
The term idiopathic refers to an unknown aetiology.3 However, the literature describes some cases4 in the context of a systemic disease which required prolonged treatment with corticosteroids or chemotherapy.
At present, most authors agree that a previous history of trauma to the wrist excludes the diagnosis of Preiser's disease. Moreover, the vulnerability of the scaphoid to necrosis is widely accepted, especially in its proximal pole due to the anatomical characteristics of its vascularisation.5 Although avascular necrosis in Preiser's disease occurs without a prior history of traumatic injury, sometimes the symptoms make it difficult to exclude the possibility of minor or repetitive trauma.6
Currently, the definition of Preiser's disease consists in scaphoid osteonecrosis without a known aetiology. Often, its clinical presentation is as a mild wrist pain with an insidious onset.
Materials and methodsCase reportWe report the case of a 20-year-old, right-handed female who presented pain in the right wrist of 4.5 years evolution. She had no relevant medical history and did not report suffering any previous trauma.
The patient reported that, since the beginning of the symptoms 2 years earlier, she had been diagnosed with tendinitis and treated conservatively with anti-inflammatory agents, immobilisation with casts, physical therapy and injections, without any improvement.
Following those 2 years, at the time of examination, she suffered pain in the dorsal region of her right wrist and in the anatomical snuff box (foveola radialis), there was no loss of strength compared to the contralateral hand and the neurological examination was normal. Joint balance was limited: 10° dorsal flexion, 20° palmar flexion and complete and painless prono-supination. Radial deviation to 25° and ulnar deviation to 40° were both painful.
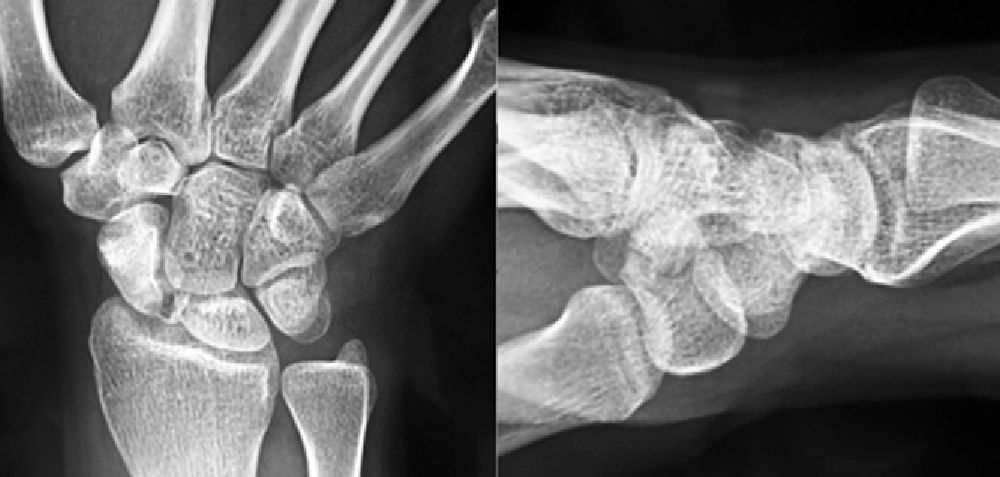
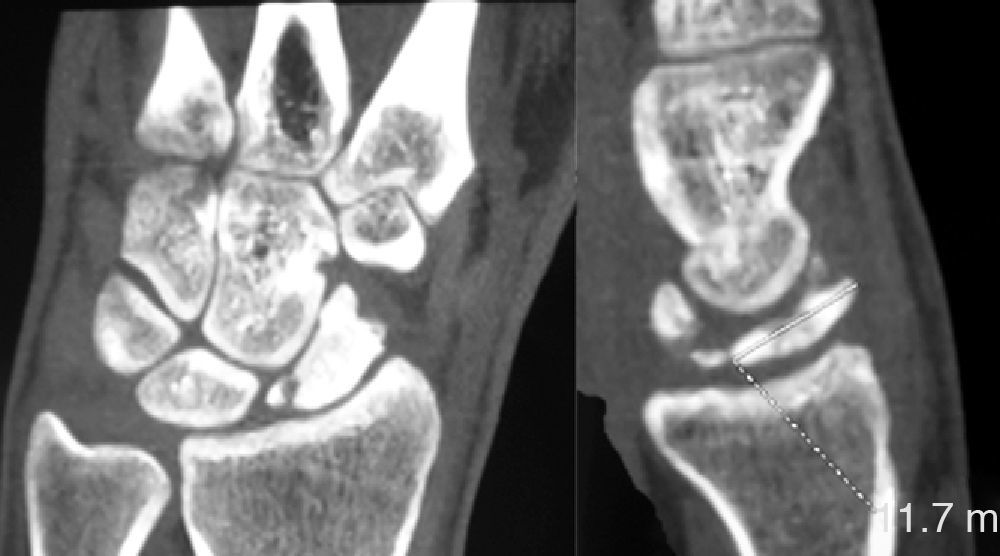
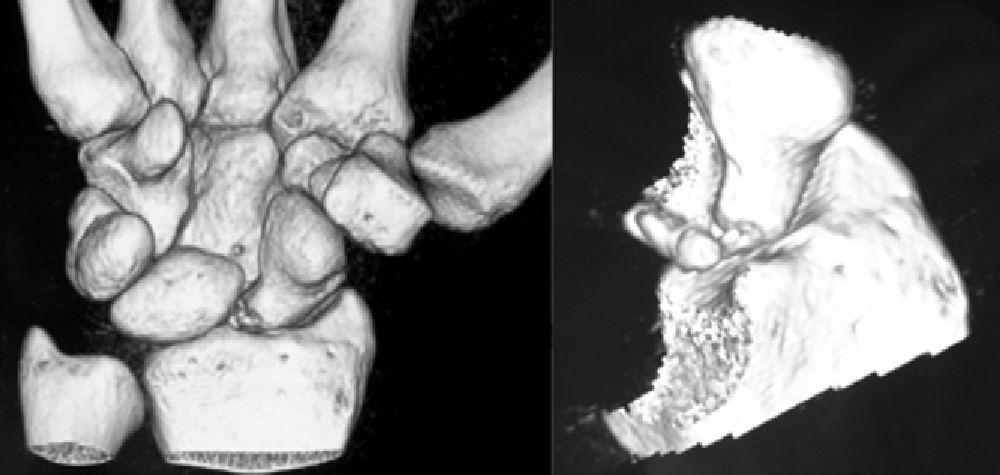
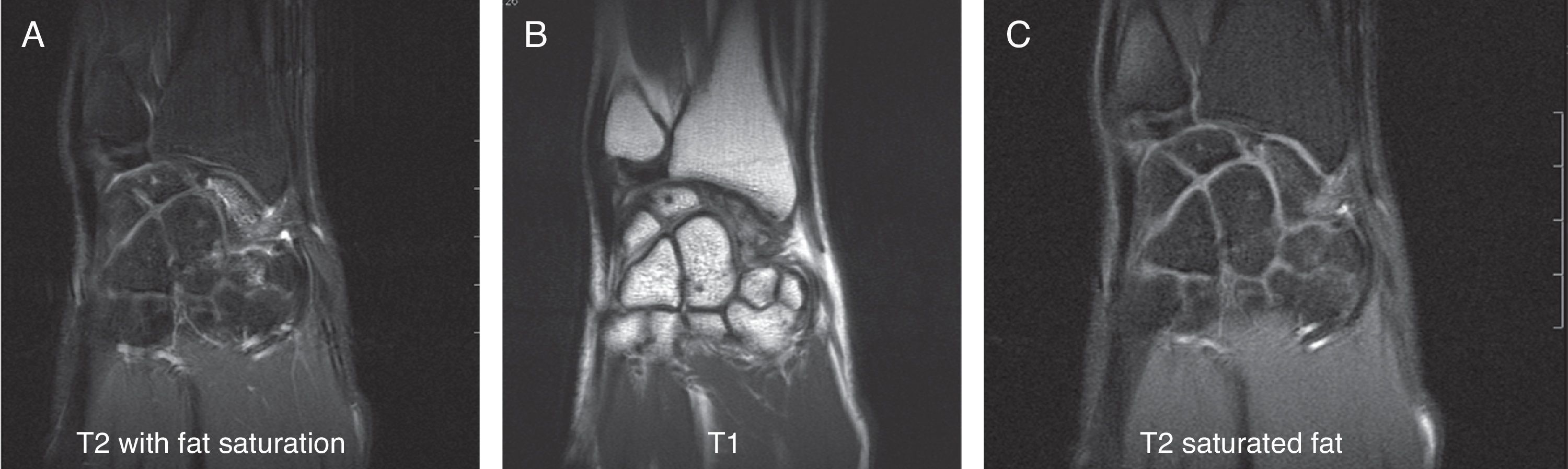
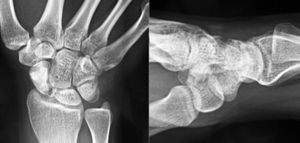
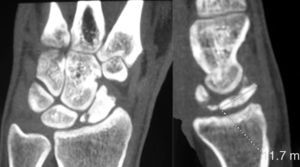
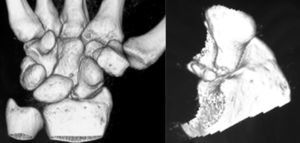
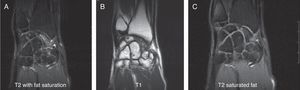
Various imaging studies including plain radiography (Fig. 1), computed tomography (CT) (Figs. 2 and 3) and magnetic resonance imaging (MRI) both standard and with contrast (Fig. 4), were requested. The findings on plain radiographs included an abnormality in the proximal pole of the scaphoid which originated from a fragmentation of the bone. This fragmentation was confirmed in more detail through the CT scan. MRI was the crucial test for diagnosis. The changes observed in the MRI in sequences T2 with fat saturation (a), T1 (b) and T1 with fat saturation following intravenous administration of paramagnetic contrast (c) (Fig. 4), confirmed image changes compatible with Preiser's disease, as described by Schmitt et al.5 Our case presented a decrease in signal intensity in simple sequences of the scaphoid, with an enhancement of the signal after application of paramagnetic contrast in the distal fragment of the bone. This indicated its vascularisation and, therefore, its viability. However, the proximal fragment which also presented alterations in the simple sequence did not show contrast enhancement, thus being compatible with necrosis.
Coronal MRI scan in sequences T2 with fat saturation (A), T1 (B) and T1 with fat saturation after intravenous administration of paramagnetic contrast (C). In simple sequences it is possible to observe an alteration in signal intensity in the distal fragment of the scaphoid following contrast enhancement. These indicate vascularisation of the fragment, thus pointing to its viability. The proximal fragment is also altered in simple sequences and shows no contrast enhancement, thus pointing to necrosis.
Based on the results of the imaging tests,5 where the simple sequences showed a signal alteration not only in the proximal pole but throughout the entire scaphoid, as well as the image of cortical avulsions in the periphery in CT scans, and added to the absence of previous trauma, we ruled out the possibility of a posttraumatic osteochondral fracture of the proximal pole of the scaphoid. Instead, the diagnosis was of Preiser's disease (advanced stage).5
At present, there are some authors who support the presence of osteonecrosis only in the proximal pole of the scaphoid in Preiser's disease.7
The latest advances in technology have enabled us to obtain detailed information about the microstructure of the scaphoid bone and its viability through the use of high resolution MRI with contrast and CT scans. In our case, these techniques were pivotal in conducting the differential diagnosis of osteochondral fractures in the proximal pole of the scaphoid,8 the first point to be ruled out given the images obtained through plan radiography. Following the classification of Schernberg et al.,9 we found 2 types of lesion: type IA or fracture without scapholunate dissociation (with integrity of the scapholunate ligament) and type IB with scapholunate dissociation (fracture of the proximal third of the scaphoid, whose route passes distally to the insertion point of the scapholunate ligament).
Both contrast MRI and high-resolution CT allow physicians to distinguish which anatomical areas of the scaphoid suffer vascular involvement. Furthermore, when fractures are present, their imaging features enable differentiation between posttraumatic and pathological fractures, through observation of alterations in vascularisation. Therefore, these techniques are crucial in the early detection of Preiser's disease, as well as in its staging.
According to Schmitt et al.,5 there are 3 different evolutionary phases or stages in Preiser's disease, based on contrast MRI and CT imaging. The initial stage features proximal osteosclerosis with areas of oedema. In the advanced stage, the proximal pole collapses and its main characteristic is the presence of pathological fractures with volume loss. These can be differentiated from posttraumatic proximal osteochondral fractures by their paths, which are parallel or oblique to the longitudinal axis of the scaphoid, the presence of sclerosis areas on both sides of the fracture line and by their peripheral location, of cortical avulsion type.5 Lastly, the final stage of Preiser's disease involves complete or nearly complete osteonecrosis of the scaphoid, without MRI signal enhancement after the application of contrast.
In our case, the images obtained were compatible with the advanced stage of Preiser's disease, with fragmentation of the proximal pole. The distal fragment was viable.
We opted for surgical treatment. We performed a dorsal approach between the third and fourth compartment, plus capsulotomy. We identified the proximal pole of the scaphoid which was fully integrated into the distal fragment, although with significant irregularities in the osteochondral surface, especially at the dorsal level. The scapholunate ligament presented indemnity in two-thirds, as well as stability which was verified by observing the block movement of the scaphoid and semilunate during flexion-extension movements of the wrist. We performed excision of the proximal fragment (preserving the scapholunate ligament), plus neurectomy of the posterior interosseous nerve. Following surgery, the limb was immobilised with a forearm splint for 3 weeks, and after this period the patient began rehabilitation therapy.
At 2 years of surgery, the patient is fully asymptomatic and presents complete recovery of the wrist joint balance, having regained her normal daily activity.
DiscussionWhen we speak of idiopathic avascular necrosis of a carpal bone, we usually refer to the lunate or Kienböck disease. Despite the high frequency of necrosis of the proximal pole in scaphoid fractures, owing to the high risk from the vascular point of view, it is rarely affected by idiopathic avascular necrosis.10 Some cases described in the literature report avascular necrosis of the scaphoid in a context of systemic disease with intake of steroids or chemotherapy.7
In any case, an inadequate vascular supply is a common denominator in all case series of necrosis of the scaphoid published so far. This is the reason why several authors have studied its vascular anatomy. Gelberman and Menon11 showed that between 70% and 80% of the vascular supply of the proximal pole of the scaphoid comes from a branch of the radial artery which penetrates through the dorsal ridge, so that a fracture through the ridge or proximal pole could produce a complete disruption of the vascularisation of this anatomical area and, ultimately, lead to avascular necrosis.
Furthermore, Panagis et al.12 studied the size and distribution of intraosseous anastomoses and noted that those individuals who presented large areas depending on a single vessel suffered a higher incidence of avascular necrosis.
The scaphoid is a crucial element in wrist stability, for both kinetics and kinematics. In order to maintain its carpal relationships, the scaphoid has 5 joints, which means that it is covered with cartilage throughout the majority of its surface, thus hindering vascular access. Gelberman and Menon11 described only 2 perforating vessels through the ligamentous insertions, areas not covered by cartilage. In their studies, Taleisnik and Kelly13 noted 2 small additional vessels entering the proximal pole of the scaphoid through ligaments. However, this proximal vascularisation is insufficient by itself. Therefore, a large amount depends on a retrograde vessel, a branch of the radial artery which is inserted into the tuberosity at the dorsal level. This vascular anatomy plays an important role in the development of idiopathic avascular necrosis.
In 2001, Buttermann et al.14 confirmed previous anatomical studies and noted that there could be an extrinsic pressure generated by the ligaments and narrow membranes which the vessels had to pass through in order to penetrate into the scaphoid. This pressure could increase with certain positions and movements of the wrist, even collapsing the vessels supplying nutrition to the scaphoid at their entry point.
All these contributions speculate on the aetiology of Preiser's disease, in which small and repetitive microtrauma on anatomical structures could hinder the blood supply to the scaphoid and trigger the development of idiopathic avascular necrosis in patients with a greater predisposition.6
Herbert and Lanzetta10 hypothesised that a certain percentage of patients could receive vascularisation of the proximal pole of the scaphoid through the scapholunate ligament complex, so that it could remain viable in case of fracture. This would explain the fact that proximal pole necrosis may occur without a previous fracture. In 1993, they studied 8 patients with avascular necrosis of the proximal pole of the scaphoid. In this series, plain radiographs found no signs of previous fracture of the scaphoid. This segmental necrosis would support their hypothesis, in which a traumatic avulsion of the scapholunate ligament could compromise the vascularisation of the proximal poles of those scaphoid bones with this anatomical variant. Given that only this area was affected, this type of avascular necrosis was considered in our case as a possible aetiology. However, during surgery we observed that two-thirds of the scapholunate ligament were intact and, therefore, in the event that we were facing such an anatomical variant, vascularisation would have no reason to be affected.
In 1991, Nakamura et al.15 evaluated the influence of ulnar variation as a possible causative factor in the development of Preiser's disease in an extensive series of 23 patients suffering idiopathic avascular necrosis, and concluded that it was excluded as a participating element in the pathogenesis.
From a diagnostic standpoint, we can currently rely on more advanced diagnostic techniques than Preiser had at his disposal in 1910. In fact, a critical reading of his original work2 reveals that he attempted to explain why some scaphoid fractures did not appear on plain radiographs until months after the traumatism. Preiser believed that the source was an avascular state which originated after the damage. Currently, this definition is not valid because this disease is understood as osteonecrosis without a known aetiology and, therefore, prior trauma excludes it.
Considering the quality of plain radiographs and the aid of CT and MRI with and without contrast, it would not be unreasonable to think that, nowadays, the 5 patients described by Preiser would be diagnosed with acute fracture of the scaphoid.6
At present, MRI scans are used for patients in whom a scaphoid injury is suspected and where the initial plain radiograph is normal. The image produced by MRI allows us to discriminate occult fractures from bone contusions and even torn ligaments. In turn, paramagnetic contrast MRI (crucial to describe vascular status) can differentiate various viability states of the proximal pole versus avascular necrosis, and even detect the presence of a previous fracture, which is crucial in therapeutic planning.16 Occult scaphoid fractures are present in 16% of cases. MRI techniques can detect these fractures with a sensitivity and specificity of 100%, as well as bone oedemas and contusions.12
Although Preiser's disease is initially considered idiopathic, it is accepted that one of its main causes could be repetitive microtrauma, as well as complications derived from the intake of certain types of medication, which are linked to altered vascularisation patterns in the proximal pole.17 Due to its capacity to detect changes in vascularisation, as well as occult fractures, MRI with and without contrast is the essential diagnostic test to detect this rare entity.
In turn, it allows us to differentiate between 2 different patterns: type I, characterised by ischaemia and/or necrosis signal changes affecting the entire scaphoid, and type II, in which such signal changes are limited to 50% or less, with a predominance of the proximal pole, and with such patients having a more favourable diagnosis.5,17
Our case corresponded to the latter, type II, where the vascularisation of the larger, distal fragment was preserved, thus fulfilling the criteria for severe ischaemia/necrosis of the proximal fragment.
Herbert and Lanzetta10 elaborated the first staging of the disease based on simple radiographic images. They established a division into 4 stages, from a normal image in the initial stage to a complete collapse pattern with osteoarthritis in stage IV.
There is a wide variety of treatment options, ranging from conservative treatment with immobilisation to different surgical techniques, including revascularisation, radial osteotomies, silicone arthroplasties, 4-corner arthrodesis and scaphoid excision or carpectomy of the proximal row.6 This treatment is not standardised, mainly due to the large number of factors on which the aetiology is based and to the different stages of the disease.
Revascularisation procedures would be indicated in the initial stages, when there is necrosis with very mild or no degenerative changes (stages I and II), in which the process may still be reversible. When carpal collapse and irreversible changes appear (stages III and IV), reconstruction is no longer viable. In such cases we must resort to salvage procedures, such as excision of the scaphoid or proximal carpectomy.
Our case could be considered as a stage I. We conducted excision of the necrotic fragment and posterior interosseous neurectomy, which we considered as a mildly aggressive technique and which did not exclude other surgical options in the future. The patient is currently free of clinical symptoms and presents a full range of motion and strength at 2 years postoperatively.
The literature reports various results for the different treatment options. In their series, De Smet et al.4 gathered 4 cases in stage III which were treated with proximal carpectomy, 3 of which obtained very successful results.
For their part, in 2006 Moran et al.17 published a work reflecting their results after using vascularised distal radius grafts in 8 patients with idiopathic avascular necrosis in stages II and III. They concluded that this option should be reserved for patients in Herbert stages I and II without evidence of radiocarpal osteoarthritis or carpal instability. The presence of osteoarthritis in the radiocarpal or midcarpal joint would represent an indication for scaphoid excision and arthrodesis.18
Viegas19 in 1988 and subsequently Menth-Chiari and Poehling20 in 2000 conducted arthroscopic debridement of the entire necrotic surface of the scaphoid in early stages, improving function and obtaining a complete relief of pain.
ConclusionsMRI has represented a major advance in the diagnosis of Preiser's disease. There is currently no standardised treatment algorithm for these patients, and there is a wide variety of treatments depending on the degenerative changes observed. Our experience with excision of necrotic fragments associated to neurectomy has been favourable.
Level of evidenceLevel of evidence v.
Ethical responsibilitiesProtection of people and animalsThe authors declare that this investigation did not require experiments on humans or animals.
Confidentiality of dataThe authors declare that they have followed the protocols of their workplace on the publication of patient data and that all patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors declare that this work does not reflect any patient data.
Please cite this article as: Andrés Grau J, et al. Enfermedad de Preiser. A propósito de un caso. Rev Esp Cir Ortop Traumatol. 2013;57:61–6.