There are increasingly more patients with prosthetic implants (orthopaedic prostheses, lumbar instruments, osteosynthesis material). In the last decade, infections caused by carbapenem resistant Enterobacteriaceae have increased (bacteriaemia, abscesses, urinary tract infections…) with great difficulty in treatment and important associated comorbidity. We present the first case of infection of a lumbar instrumentation by Klebsiella pneumoniae producing carbapenemase D, OXA-48 type, and successfully treated.
Cada vez son más los pacientes portadores de implantes protésicos (prótesis ortopédicas, instrumentaciones lumbares, material de osteosíntesis). En la última década se han incrementado las infecciones producidas por enterobacterias resistentes a carbapenémicos (bacteriemias, abscesos, infecciones urinarias, etc.) con gran dificultad para el tratamiento y una importante comorbilidad asociada. Presentamos el primer caso de infección de una instrumentación lumbar por Klebsiella pneumoniae productora de carbapenemasa D, tipo OXA 48, exitosamente tratada.
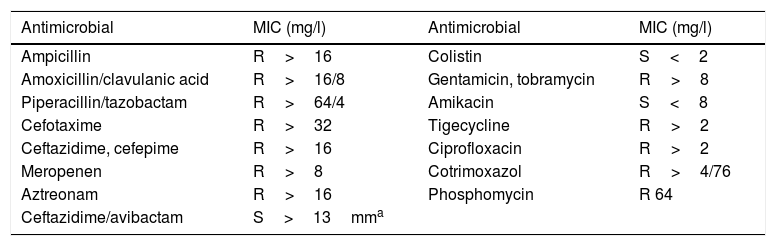
Although surgical wound infection is a rare complication, it causes a high level of morbimortality, prolonged hospitalisation, patient suffering and increased medical spending. Infections by multi-resistant bacteria have increased over the last decade. These infections are very difficult to treat medically-surgically, and they are also an epidemiological problem in hospitals. We present the case of a 29-year-old woman with a history of psychotic disorder, smoking and habitual cannabis consumption. She had multiple trauma following a fall in a suicide attempt, with fractures of both ankles, the right cubitus and distal radius, burst fracture of L2 with occupation of the channel without a neurological lesion at this level and fracture of the sacrum bone with spinal-pelvic disassociation and instability, as well as injuries to sacral roots S1, S2 and S3, requiring multiple surgical operations. The consequences included urinary and faecal incontinence, and permanent bladder catheterisation was necessary. On day 7 after admission, once the patient had attained overall stabilisation surgery with lumbopelvic instrumentation was performed from L1 up to both iliac bones, with pediculated screws at all levels from L1 to S1, pelvic screws, partial reduction of the fracture in hours and bilateral sacroiliac joint stabilisation using computed tomography and intraoperative navigation (O-Arm, Medtronic), followed by the decortication of joint surfaces and the addition of local graft and frozen spongy allograft. There were no intraoperative incidents and the antibiotic prophylaxis recommended in our hospital was applied, with 2g IV cefazoline in induction followed by every 8h until the withdrawal of the Redon wound drains. During the first week after surgery she had daily peaks of fever, with the appearance of haemopurulent exudate from the lumbar surgical wound and increased C-reactive protein (CRP) 35.96mg/l. A culture was made of the surgical wound, together with hemocultures, uroculture, replacement of the bladder catheter and those giving venous access. Empirical treatment commenced with vancomicine and IV cefepime, in spite of which the patient had the clinical symptoms of sepsis which made emergency cleaning surgery and debridement necessary. The graft was removed and washed and immersed in 240mg gentamicine diluted in 250cc saline solution. It was kept in this solution for 30min. After this time the graft was once again placed in the previous surgical bed, with the aim of creating the biological conditions for bone fusion of the instrumented area. All of the implanted instrumentation was also aggressively washed, keeping the osteosynthesis material. All of the cultures were negative (the uroculture, hemocultures and the surgical wound surface culture) except for the intraoperative samples and the rectal colonisation study, which showed the sole growth of multiresistant Klebsiella pneumoniae (K. pneumoniae) with MIC >8μg/ml for meropenem and producing broad spectrum betalactamase (BSBL). It was only sensitive to amikacine and colistine (system, Wider®, Soria Melguizo, Madrid, Spain) (see Table 1, antibiogram). Multiple CRP was performed in real time that confirmed the presence of class D carbapenemase, OXA 48 type (Progenie Molecular, Valencia, Spain). Treatment commenced with 2g iv/8h meropenem in extended infusion and 1000mg IV/24h amikacine, without achieving appropriate pharmacological levels or control of the infection. An in vitro disc diffusion study was performed for sensitivity to ceftazidime/avibactam. Sensitivity was detected, so that treatment was prescribed of 2g IV/8h together with colistimethate sodium 3.5MU/12h. The patient improved clinically and analytically, without toxicity data. She was treated for 8 weeks and then clinically monitored for one year, remaining stable and without data showing infection. No further cleaning surgery was required and the implanted osteosynthesis material has remained in situ without the need to remove it or modify it. No additional follow-up data is available as the patient returned to her country of origin (Italy).
Antibiogram.
| Antimicrobial | MIC (mg/l) | Antimicrobial | MIC (mg/l) |
|---|---|---|---|
| Ampicillin | R>16 | Colistin | S<2 |
| Amoxicillin/clavulanic acid | R>16/8 | Gentamicin, tobramycin | R>8 |
| Piperacillin/tazobactam | R>64/4 | Amikacin | S<8 |
| Cefotaxime | R>32 | Tigecycline | R>2 |
| Ceftazidime, cefepime | R>16 | Ciprofloxacin | R>2 |
| Meropenen | R>8 | Cotrimoxazol | R>4/76 |
| Aztreonam | R>16 | Phosphomycin | R 64 |
| Ceftazidime/avibactam | S>13mma |
Infection of the surgical wound in spinal column surgery is a severe but rare complication. It occurs at a higher rate in surgery with instrumentation, at from 2.1% to 8.5%.1 In a study published by Abdul-Jabbar et al., approximately 70% of cases are caused by Gram-positive bacteria, chiefly Staphylococcus aureus (45.2%), followed by Staphylococcus coagulasa negative (31.4%). The percentage of methicillin resistance stands at 34.3%. Gram-negative bacteria cause 30.5% of cases. Spinal column and sacrum surgery are associated more often with polymicrobial infections or ones caused by Gram-negative micro-organisms.2 The infections that commence before 90 days are considered to be early, while those which occur following 90 days after surgery are classified as late. For early infections Mok et al. recommend surgical cleaning and debridement while retaining implants, followed by appropriate antibiotic treatment during 4–6 weeks until vertebral fusion occurs, with 80% rate of cure.3 In late infections it is recommended that instrumentation be removed once vertebral fusion has been achieved.1,4 On the other hand, an increase has occurred over the past decade in infections caused by carbapenem resistant enterobacteria, leading to an increase in morbimortality and a public health problem.5 Treatment has to be on an individual basis depending on sensitivity, the focus of the infection and patient comorbidity. In infections caused by carbapenemase-producing K. pneumoniae combined treatment is recommended using a carbapenem, for example, meropenem if the MIC≤8mg/l, a double dose, i.e., 2g/8h in a prolonged infusion together with another active antibiotic.6–8 Our case occurred in the context of a hospital infection that has lasted for a decade.9 Ceftazidime/avibactam is a new betalactam antibiotic, approved by the Food and Drug Administration and the European Medicines Agency10 and recently commercialised in Spain. It is used to treat complicated intra-abdominal infections, complicated infections of the urinary tract, hospital-acquired pneumonia and infections by resistant Gram-negative micro-organisms in adult patients with limited therapeutic options.10 Only one publication, by De Sanctis et al., describes 3 knee prosthesis infections infected by carbapenem-resistant K. pneumoniae. Two patients died and the third required amputation, in spite of multiple surgery and prolonged treatments with antibiotics.11
We present the first case, to our knowledge, of infection of the instrumented surgical site in the spinal column by multi-resistant K. pneumoniae (producing class D carbapenemase, type OXA-48 and BSBL). The interest and complexity of our case arise due to the combination of major instrumented spinal column surgery and infection of the surgical bed caused by a multi-resistant pathogen, together with the resolution of the same without the need to remove the implant thanks to aggressive local treatment and antibiotic treatment that was appropriate in terms of drugs and dose used. Although it is true that prosthetic knee infection differs in some ways from infection of a surgical bed with implants in the lumbar spinal column, above all in terms of mobility, our case consists of far broader and more aggressive surgery on a traumatic bed with a far higher implant density than is the case with knee prosthesis. Due to all of the above factors the successful resolution of this case is especially relevant for the future. Infections of this type are considered to be extremely severe, and our case shows that although the results could probably not be reproducible in all patients, the combination of correctly applied local and general measures based on multi-disciplinary agreement is able to successfully treat extremely severe infections.
Level of evidenceLevel of evidence v.
Please cite this article as: Rico-Nieto A, Moreno-Ramos F, Fernández-Baillo N. Infección de artrodesis lumbar por Klebsiella pneumoniae multirresistente, exitosamente tratada con retención del implante y ceftazidima/avibactam. Rev Esp Cir Ortop Traumatol. 2018;62:471–473.