(1) To recall the epidemiology and signs of osteochondromas of the proximal humerus (OPH); (2) determine treatment indications; (3) and make recommendations for surgical treatment.
MethodsRetrospective, observational and longitudinal study of 20 solitary and 12 multiple osteochondromas of the proximal humerus. We analysed the epidemiological, clinical and imaging characteristics and treatment results with an average time of follow-up of the operated cases of 45 months.
ResultsEleven (55%) males and 9 (45%) females with an average age of 21 years presented solitary osteochondromas. Twelve (60%) cases were operated on at a mean age of 23 years because they were symptomatic or, in one case, malignancy was suspected. Two solitary osteochondromas could have spontaneously regressed. Multiple osteochondromas were found in 11 (92%) males and one (8%) female of whom 3 required surgery. There were no complications or recurrences. Functional outcome was excellent in all patients.
DiscussionOsteochondromas of the proximal humerus are relatively common, although most publications are case reports or short series.
ConclusionsOsteochondromas of the proximal humerus do not differ from those in other locations. Symptomatic cases and those in which malignancy is suspected would be operated, the former preferably at the end of growth. The surgical treatment is summarised in planning the approach, using CT and/or MRI, extraperiosteal en bloc resection, and eventual bone reconstruction, ideally with allograft.
1) Recordar la epidemiología y semiología de los osteocondromas del extremo proximal del húmero (EPH); 2) determinar las indicaciones de su tratamiento y 3) hacer recomendaciones relativas al tratamiento quirúrgico en ese asiento.
Material y métodoEstudio retrospectivo observacional y longitudinal de 20 osteocondromas solitarios y 12 múltiples del EPH. Se analizaron las características epidemiológicas, clínicas y de imagen de los pacientes de la serie, y los resultados del tratamiento, con un tiempo medio de seguimiento de los casos intervenidos de 45 meses.
ResultadosLos osteocondromas solitarios correspondieron a 11 hombres (55%) y 9 mujeres (45%), con una edad media de 21 años. Doce (60%) fueron intervenidos a una edad media de 23 años por ser sintomáticos o, en un caso, por sospecharse malignización. Dos casos solitarios no intervenidos pudieron haber involucionado espontáneamente. Las formas múltiples ocurrieron en 11 hombres (92%) y una mujer (8%) y 3 fueron intervenidos. No hubo complicaciones ni recidivas y el resultado funcional fue excelente en todos los pacientes.
DiscusiónLos osteocondromas del EPH son relativamente frecuentes, aunque la mayoría de las publicaciones son de casos clínicos aislados o de series cortas.
ConclusionesLos osteocondromas del EPH no difieren de los de otra localización. Los casos sintomáticos y en los que se sospecha malignización serían intervenidos, aquellos mejor con el crecimiento finalizado. El tratamiento quirúrgico se resume en la planificación del abordaje mediante TC o RM, resección en bloque extraperióstica y una eventual reconstrucción ósea, idealmente con homoinjerto.
Osteochondromas are benign bone tumours composed of cartilage tissue. They are estimated to represent up to 35–40% of all benign skeletal tumours, and are present in 2–3% of the general population, in 15% of occasions within the context of a multiple hereditary osteochondromatosis (MHOC). Cases are usually diagnosed in the metaphysis beside the cartilage where growth is the most fertile, generally around the knee, although it may originate in any bone with endochondrial ossification. The majority of cases are discovered during childhood and adolescence, with a slight predominance of males over females; cases may be asymptomatic or become complicated by fractures, neurological or vascular lesions, bursitis or tendon damage. They may also become malignant, which occurs in almost 1% of solitary forms and in 3–25% of multiple forms.1
Osteochondromas of the proximal end of the humerus (PEH), which are relatively common, have received relatively little attention as a specific entity in the scientific literature. The majority of papers describe isolated cases with a complication (bursitis,2 tendonitis,3 tendon tear,4 radial paralysis,5 pseudoaneurisms6) or short series of cases, usually in immature skeletons.7 This may be due to the dispersion of cases, which are usually treated in the hospital where they are diagnosed. It may also be because treatment of them is understood to be the same as treatment of distal femur or proximal tibia osteochondromas, which are together more frequent than cases of the proximal humerus. Although this is true, the local anatomy differs in each site.
This work has three aims: (1) to review the epidemiology and semiology of PEH osteochondromas; (2) to determine the indications for treatment, and (3) to make recommendations on surgical treatment at this site.
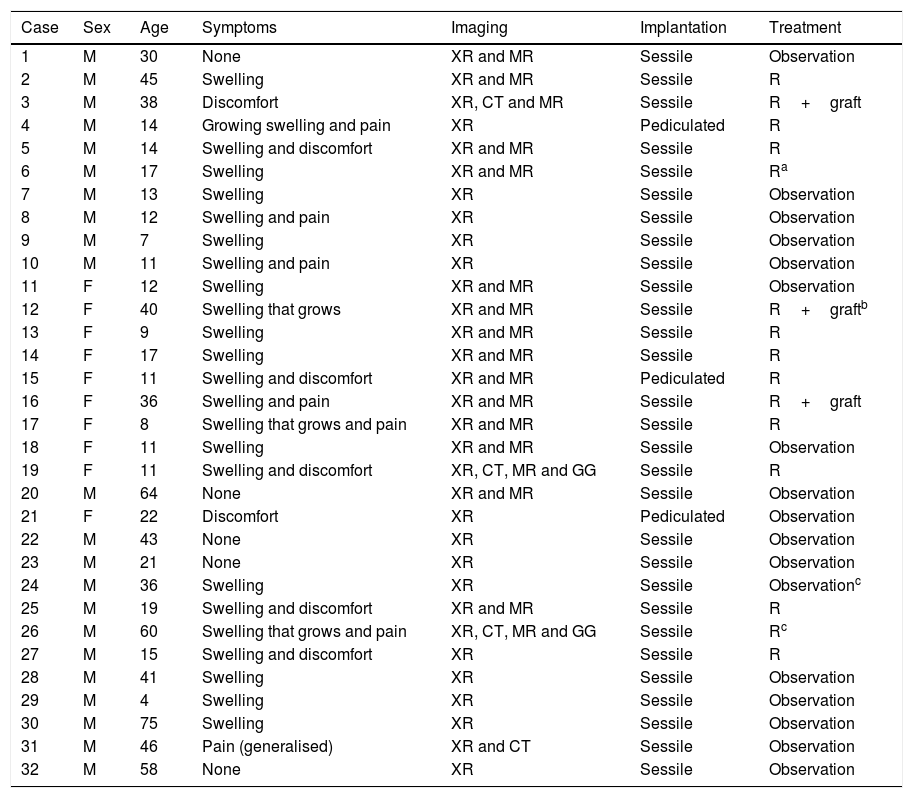
Material and methodsWe perform a retrospective, observational and longitudinal study of all the patients with PEH osteochondromas diagnosed in our Musculoskeletal Tumour Unit from July 2006 to June 2016. The total number of patients with osteochondromas at any site treated during this period amounted to 176, and in 24 of them the disease was multiple with varying numbers of osteochondromas (at least 2) in different locations. Of the 152 solitary osteochondromas (SOC), 86 (56.58%) were located close to the knee (the distal femur, proximal tibia and proximal fibula). The sample studied was composed of 32 patients, 20 with SOC and 12 with involvement of one or both humeri in the context of multiple exostosant disease or MHOC. The diagnosis was radiological in all cases, with pathological confirmation in the cases operated: 12 of the SOC cases (60%) and 3 in patients with MHOC (25%). Of the SOC cases, one patient was operated again due to incomplete resection of the tumour in another hospital, while another patient was operated due to the suspicion of malignisation due to growth and radiological changes during follow-up, with a MR image showing a chondral cap almost 2cm thick, although no malign cells were detected in the resected piece. One MHOC patient aged 36 years old had been operated in both shoulders in another hospital at the age of 12 years old; another was operated in the right shoulder had already been operated in the left shoulder, once again in another hospital, 30 years previously. Neither of these operations qualified for inclusion in the statistics as specific information about them was lacking. Table 1 contains a summary of the most outstanding epidemiological and clinical data of the series.
Summary of the epidemiological and clinical data of the cases in the series.
| Case | Sex | Age | Symptoms | Imaging | Implantation | Treatment |
|---|---|---|---|---|---|---|
| 1 | M | 30 | None | XR and MR | Sessile | Observation |
| 2 | M | 45 | Swelling | XR and MR | Sessile | R |
| 3 | M | 38 | Discomfort | XR, CT and MR | Sessile | R+graft |
| 4 | M | 14 | Growing swelling and pain | XR | Pediculated | R |
| 5 | M | 14 | Swelling and discomfort | XR and MR | Sessile | R |
| 6 | M | 17 | Swelling | XR and MR | Sessile | Ra |
| 7 | M | 13 | Swelling | XR | Sessile | Observation |
| 8 | M | 12 | Swelling and pain | XR | Sessile | Observation |
| 9 | M | 7 | Swelling | XR | Sessile | Observation |
| 10 | M | 11 | Swelling and pain | XR | Sessile | Observation |
| 11 | F | 12 | Swelling | XR and MR | Sessile | Observation |
| 12 | F | 40 | Swelling that grows | XR and MR | Sessile | R+graftb |
| 13 | F | 9 | Swelling | XR and MR | Sessile | R |
| 14 | F | 17 | Swelling | XR and MR | Sessile | R |
| 15 | F | 11 | Swelling and discomfort | XR and MR | Pediculated | R |
| 16 | F | 36 | Swelling and pain | XR and MR | Sessile | R+graft |
| 17 | F | 8 | Swelling that grows and pain | XR and MR | Sessile | R |
| 18 | F | 11 | Swelling | XR and MR | Sessile | Observation |
| 19 | F | 11 | Swelling and discomfort | XR, CT, MR and GG | Sessile | R |
| 20 | M | 64 | None | XR and MR | Sessile | Observation |
| 21 | F | 22 | Discomfort | XR | Pediculated | Observation |
| 22 | M | 43 | None | XR | Sessile | Observation |
| 23 | M | 21 | None | XR | Sessile | Observation |
| 24 | M | 36 | Swelling | XR | Sessile | Observationc |
| 25 | M | 19 | Swelling and discomfort | XR and MR | Sessile | R |
| 26 | M | 60 | Swelling that grows and pain | XR, CT, MR and GG | Sessile | Rc |
| 27 | M | 15 | Swelling and discomfort | XR | Sessile | R |
| 28 | M | 41 | Swelling | XR | Sessile | Observation |
| 29 | M | 4 | Swelling | XR | Sessile | Observation |
| 30 | M | 75 | Swelling | XR | Sessile | Observation |
| 31 | M | 46 | Pain (generalised) | XR and CT | Sessile | Observation |
| 32 | M | 58 | None | XR | Sessile | Observation |
Multiple forms are shown in grey boxes.
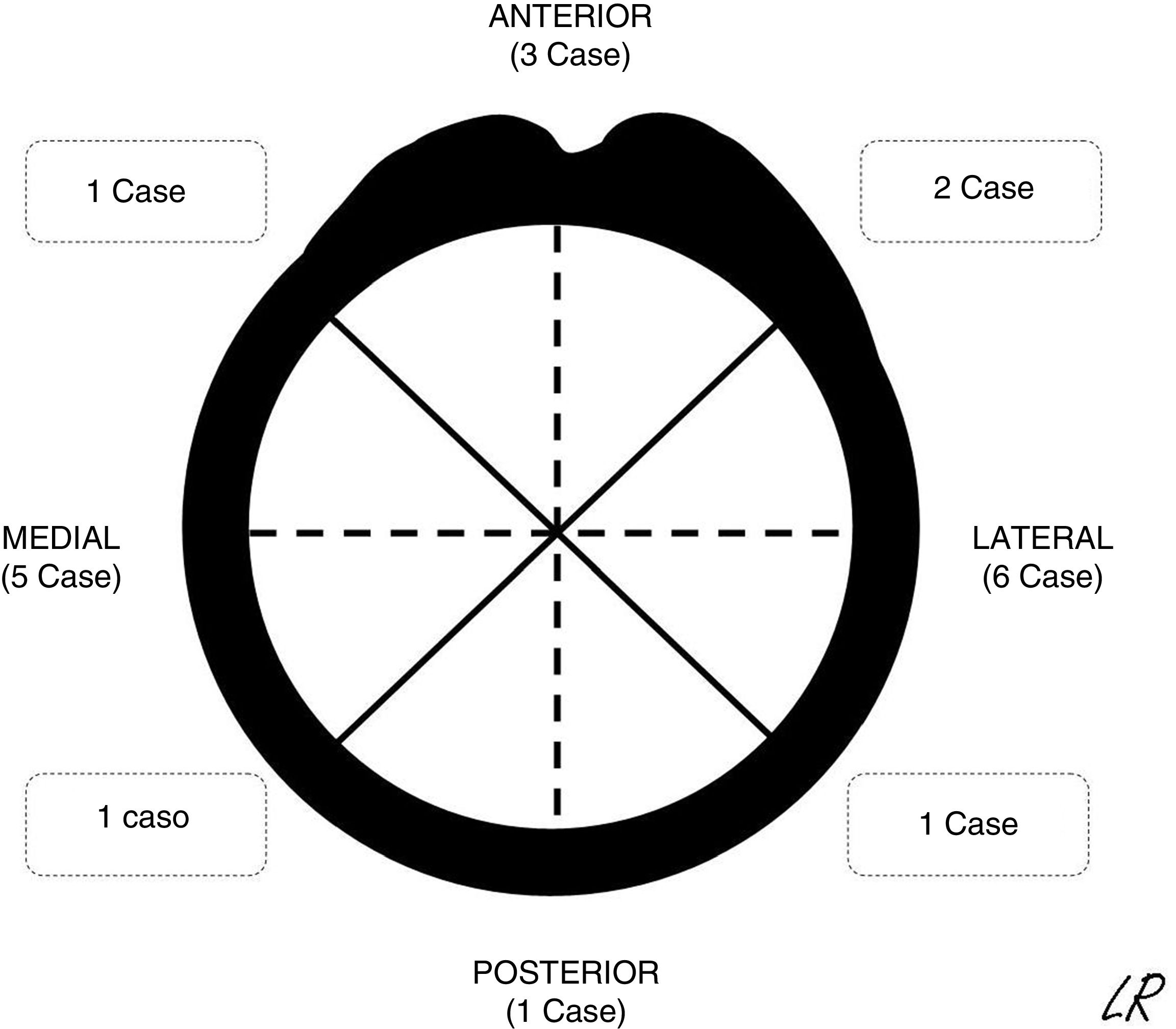
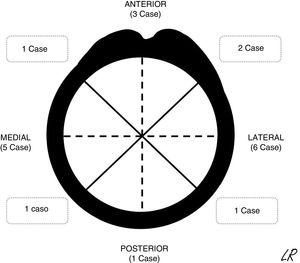
The patients were continuously detected and identified in the patient registry of the unit. According to the ethical norms governing research procedures, data was obtained by reviewing clinical histories. The epidemiological, clinical and imaging characteristics of the patients in the series were recorded. In SOC cases the morphology was described together with an axial plane CT scan or MR image in those cases in which one had been requested, together with its location (Fig. 1) and the size of its implantation base. Location was described using quadrants (anterior, medial, lateral and posterior) while size was given according to the maximum percentage of the perimeter of the affected humerus in axial slices. The radiographic characteristics of the multiple osteochondromas were not specified, due to the difficulty of distinguishing them in isolation because of their number and, sometimes, superimposition. They were described and the treatments applied were justified, while the results of surgical treatment were described in terms of complications, relapse or not of the tumour, and function using the Musculoskeletal Tumour Society scale.8 The average time of follow-up of the cases operated was 45 months (IQR: 12–116 months). In the SOC cases that were not operated after the first consultation in our unit (8 cases) the average time of follow-up was 49 months (14–120 months).
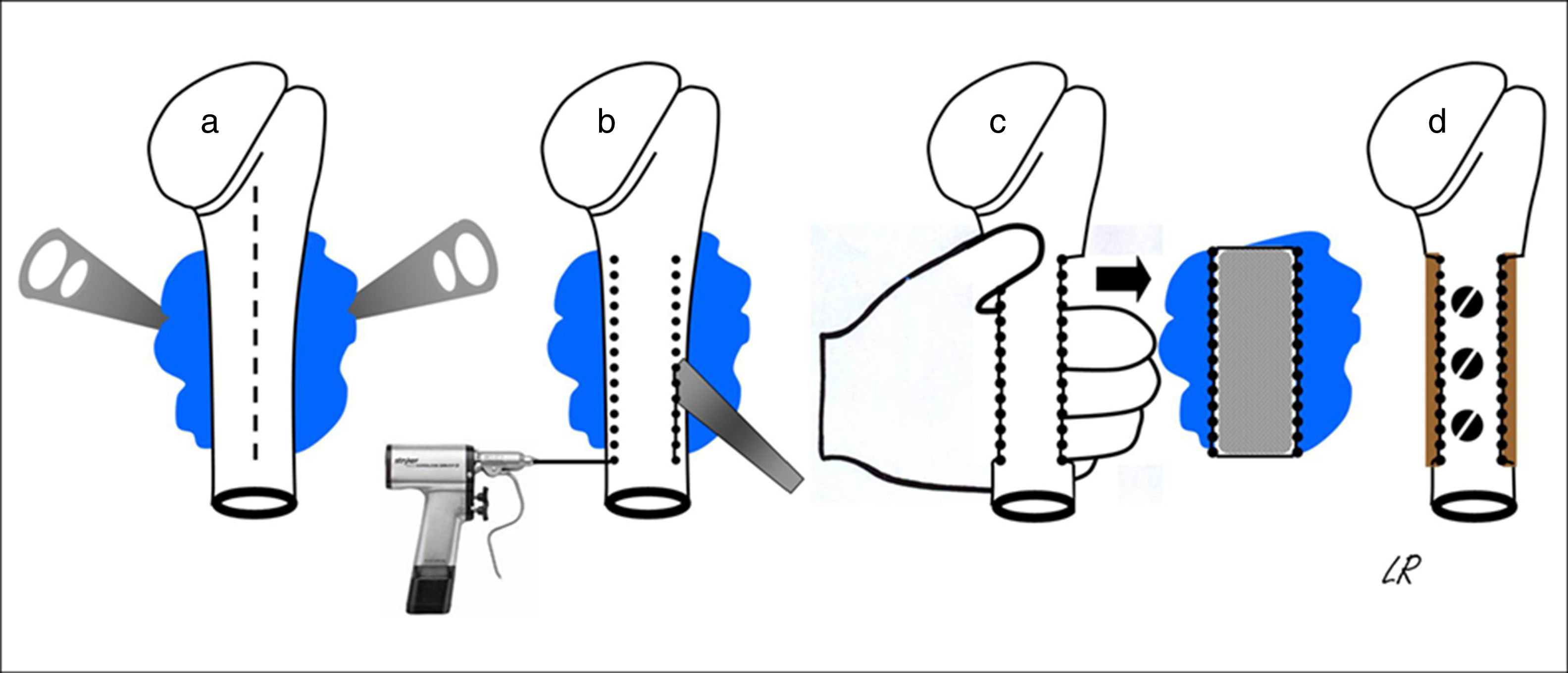
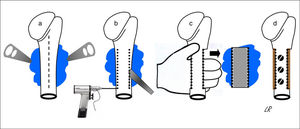
All of the patients were operated under general anaesthetic, in a semi-seated position or in supine decubitus with the trunk slightly angled up and the arm free so that it could be rotated and separated. The cutaneous incision was always longitudinal and was prolonged distally in the anterior half of the circumference of the proximal third of the arm, more or less medialised or lateralised depending on the tumour implantation base. When the latter was broadly posterior the incision was strictly anterior so that, from here, the whole osteochondroma implantation base could be accessed from within and from outside the humeral diaphysis, without finding or tensing the radial nerve. The medial approach almost always made it necessary to disinsert the greater pectoral and great round muscles, while a lateral approach only required the separation of the deltoids or some of the fibres in its anterior third. Once the tumour had been reached its implantation base was identified and the periostium around it was cut, separating it with a curette to expose the healthy cortical bone, and the osteotomy was either performed directly with a chisel or after a series of perforations. The resection of the tumour block was completed by extracting it manually after carefully freeing it from the soft tissues that remained inserted in it, while always respecting its chondral cap and the perichondrium cover. Fig. 2 shows the procedure for resecting osteochondromas with a posterior seating in graphic form. When half or more of the circumference of the humerus was resected in cases of broad implantation of the osteochondroma in the cortical perimeter of the humerus, and even though this is not a load-bearing bone, the segment of the humerus was reconstructed using an inserted autogenous graft that was formed or not in the proximal epiphysis and affixed with screws (4 cases). In all cases the operation concluded with the reinsertion of the tendons that needed this, the positioning of a Redon-type drainage and immobilisation of the shoulder.
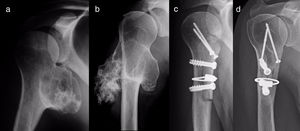
Diagrammatic representation of the block resection of an osteochondroma implanted in more than half of the posterior face of the proximal humerus: an anterior longitudinal approach (discontinuous line) and exposure of the osteochondroma using levers (a); humeral osteotomy with a chisel after drilling perforations (b); manual extraction of the osteochondroma (c); and reconstruction using carved homograft fixed with 3 screws (d).
In all of the patients who had been subjected to tendon reinsertion or bone reconstruction, the shoulder was immobilised during 3–4 weeks, although pendular movements were permitted immediately. Immobilisation in the other patients took the form of a simple sling. Only the patients who had received a graft required the assisted rehabilitation of the shoulder. All of the patients were examined as out-patients until their medical discharge when, in the absence of complications, it was considered that functional recovery could not improve any further. They were all re-evaluated at the present time by means of a clinical or clinical interview for this work.
Statistical analysisInformation was collected in a database created using the Access 2000 programme by Microsoft. Once data had been revised and processed statistical analysis consisted of the descriptive analysis of the variables, calculating the distribution of frequencies for qualitative data and by using the median and interquartile range (IQR) for quantitative data.
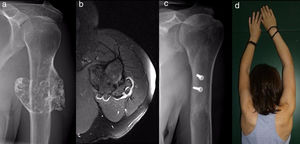
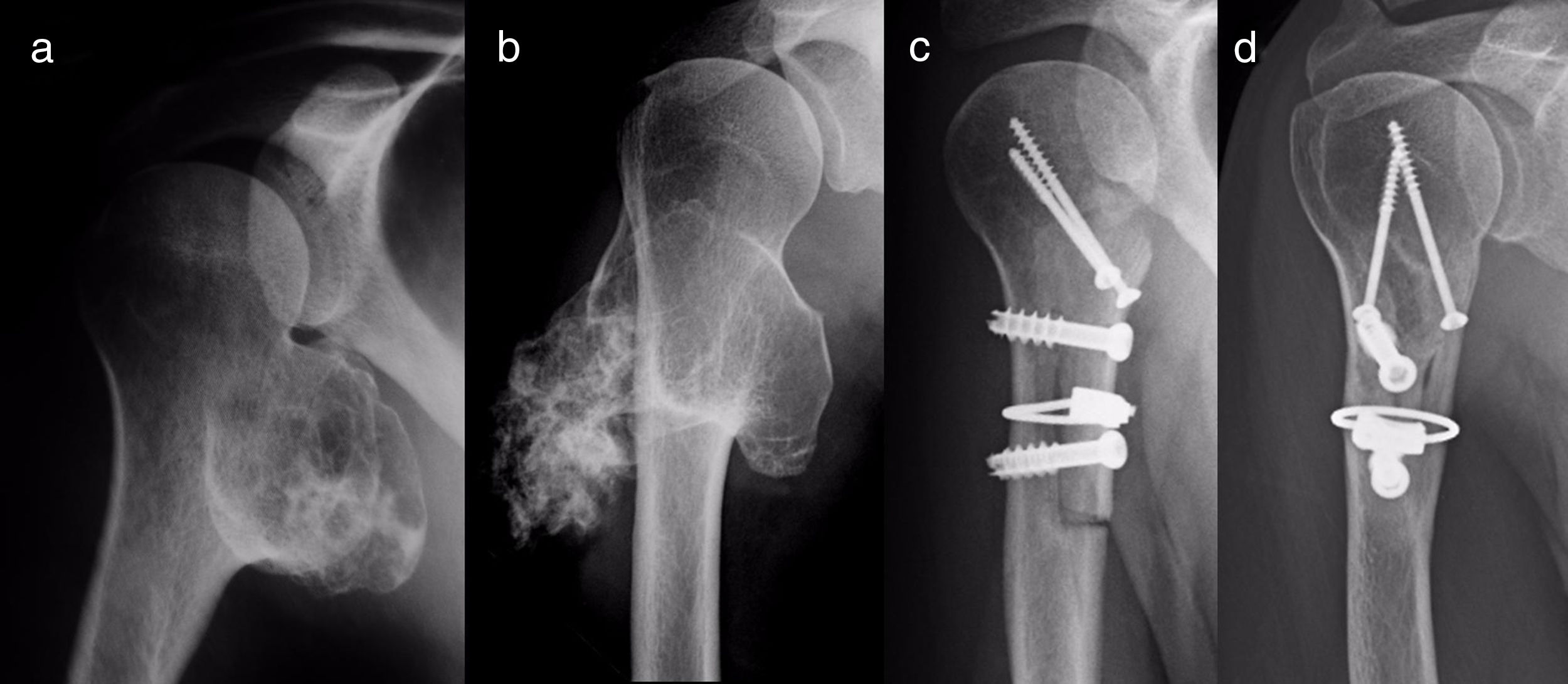
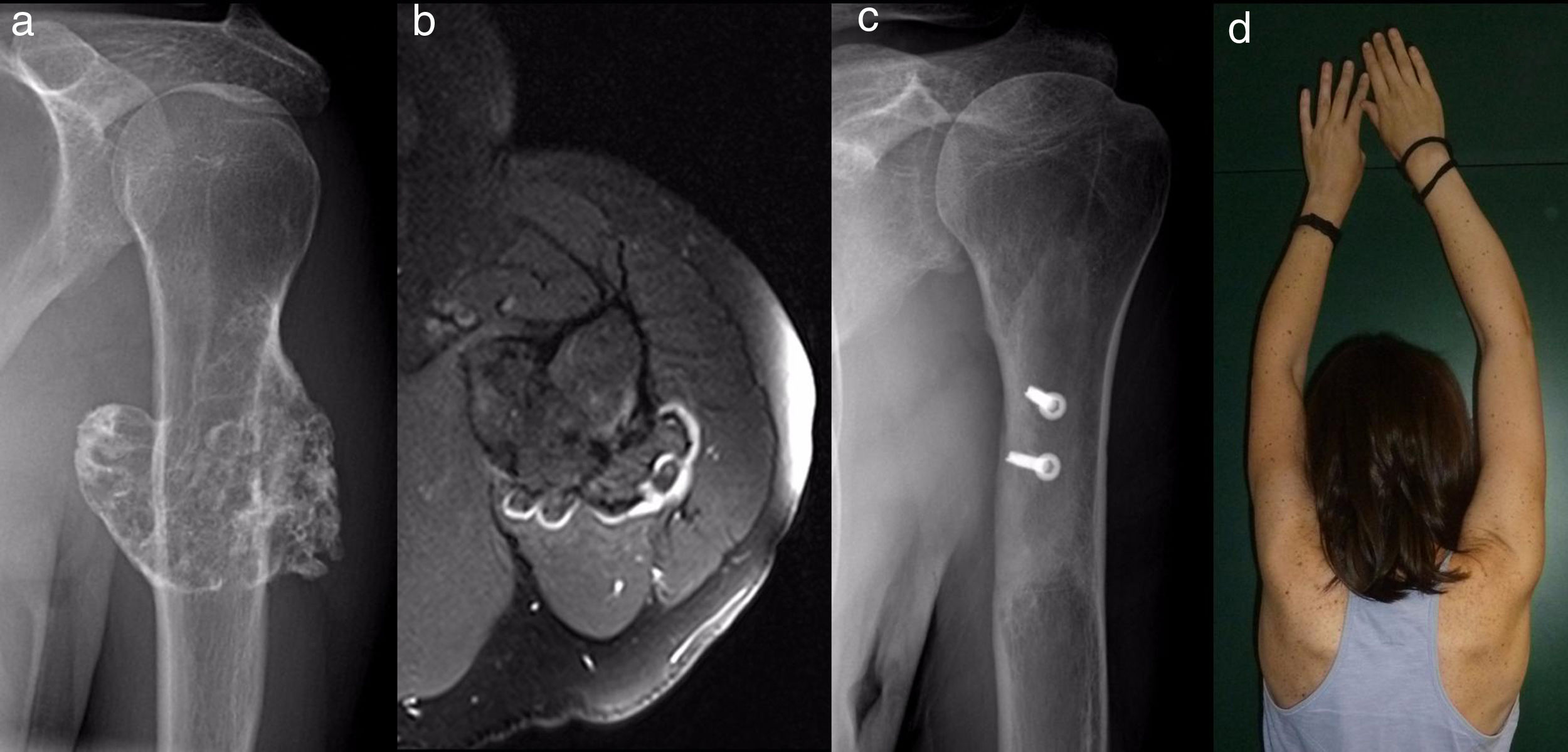
ResultsEpidemiological and clinical results together with therapeutic indications11 Of the patients with SOC were men (55%) and 9 were women (45%), with an average age at diagnosis of the tumour in our unit 21 years old (IQR: 7–64 years old). Eighteen (90%) patients mentioned pain or swelling as the primary symptom. In 2 patients the diagnosis was accidental, due to an X-ray taken for another reason. 9 patients (45%) said that the pain was slight, and they sometimes described it as discomfort generally associated with physical activity. 17 patients mentioned the swelling that they, their mother or in one case a physiotherapist had noticed. Three of them, 2 of whom were still growing, expressed the feeling that the tumour had grown in size. Scapulohumeral mobility was conserved in all cases. The patient with an unconfirmed suspicion of a secondary chondrosarcoma was 40 years old and had a known osteochondroma from the age of 24 years old, which had grown and changed radiographically over the previous 2 years (Fig. 3a and b).
All of the patients were informed about the disease and the possibility of surgical or conservative treatment. This decision was taken in agreement with the patient and their family, depending on their symptoms and the intensity of the same and the suspicion or not of degeneration. Twelve of the patients (60%) with SOC were operated, the majority because of their symptoms. One had the suspicion of degeneration due to increase in tumour size and changing radiological appearance. The average time from their first visit to our unit and surgical treatment was 272 days (IQR: 8 days-5 years and 3 months), with an average age at the moment of surgery of 23 years old (IQR: 10–46 years). The 8 patients (40%) who were not operated as they were asymptomatic or had occasional well-tolerated discomfort were monitored clinically and radiographically in our unit or their General Practitioner.
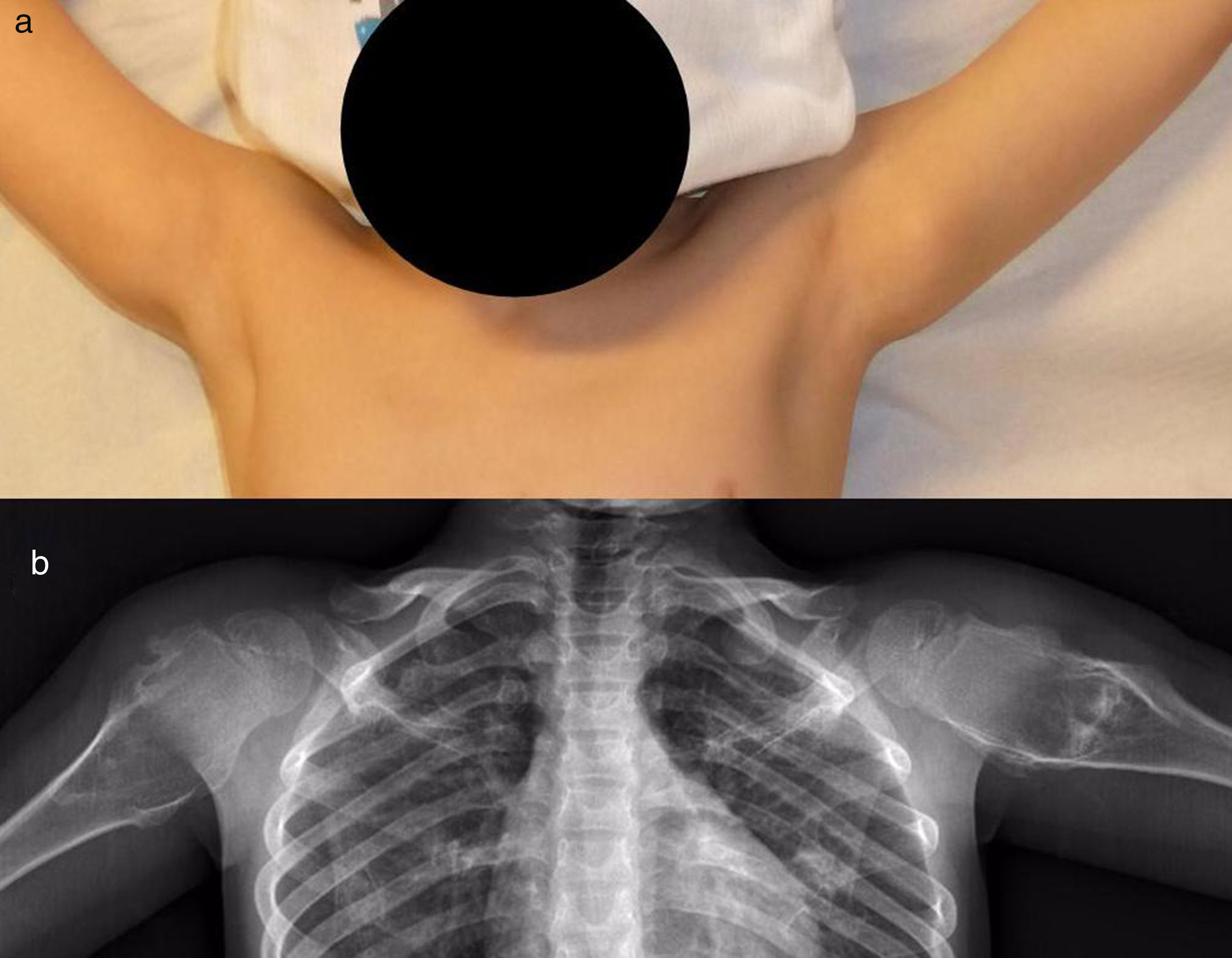
The patients with MHOC were 11 men (91.67%) and one woman (8.33%), with an average age at their first visit to our unit of 37 years old (IQR: 4–75 years old). Eight (67%) mentioned some type of pain or swellings (Fig. 4); the 3 most symptomatic (25%) patients were operated after an average of 29 months after their first visit. The average age of these patients at the moment of surgery was 34 years old (IQR: 16–25 years old). It was decided to keep the patients with few symptoms and without the suspicion of malignisation under observation, so they were monitored clinically and radiographically.
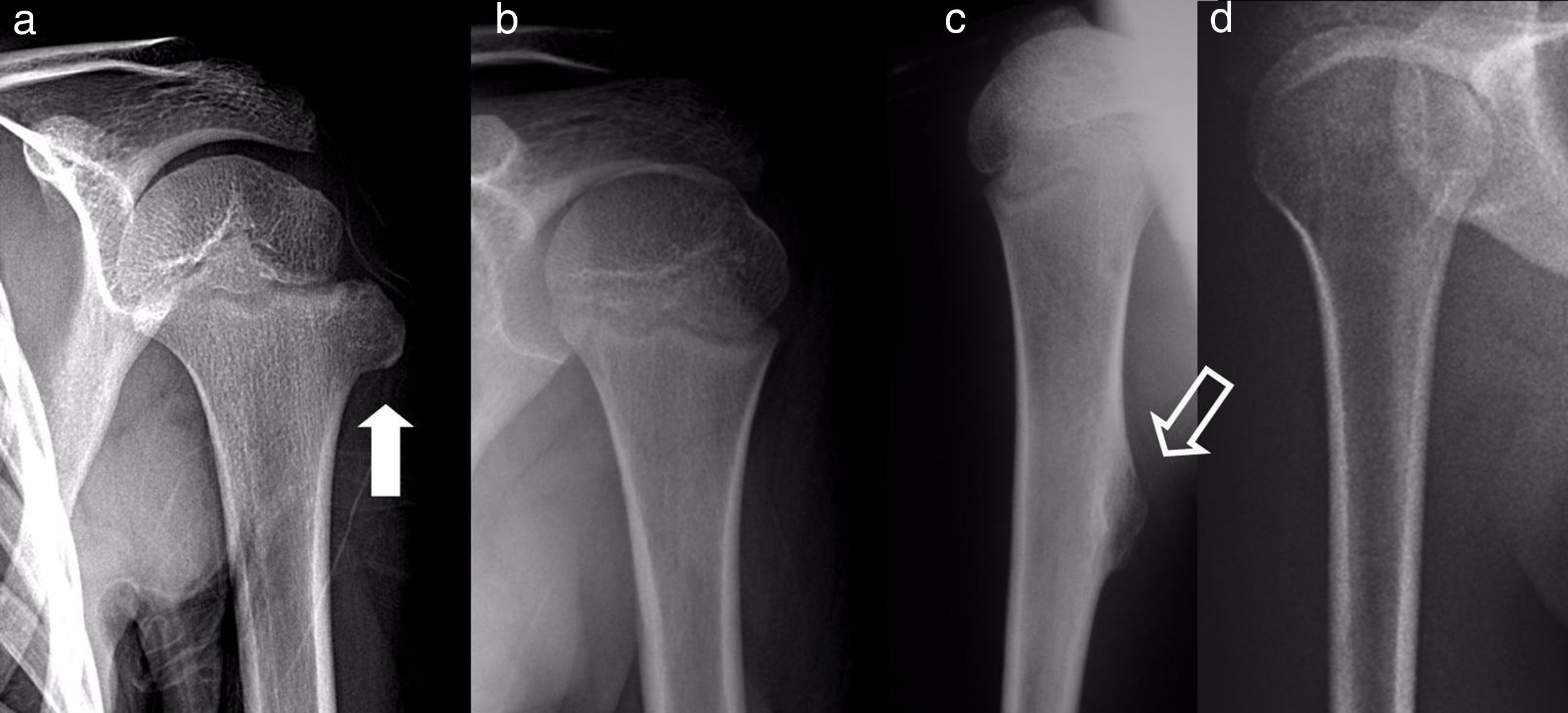
Radiographic resultsEighteen of the SOC tumours were sessile and 2 were pediculated. They were all based on the metaphysis or the proximal diaphyseal third, and they were implanted in all of the quadrants of the humerus circumference (Fig. 1). In the 15 cases in which CT or MR imaging was performed, the implantation base of the tumour occupied an average of approximately 30% of the perimeter of the humerus circumference in an axial plane.
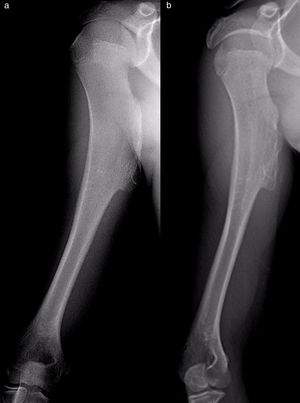
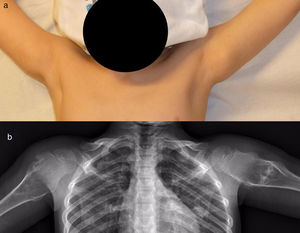
The results of treatmentTwo of the patients who were not operated seemed to experience the spontaneous regression of the osteochondroma: the tumour symptom with which they had presented ceased to be palpable and it disappeared from radiographic study repeated over time (Fig. 5). In the other cases the osteochondroma did not change and the patients remain under observation (Fig. 6) or have been discharged for observation by their General Practitioner with the instruction to consult again in case of pain, if the swelling increases in size or any other eventuality. The patients with MHOC continue with yearly check-ups due to their higher risk of malignisation.
All of the patients who were operated were admitted to hospital the day before surgery and were discharged from hospital after an average time of 2.5 days (IQR: 1–7 days) after surgery. Both pediculated forms were easily removed and there were no postoperative complications in any case, although one patient suffered an incomplete fracture as the result of a fall 2 months after surgery. The fracture set while keeping the arm in a sling, as did the autogenous grafts used in reconstruction in the 4 patients for whom this was indicated (Figs. 3–7c and d). At the end of the study there was no relapse of the tumour and the functioning of all the patients was excellent according to the Musculoskeletal Tumour Society8 scale.
DiscussionOsteochondromas are the most common benign bone tumours.9 The prevalence of the solitary forms in the population is calculated to stand at 3%, although this figure is an underestimate as many such tumours are asymptomatic and are not diagnosed. This is also the case in the proximal humerus, where 10–35% of all osteochondromas7 occur: these are the most common tumours in this bone segment. In our series they account for 13.16% of the total number of SOC that we treated during the study period. It is harder to calculate the incidence of humerus involvement in multiple forms of the tumour. Clement et al.10 identified 5361 osteochondromas in 172 patients belonging to 78 families with MHOC, of which 6.9% were located in the proximal humerus. In contrast with these data, the majority of the publications on osteochondromas of the PEH cover isolated cases or short series. The largest study we are aware of is the one by Bae et al.,7 which covered 31 patients with an average age of 13 years old who were treated surgically.
The symptoms of osteochondromas of the PEH, which are easy to diagnose based on an X-ray of a lesion in the surface of the bone that prolongs its cortical and spongy tissue in the humerus, may be restricted to a hard swelling adhered to the deep bone plane or a local more or less clear deformity. On other occasions, which are rare according to the literature and our experience, the symptoms may arise due to the compression or irritation of neighbouring anatomical structures such as vessels,6 vessels containing serum,2 nerves5 or tendons.3,4 When these tumours grow after bodily growth has ceased, if they become unexpectedly painful or change their radiographic appearance, if previous X-ray images are available, then the possibility of sarcomatose degeneration must be considered. This makes it necessary to perform MR imaging tests to determine the thickness of the cartilaginous cap of the osteochondroma. Although it is controversial, when this is more than 2cm thick in an adult or 3cm thick in a child11 the suspicion is very strong and may justify treatment without the need for a biopsy, taking into account the difficulty of a histopathological diagnosis.12 This study did not intend to examine this complication, which occurs in fewer than 1% of solitary forms and in 3–25% of the multiple hereditary forms,1 with special risk in scapular sites,10 and which one of our patients may have experienced. This case shows how important it is to suspect malignisation13 and the need, given that there is no specific moment at which the chromosome instability that will genetically trigger it becomes manifest,14 to anticipate the pathological changes that will confirm this suspicion. The development of immunohistochemical diagnostic tests (such as Bcl-2 marker expression), flow cytometry tests and cytogenetic tests of chondrocyte phenotype and the expression profiles of different types of collagen to foresee the biological behaviour of a chondral tumour may change the way these tumours are managed. This would involve the early treatment of those osteochondromas which have the possibility of becoming malign.14
Once an osteochondroma has been diagnosed by clinical and radiographic data, if it is symptomatic it should be resected in an extraperiosteal block around its implantation base: this should be planned in detail using CT or MR imaging techniques to determine its exact location and extension on the humerus, which may require reconstruction. The location and state of adjacent vascular and nerve structures that have to be respected must also be determined. Asymptomatic cases may be monitored, informing the patient of the disease and maintaining clinical check-ups and imaging tests. Parents’ worries must be taken into account, above all for young patients, and this may make excision recommendable in selected cases. If this is so it would be prudent to delay surgery until skeletal maturity and plan it as convenient to minimise the risk of local relapse15 and damage to the proximal physis of the humerus, which would be less important. Another justification for avoiding early surgery and preventing unnecessary risks is the possibility that the tumour will remit spontaneously, as 25 such cases were reported prior to 2014,16,17 and this cannot be ruled out in 2 of our patients. The theoretical mechanism for this would be inclusion of the tumour within the humerus by a bone remodelling process when tumour growth ceased before skeletal maturity. When the decision is taken to apply conservative treatment in the multiple form of the disease, annual check-ups are obligatory for the patients. These have to be thorough due to the above-mentioned increased risk of malignisation in some of the osteochondromas.
In any case, analysis of the symptoms and therapeutic indications of the patients in our series seem to show that surgery is indicated more often in cases of SOC, and that it takes place in younger patients than is the case for MHOC, with figures of 60% and an average age of 23 years old, respectively, in the former and 25% and an average age of 34 years old in the latter. As the proportion of symptomatic cases is the similar in both groups, the explanation for this may be that a single tumour, whether or not it is symptomatic, is more worrying than the same tumour in the context of multiple exostosant disease. In such disease the patient tolerates the discomfort better and it is considered to be relatively unimportant by the doctor, who centres his attention on the possibilities of malignisation. Due to this, when there is no suspicion of malignisation doctors will delay or rule out the indication of excision for osteochondroma. It may therefore be inferred that the excision of some hardly symptomatic SOC may be unnecessary.
The decision on the approach to be used is an essential part of the planning to ensure effective and safe excision of a PEH osteochondroma. Effective means that the tumour is removed in a single piece and without harming its chondral cap to prevent incomplete excision6 and local relapse, which are estimated to occur in less than 2% of occasions.18,19 A safe excision means that no fractures or iatrogenic neurovascular damage is caused. The incision is usually deltopectoral, although it may also be lateral, medial, posterior or a combination of these,7 depending on the seat of the tumour. When it is posterior, small and originates in the proximal 8cm of the humerus, Berger and Buckwalter's posterior route may be useful.20 When it is posteromedial, access—which is narrow and risks neurovascular damage—may be direct or indirect. A direct medial approach, which has the additional advantage of hiding the scar in the axilla, should not go beyond the axillary fold and should use the wide dorsal muscle as its guide.7 An indirect approach uses the deltopectoral and makes it possible, after the necessary tenotomies (of the greater pectoral muscle, the wide dorsal muscle, the greater round and subscapular muscles), to externally rotate the shoulder and expose the tumour relatively safely, in the opinion of those who support this technique.21 In our series all of the cases used an approach through the anterior third of the arm circumference. We believe it is noteworthy that even in 2 cases of broad posterior implantation the purely anterior route was used.
For reconstruction, when a major part of the diaphyseal cortical circumference of the humerus has been sacrificed—which usually occurs when block resection of an osteochondroma which surrounds and often has a sessile implantation is attempted—it seems prudent to use reinforcing grafts. Taminiau et al.22 use 2 fragments of autogenous tibia lodged in the defect and complemented with spongy autogenous graft, while Gebhart et al.23 use autogenous graft from the free fibula, which they divide in 2 and fix with 3 tirings. We prefer structural carved homograft fixed with screws, to prevent the morbidity of an autogenous graft, accepting slower consolidation and remodelling due to its lower osteogenic potential.
The results of the surgical treatment of PEH osteochondroma are good when the above recommendations are followed. Nevertheless, it is not free of complications: residual pain (6%), rigidity, neurovascular damage and fractures, etc.7 In our series, and ruling out local relapse as an intrinsic complication and an incomplete fracture due to a fall, we recorded no noteworthy relapses and the functional results were good in general.
The chief strength of our study is that all of the cases were diagnosed and treated by the same team, so that the overall treatment of the entity was uniform. The referral of all tumours or lesions with the suspicion of being tumours in our health catchment area to our unit may be considered to be a strong point of the epidemiological study, although the sample also includes a case from other catchment areas within the autonomous community of Castile and León, while the majority of other asymptomatic lesions will surely have escaped diagnosis. All of our surgical cases were subjected to CT or MR imaging tests, as opposed to the few cases in which this was so in the series of Bae et al.,7 who only made measurements using X-ray images, which is a limitation due to the variations in radiographic orientation. MR or CT imaging tests, we insist, are of diagnostic interest in some cases and are fundamental in the planning of surgery.
Our study has several limitations. The first of these is its retrospective design and low level of evidence as it covers a series of cases. The second limitation is the size of the sample, which is relatively small. It would be even more limited if it were divided into subgroups according to skeletal maturity and number of osteochondromas. Without this being a justification, these limitations are usually inevitable in tumour diseases. Nevertheless, the sample is comparable with those of the few other studies of this entity, as was pointed out above. For statistical purposes there would be a distortion in the patients with multiple exostosant disease who were examined in our unit as not all of them were systematically examined for the presence of osteochondromas in the proximal humerus: it is very probable that there would be more cases than the ones included in the study sample. As radiographic measurements were not taken in these cases this may also distort the results, although we understand that such measurements may, due to the difficulty in taking them, have distorted those of the SOC cases, which are of sufficient importance to be considered apart. However, the radiographic measurement method was neither exact nor validated. Respecting the duration of the follow-up period, which may in some cases be understood to constitute a cut-off point, above all for the youngest patients, we believe that this is not the case due to the benign nature of the entity in question and because the majority of the operations on patients took place after skeletal maturity. We therefore believe this work to be appropriate and that it meets the set objectives.
To conclude, the semiology and epidemiology of PEH osteochondromas do not differ significantly from those of osteochondromas in other locations. Asymptomatic cases can be monitored, and it is prudent to delay surgical excision in symptomatic cases until growth has terminated, to prevent damage to the physis and to reduce the risk of local relapse, in the knowledge that spontaneous regression of the tumour is theoretically possible. There should be no delay when malignisation is suspected. Surgical treatment may be summarised to consist of planning of the approach using CT or MR imaging techniques, resection of the extraperiosteal block and eventual reconstruction of the bone, which may take place using a homograft.
Level of evidenceLevel of evidence III.
Ethical responsibilitiesProtection of human and animal subjectsThe authors declare that the procedures followed comply with the ethical norms of the responsible human experimentation committee, the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that they followed the protocols of their centre of work governing patient data publication.
Right to privacy and informed consentThe authors obtained the informed consent of the patients and/or subjects referred to in this paper. This document is held by the corresponding author.
Conflict of interestsThe authors received no financing for this work and have no conflict of interests to declare.
We would like to thank the traumatologists who refer their patients to us in trust.
Please cite this article as: Ramos-Pascua LR, Sánchez-Herraéz S, Casas-Ramos P, Mora-Fernández M, Izquierdo-García FM. Osteocondromas del extremo proximal del húmero. Manejo diagnóstico y terapéutico. Rev Esp Cir Ortop Traumatol. 2018;62:168–177.





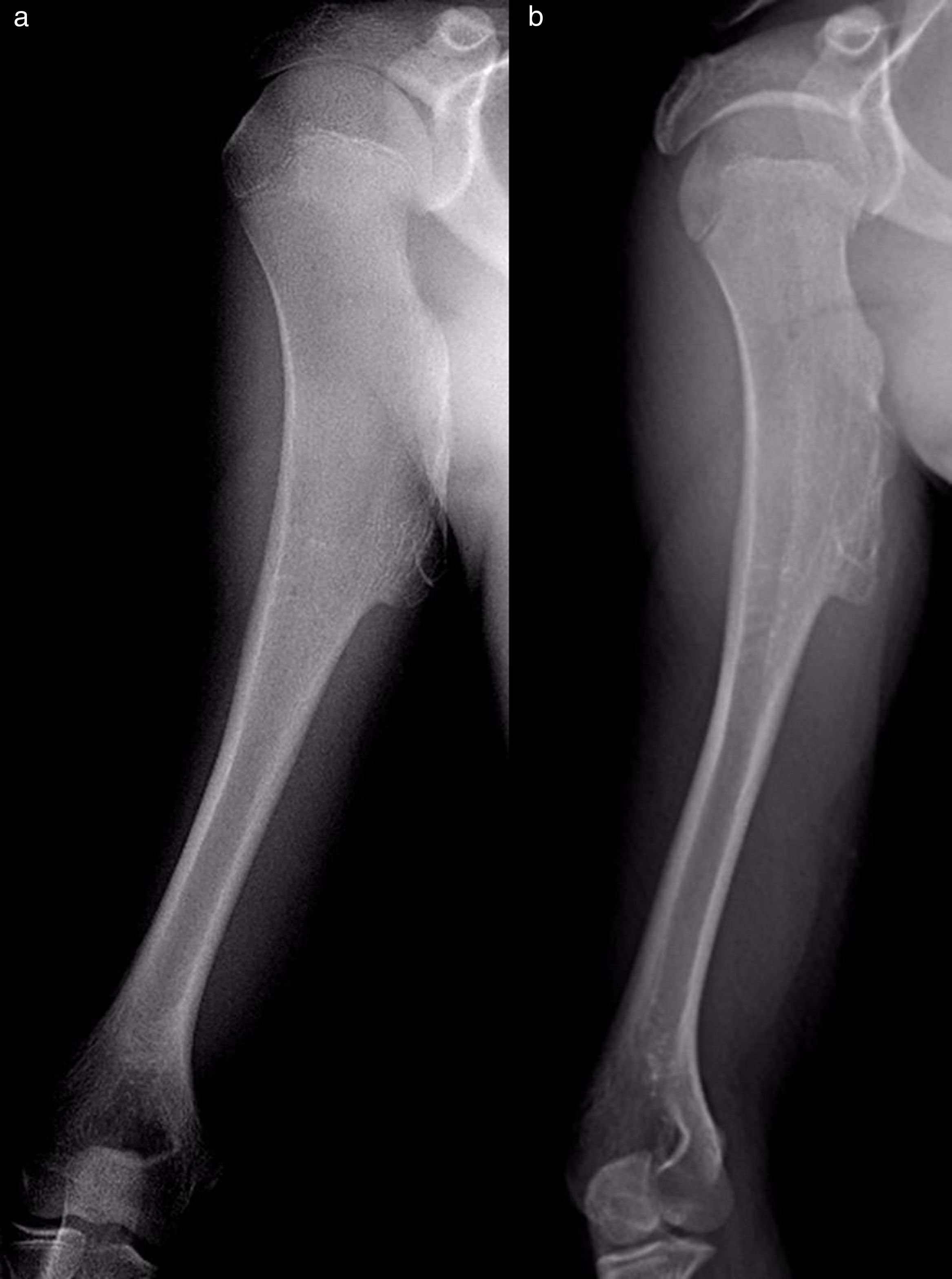
![Osteochondromas of the proximal end of the humerus in a 7 year-old boy (black arrow [a]) and in an 11 year-old girl (outlined arrow [c]), with spontaneous regression 1 and 2 years later, respectively. Radiographic imaging tests 2 and 6 years later (b and d). Osteochondromas of the proximal end of the humerus in a 7 year-old boy (black arrow [a]) and in an 11 year-old girl (outlined arrow [c]), with spontaneous regression 1 and 2 years later, respectively. Radiographic imaging tests 2 and 6 years later (b and d).](https://static.elsevier.es/multimedia/19888856/0000006200000003/v1_201805060426/S1988885618300300/v1_201805060426/en/main.assets/thumbnail/gr5.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)