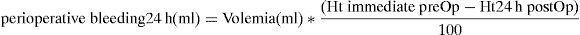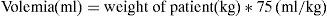A review of the perioperative management of patients with hip fractures and concomitant therapy with antiplatelet agents, and to analyse the differences in mortality and perioperative bleeding in early surgery (<48h) versus delayed surgery (>5 days).
Platelet aggregation was measured on admission and immediately before surgery in all patients included in the study
Patients and methodsA total of 175 patients over 65 years old, with low energy hip fracture were randomised into 3 groups: Patients on antiplatelet therapy undergoing early surgery, patients on antiplatelet therapy undergoing delayed surgery, and patients not on antiplatelet therapy undergoing early surgery. The same clinical and laboratory data were collected prospectively up to 12 months for all the patients.
The platelet aggregation was determined by a semi-quantitative computerised system based on impedance aggregometry in whole blood.
ResultsBleeding, transfusion requirements and analytical results showed no significant differences between groups.
More than half (59.8%) of the patients not taking antiplatelet therapy had normal platelet aggregation on admission, while 13.5% of those taking antiplatelet agents did not have that.
Multivariate analysis showed increased mortality at 12 months for the variables, low Barthel index before hip fracture (OR: 0.9–0.9) and number of transfusions (OR: 1.1–1.5).
The average length of stay was 4.1 days greater in the delayed surgery group.
ConclusionEarly surgery for patients receiving antiplatelet therapy has similar clinical outcomes to the delayed, but improves hospital efficiency by reducing the average length of stay.
The antiplatelet drug reported by the patient showed low concordance with the determination of the platelet aggregation.
Revisar el tratamiento perioperatorio de los pacientes con fracturas de cadera y tratamiento concomitante con antiagregantes plaquetarios, así como analizar las diferencias de mortalidad al año, y el sangrado perioperatorio según la pauta de cirugía precoz (<48h) vs. demorada (>5 días).
Paralelamente, determinar al ingreso y en el preoperatorio inmediato la agregabilidad plaquetaria en todos los pacientes incluidos en el estudio.
Pacientes y métodoSobre 175 pacientes mayores de 65 años con fractura de cadera de baja energía se aleatorizaron 3 grupos: antiagregados con cirugía precoz, antiagregados con cirugía demorada, y no antiagregados con cirugía precoz; se recogieron prospectivamente los mismos datos clínicos y analíticos para todos ellos.
La agregabilidad plaquetaria se determinó mediante un sistema informatizado semicuantitativo basado en la agregometría por impedancia en sangre completa.
ResultadosEl sangrado, los requerimientos transfusionales y los resultados analíticos no mostraron diferencias estadísticamente significativas entre los grupos.
Un 59,8% de los pacientes que no referían tomar antiagregantes se encontraban analíticamente antiagregados al ingreso, mientras que un 13,5% de los que tomaban antiagregantes no se encontraban correctamente antiagregados.
El análisis multivariante mostró mayor mortalidad a 12 meses para las variables del índice de Barthel bajo previo a la fractura (OR: 0,9-0,9) y número de transfusiones (OR: 1,1-1,5).
La estancia media fue de 4,1 días mayor en el grupo demorado.
ConclusiónLa pauta de cirugía precoz para los pacientes en tratamiento antiagregante tiene resultados clínicos parecidos a la demorada, pero mejora la eficiencia hospitalaria al reducir la estancia media.
La antiagregación farmacológica referida por el paciente resultó poco concordante con la determinación de la agregabilidad.
Hip fractures (HF) are considered the most serious osteoporotic fractures because they are associated with increased morbidity and mortality,1,2 and have a significant economic impact on society, both due to their frequency3,4 and to the direct and indirect health costs they generate.
Due the widespread use of antiplatelet agents (AP) and the high incidence of HF among the elderly population, it is common for both events to coincide in the same patient. This necessitates taking into account the theoretical risks associated with surgical bleeding and, therefore, influences the choice of anaesthetic technique. The lack of consensus on perioperative AP treatment makes it into a key issue in medical literature.5,6
Surgical delay in the treatment of hip fracturesThe standard treatment for HF is surgical intervention within the first 24–36h following admission, so as to minimise pain, reduce hospital stay, limit pharmacological requirements, reduce morbidity and mortality, and anticipate functional recovery.7–12 Some studies go further and recommend ignoring any interference which may cause a delay in surgery more than 48h unless it is life threatening, thus considering HF as a genuine surgical emergency.13 Most protocols and clinical guidelines agree that the optimal moment for intervention is whenever the patient is in the best possible condition. Moreover, they also agree that, if a reversible complication takes place, it is reasonable to delay the intervention until the condition is resolved.14–16 Nevertheless, a British study from 2007 concluded that, in up to 75% of cases, this early approach could not be applied due to unavailability of the surgical area.17
There are also numerous publications which take the opposite view to that recommended in the guidelines.18 The study with the largest number of patients describes a higher mortality for the early surgery pattern.19
The time delay until the intervention is just one of the factors involved in perioperative complications and the final outcome. Other factors such as age, nutritional status, previous comorbidities, socioeconomic status and even the experience of the surgical team also affect morbidity and mortality in HF,20–22 so that, despite the theoretical advantages of an early intervention, the ideal time for surgical treatment remains controversial and still under review and study.
Antiplatelet agentsAntiplatelet drugs are used for prophylaxis or treatment of arterial thrombosis episodes,23 which are especially prevalent among the elderly. Almost 50% of HF cases among the elderly associate their admission with the concomitant use of an AP drug.24
AP agents act on primary haemostasis by blocking one of the multiple platelet activation pathways,25 thus producing a partial inhibition of aggregability which results in a lengthening of bleeding time, although this is not highly significant in clinical terms.26 In addition, there is a wide range of responses to AP agents, either due to idiosyncrasy, to not following the treatment regime, to drug interactions or to environmental and dietary factors. It is because of these factors that platelet aggregation objectified by an analytical study does not always correspond with expectations in a given patient, depending on whether or not the patient is following treatment with an AP drug.
Until few years ago, measurement of platelet activity had been carried out through bleeding time: a qualitative and poorly reproducible method, so it was not performed routinely. The platelet function analyser through high flow on an in vitro membrane support (PFA-100®, Siemens) was only used in some cases, but at the time of the study design it was only validated for measuring acetylsalicylic acid (ASA).27
A platelet aggregation measuring system based on aggregability induced by impedance28 on whole blood has been developed in recent years. It is easy to apply, has high reproducibility and sensitivity, and can also measure other platelet aggregation pathways, so it has been included in recent clinical guidelines of platelet function analysis.29 At present, this system is still not widely available in hospital laboratories, so it is not routinely employed.
Anaesthetic considerationsThe type of anaesthesia employed in HF has also undergone reviews in guides and protocols.30–32 General anaesthesia has been associated with increased bleeding in thromboembolic complications and postoperative confusion. By contrast, regional anaesthesia has shown better functional recovery and a lower rate of systemic complications. However, no significant differences have been found between the two alternatives in terms of mortality, so there is not enough scientific evidence to indicate one type of anaesthesia as standard for hip surgery.32,33 Therefore, there is no anaesthetic standard for this condition and the most appropriate technique must be individualised for each case. One of the factors to take into account when selecting the anaesthetic technique is precisely whether the patient is following treatment with AP agents.
Guidelines and protocols from the beginning of last decade34–36 recommended delaying intervention until a functional platelet pool had been recovered (5–14 days, depending on the AP agent), with the exception of low-dose ASA. In recent years, several publications have suggested that the increase in perioperative haemorrhagic risk attributed to antiplatelet therapy has been overestimated, while the increase in thrombotic risk assumed by withdrawing this treatment has been underestimated, especially in those patients with higher thrombotic risk.37–41 This change in attitude has been reflected in the new guide for perioperative management of AP agents in non-cardiac surgery from the Spanish Society of Anaesthesiology, published in May of this year.42
Objectives of the studyWe intend to establish a comparison between groups of patients with HF in terms of the 2 variables being considered: the surgical delay time (within 48h vs. more than 5 days), and the condition of platelet aggregation upon admission.
The primary objective of this study was to describe the effect of a delay of surgery on mortality in the 12 months following the intervention.
A secondary objective was to measure whether there were any differences in bleeding between the groups regarding haematocrit analysis and regarding transfusion requirements.
Another secondary objective was to assess the concordance between the analytical determination of aggregability of patients and their treatment with AP.
We believe that delaying surgery in patients with HF who are following antiplatelet treatment does not reduce mortality or increase bleeding significantly compared to the pattern of early surgery which is usually employed with patients who are not being treated with antiplatelet agents. By contrast, this delay pattern does lengthen the hospitalisation period and, consequently, direct health expenditure for each process.
Materials and methodThis was a prospective, randomised study on 206 patients aged over 65 years and admitted consecutively at Hospital Universitari Arnau de Vilanova in Lleida, Spain, with a diagnosis of low-energy HF during the year between September 2009 and September 2010.
Of these initial 206 patients, we excluded those with high-energy, pathological and periprosthetic fractures, as they were not the subject of this study. We also ruled out patients with known coagulopathies or platelet disorders, in order to reduce confusion during the measurement of platelet aggregation, as well as patients following anticoagulant treatment with coumarin agents. We include neither the patients with previous morbidities which conditioned the type of anaesthetic technique employed (such as severe chronic obstructive pulmonary disease and severe aortic stenosis) nor those patients who presented an absolute contraindication against the interruption of AP treatment (such as stroke and heart attack in the 6 months prior to the fracture and patients who carried intravascular devices). Finally, we did not include those patients who declined to be part of the study.
The study finally included 175 subjects, who were prospectively monitored from hospital admission until 12 months after surgery or until death. Follow-up was conducted by personal clinical controls in outpatient consultation at 1 month, 3 months and 6 months. The control at 12 months was conducted through a telephone interview. For those patients who could not attend personal health controls at the hospital, monitoring was conducted by telephone, with the patient or with the reference family member designated upon admission.
Patients and their families were offered the opportunity to participate in the study at the time of admission and were asked to sign an informed consent form before knowing the treatment pattern they would be assigned.
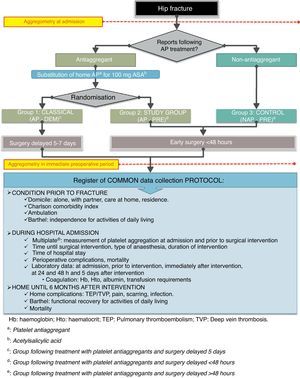
Patients were distributed according to whether they were following AP treatment upon admission or not. Patients who reported taking AP agents were randomly distributed into 2 groups using a sealed envelope system and their home-based antiplatelet treatment was replaced by 100mg of ASA (as recommended by anaesthetic guidelines42).
In addition, all patients who were not following AP treatment were assigned to a control group which followed the early treatment pattern. We ruled out the possibility of creating a control group of patients without AP treatment and a delayed surgical pattern because this was not the subject of the study and because this issue has already been extensively evaluated in other published works, as well as for ethical considerations.
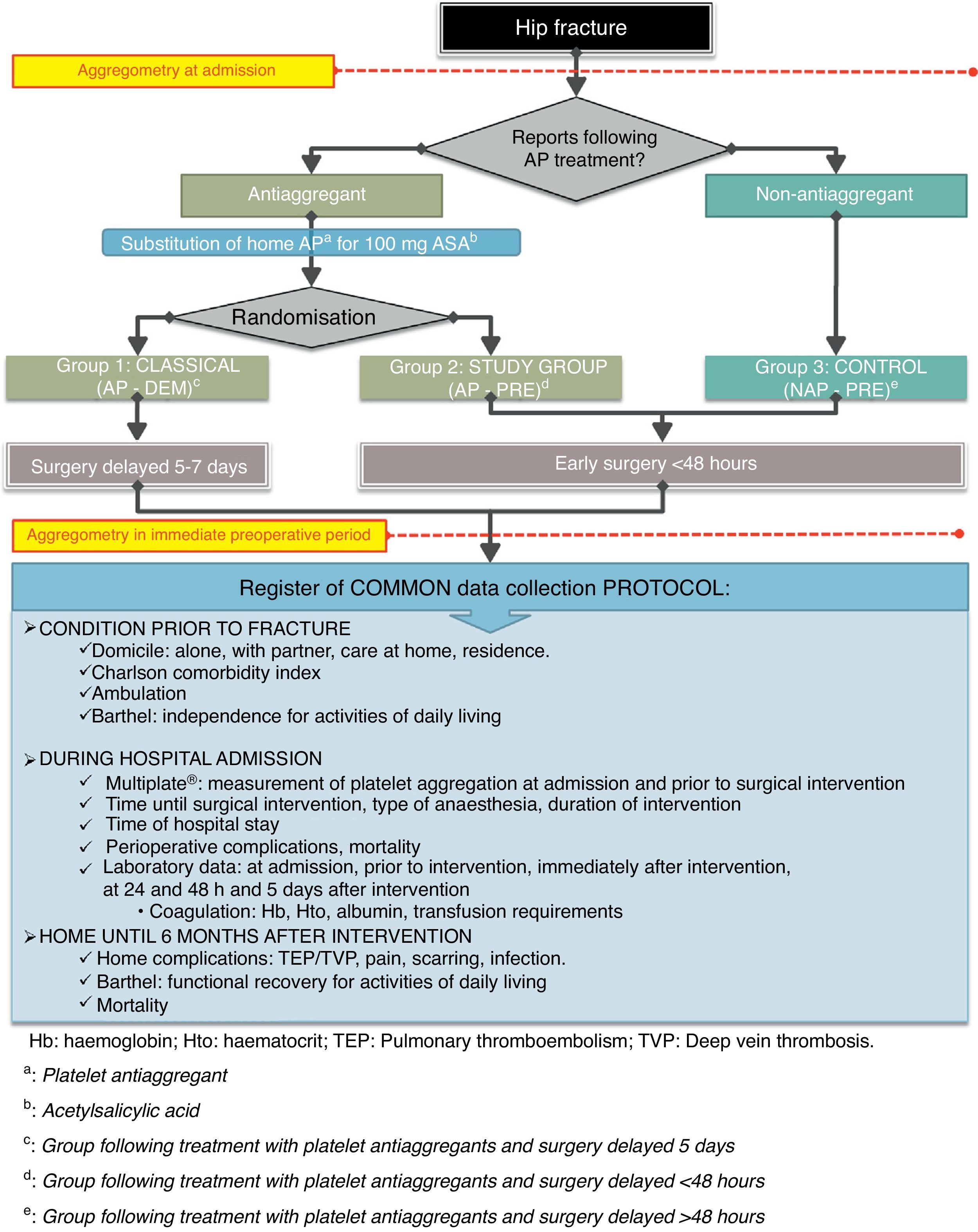
As explained, we divided patients into 3 groups, as summarised in Fig. 1 and as detailed below:
- -
Group of patients following antiplatelet therapy with a delayed surgery pattern (hereinafter AP-DEM group). We followed the usual pattern of treating patients following AP therapy by replacing their usual AP drug and delaying surgical treatment until beyond 5 days after admission.
- -
Group of patients receiving antiplatelet therapy with early surgical pattern (hereinafter AP-PRE group), which represented the study group. We performed surgical treatment within the first 48h after admission, as soon as there was availability of technical means, just as would be done in patients without antiplatelet therapy.
- -
Control group consisting of patients without antiplatelet therapy (hereinafter NAP-PRE group), in whom we followed the standard pattern of early surgery in the first hours, depending on the availability of technical means.
The choice of anaesthetic technique to be employed was left to the discretion of the anaesthesiologist, except in the AP-PRE group, since the theoretical risk of causing a spinal haematoma in case of a spinal puncture led to the use of general anaesthesia in all patients.
All procedures were conducted following the standard protocol of the Traumatology Service of Hospital Universitari Arnau de Vilanova in Lleida, in scheduled or emergency operating rooms, depending on the availability of the surgical area and randomly by physicians who were responsible for those patients. This protocol included preoperative antibiotic prophylaxis, which was administered after sampling for the measurement of platelet function, in order not to interfere with the analytical determination. We also followed the prophylaxis protocol for venous thrombosis with enoxaparin at doses adjusted according to weight and the protocol of the commission for prevention of thromboembolic disease at our centre.
During their admission, all patients were cared for by a multidisciplinary team which included members of the traumatology, geriatrics and rehabilitation services, as well as a social worker. As a general rule, we did not change the prior home treatment and did not implement guidelines for osteoporosis systematically.
We collected the same protocol for all patients included in the study, as detailed in Fig. 1.
Within the data protocol we recorded: origin upon admission, requirement for home care prior to the fracture, the Barthel Index and the Charlson comorbidity index. Regarding the analyses performed, these were the same as are conducted habitually: haemogram with cell count and composition, electrolytes and coagulation study, but we added the measurement of platelet aggregation as a new analytical determination upon admission and in the preoperative period immediately before surgery.
We used a multiple-electrode (Multiplate® Verum Diagnostica GmbH, Munich, Germany) computerised platelet function metre for the determination of platelet aggregation. This was based on impedance aggregometry on whole blood as described by Cardinal and Flower28 in 1980. This computerised, multichannel analysis system measures each platelet activation pathway, according to the variation of impedance between 2 pairs of independent, high-conductivity electrodes immersed in cuvettes containing whole blood cells at a physiological temperature. Platelets adhere to the electrodes under a specific activation in each cuvette by specific, commercially available reagents. The results are expressed as 2 superimposed graphs showing platelet aggregation, and also numerically in the form of arbitrary units called area under curve (AUC). The method was described in detail in 2006 in an article by Toth et al.43 The samples were collected in tubes containing hirudin (an anticoagulant agent, direct inhibitor of thrombin), and analysed by laboratory staff who had received prior training.
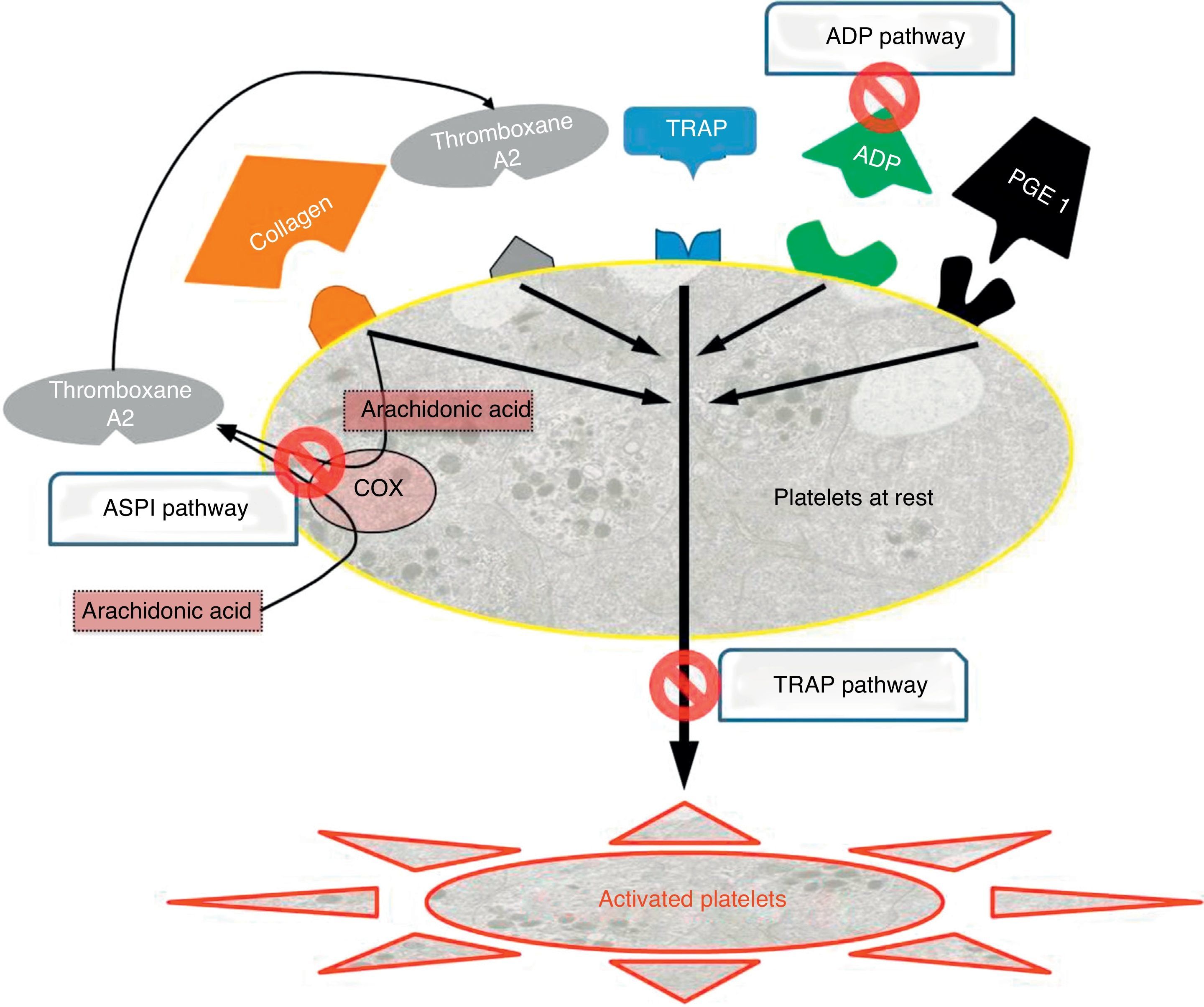
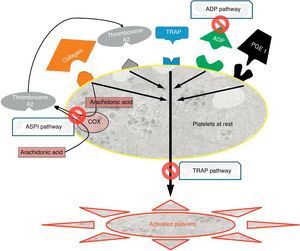
There are 3 types of drugs depending on the activation pathway which they target (Fig. 2), and these were the ones we determined systematically for all patients upon admission and before surgery:
- 1.
Adenosine diphosphate (ADP) pathway: ADP receptor inhibitors, which include, among others, clopidogrel (Plavix®, Iscover®), prasugrel and ticlopidine (Tiklid®). The reference values for the ADP pathway were taken from the study by Ranucci et al.,44 which set the cut-off value at 40 units of area under the curve (U AUC), and within the limits proposed by Gorlinger et al.45
- 2.
Arachidonic acid (ASPI) pathway: Cyclooxygenase-1 inhibitors (COX-1) represented, among others, by acetylsalicylic acid (ASA) and triflusal (Disgren®). We accepted similar levels for ASPI, namely 40U AUC.45
- 3.
Thrombin (TRAP) pathway: common IIb-IIIa pathway inhibitors, corresponding to overall platelet function, and performing the role of internal quality control. These are used intravenously in hospital environments and include abciximab, eptifibatide and tirofiban. In cases where this pathway was below functional levels (<40U AUC),45 we considered that there was a problem in overall platelet function, which could be due to multiple causes (thrombocytopenia, platelet dysfunction and drug interference, among the most frequent). Since we could not assess whether low aggregability was a consequence of the effect of AP drugs, in these cases patients were excluded from the assessment.
The aggregometry results where kept blind until the conclusion of the study, so as not to condition the decisions of the medical team responsible for each patient.
Perioperative bleeding was determined by a formula which measures the difference between the haematocrit determined in the immediate preoperative period and haematocrit at 24h postoperatively,46 and relates them with haematocrit upon admission, as well as patient volemia (or plasma volume) as detailed in the following equation:
Considering volemia as:
In addition to indirect measurement of bleeding we also recorded the transfusion requirements of each patient by counting the number of packed red blood cells transfused throughout hospitalisation.
The 3 distribution groups of patients in the study (NAP-PRE, AP-PRE and AP-DEM) allowed us to make 2 vs. 2 comparisons between:
- 1.
The patterns of early surgery vs. delayed surgery, in patients who were taking antiplatelet drugs at admission (groups AP-PRE vs. AP-DEM), in terms of bleeding, transfusion requirements and mortality.
- 2.
Patients who reported taking AP at admission vs. those did not, all of them undergoing early treatment (AP-PRE vs. NAP-PRE), in terms of bleeding, transfusion requirements and mortality.
- 3.
Regarding platelet aggregation determined by the Multiplate® system:
- •
Involvement of analytically-observed antiaggregation in terms of bleeding, transfusion requirements and mortality.
- •
Verifying the correlation between reported antiaggregation and analytical results for the population suffering hip fracture.
- •
The descriptive variables were expressed as mean±standard deviation or as percentages. The differences between the various groups were established using the Chi-square test for categorical variables and the ANOVA test for quantitative variables.
We performed a study of univariate and multivariate logistic remission, with hospital mortality as the outcome variable, calculating the odds ratio (with 95% confidence intervals) for the variables used. For the multivariate model we included all the variables in the model (full model) which were interesting for their predictive ability, and we selected the significant variables with a non-automatic system. The model was evaluated by calculating the ROC curve and the area under the curve (AUC).
We established a statistical significance level of P<.05. We used the software package SPSS® version 16.0.
The protocol for this study was approved by the Ethical Committee of Hospital Universitari Arnau de Vilanova.
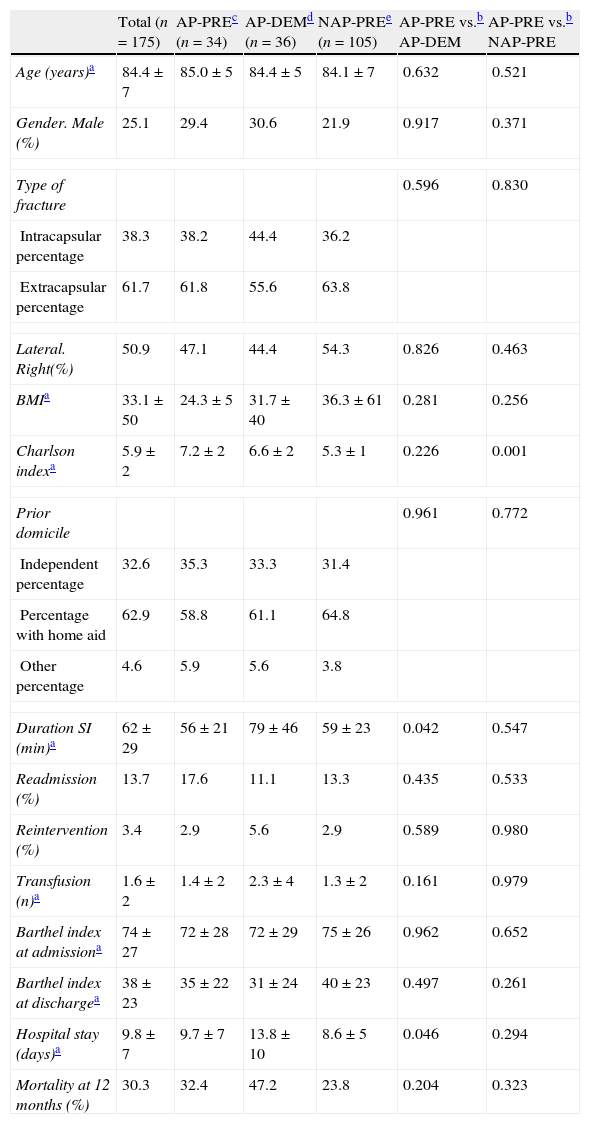
ResultsThe descriptive statistical analysis of our sample, which is detailed in Table 1, was comparable between the study groups except in 3 variables which showed statistically significant differences:
- 1.
A greater number of comorbidities in the Charlson index in the AP-PRE group following antiplatelet treatment, which was statistically significant compared to the NAP-PRE group not following antiplatelet treatment (7.2±2 vs. 5.3±1; P<.001), but with no significant differences regarding the comparison between the AP-PRE and AP-DEM groups following antiplatelet treatment (7.2±2 vs. 6.6±2).
- 2.
A longer mean admission period for the delayed surgery AP-DEM group regarding the early surgery AP-PRE group (9.7±7 vs. 13.8±10; P=.046).
- 3.
A longer mean duration of the intervention in the AP-DEM group compared to the AP-PRE group (79±46 vs. 56±21; P=.042).
Demographic characteristics (n=175).
| Total (n=175) | AP-PREc (n=34) | AP-DEMd (n=36) | NAP-PREe (n=105) | AP-PRE vs.b AP-DEM | AP-PRE vs.b NAP-PRE | |
| Age (years)a | 84.4±7 | 85.0±5 | 84.4±5 | 84.1±7 | 0.632 | 0.521 |
| Gender. Male (%) | 25.1 | 29.4 | 30.6 | 21.9 | 0.917 | 0.371 |
| Type of fracture | 0.596 | 0.830 | ||||
| Intracapsular percentage | 38.3 | 38.2 | 44.4 | 36.2 | ||
| Extracapsular percentage | 61.7 | 61.8 | 55.6 | 63.8 | ||
| Lateral. Right(%) | 50.9 | 47.1 | 44.4 | 54.3 | 0.826 | 0.463 |
| BMIa | 33.1±50 | 24.3±5 | 31.7±40 | 36.3±61 | 0.281 | 0.256 |
| Charlson indexa | 5.9±2 | 7.2±2 | 6.6±2 | 5.3±1 | 0.226 | 0.001 |
| Prior domicile | 0.961 | 0.772 | ||||
| Independent percentage | 32.6 | 35.3 | 33.3 | 31.4 | ||
| Percentage with home aid | 62.9 | 58.8 | 61.1 | 64.8 | ||
| Other percentage | 4.6 | 5.9 | 5.6 | 3.8 | ||
| Duration SI (min)a | 62±29 | 56±21 | 79±46 | 59±23 | 0.042 | 0.547 |
| Readmission (%) | 13.7 | 17.6 | 11.1 | 13.3 | 0.435 | 0.533 |
| Reintervention (%) | 3.4 | 2.9 | 5.6 | 2.9 | 0.589 | 0.980 |
| Transfusion (n)a | 1.6±2 | 1.4±2 | 2.3±4 | 1.3±2 | 0.161 | 0.979 |
| Barthel index at admissiona | 74±27 | 72±28 | 72±29 | 75±26 | 0.962 | 0.652 |
| Barthel index at dischargea | 38±23 | 35±22 | 31±24 | 40±23 | 0.497 | 0.261 |
| Hospital stay (days)a | 9.8±7 | 9.7±7 | 13.8±10 | 8.6±5 | 0.046 | 0.294 |
| Mortality at 12 months (%) | 30.3 | 32.4 | 47.2 | 23.8 | 0.204 | 0.323 |
BMI: body mass index; SI: Surgical intervention.
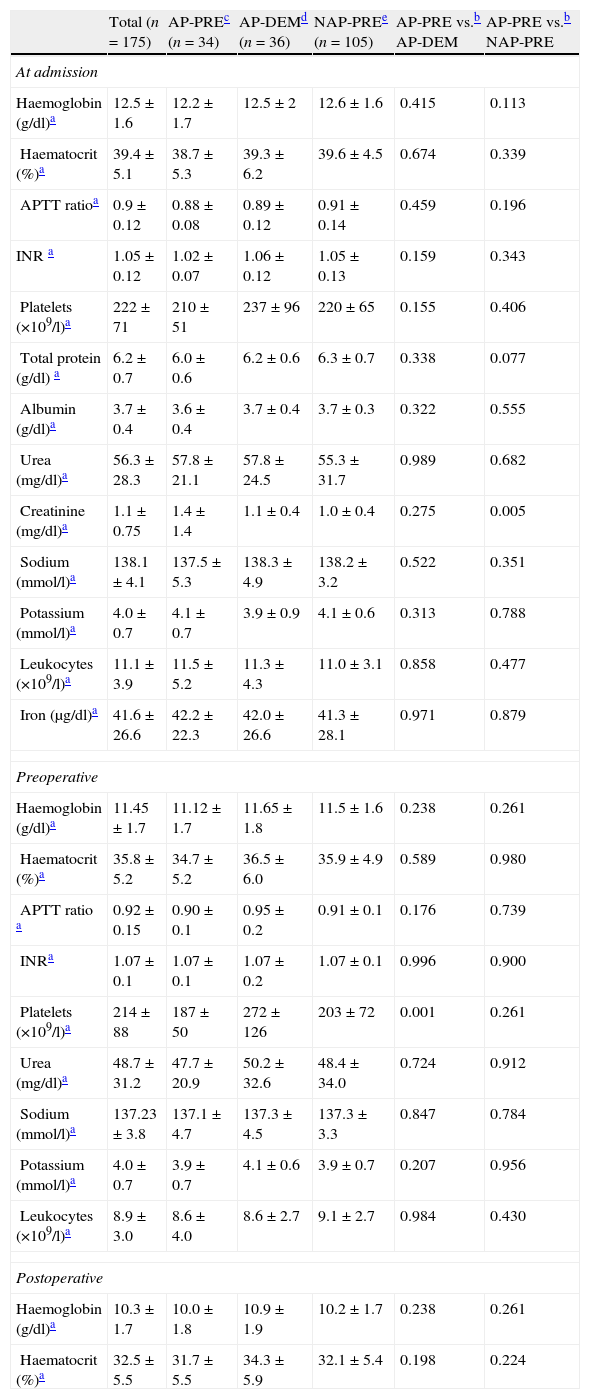
Regarding the usual laboratory values (haemoglobin, haematocrit, coagulation, platelets, urea, leukocytes, sodium, potassium, creatinine, protein, albumin and iron) collected for the common protocol upon admission and in the immediate preoperative period, we found no clinically significant differences in the comparisons between the AP-DEM vs. AP-PRE groups or between the AP-PRE vs NAP-PRE groups. The results, which are detailed in Table 1, showed some statistically significant findings, such as a difference in creatinine upon hospital admission between the AP-PRE and NAP-PRE groups (these were 1.1±0.4 vs. 1.0±0.4, respectively), which had no clinical significance. A similar statistical finding without clinical value was observed regarding the total number of platelets in the preoperative period between the AP-PRE and AP-DEM groups (187±50 vs. 272±126, respectively).
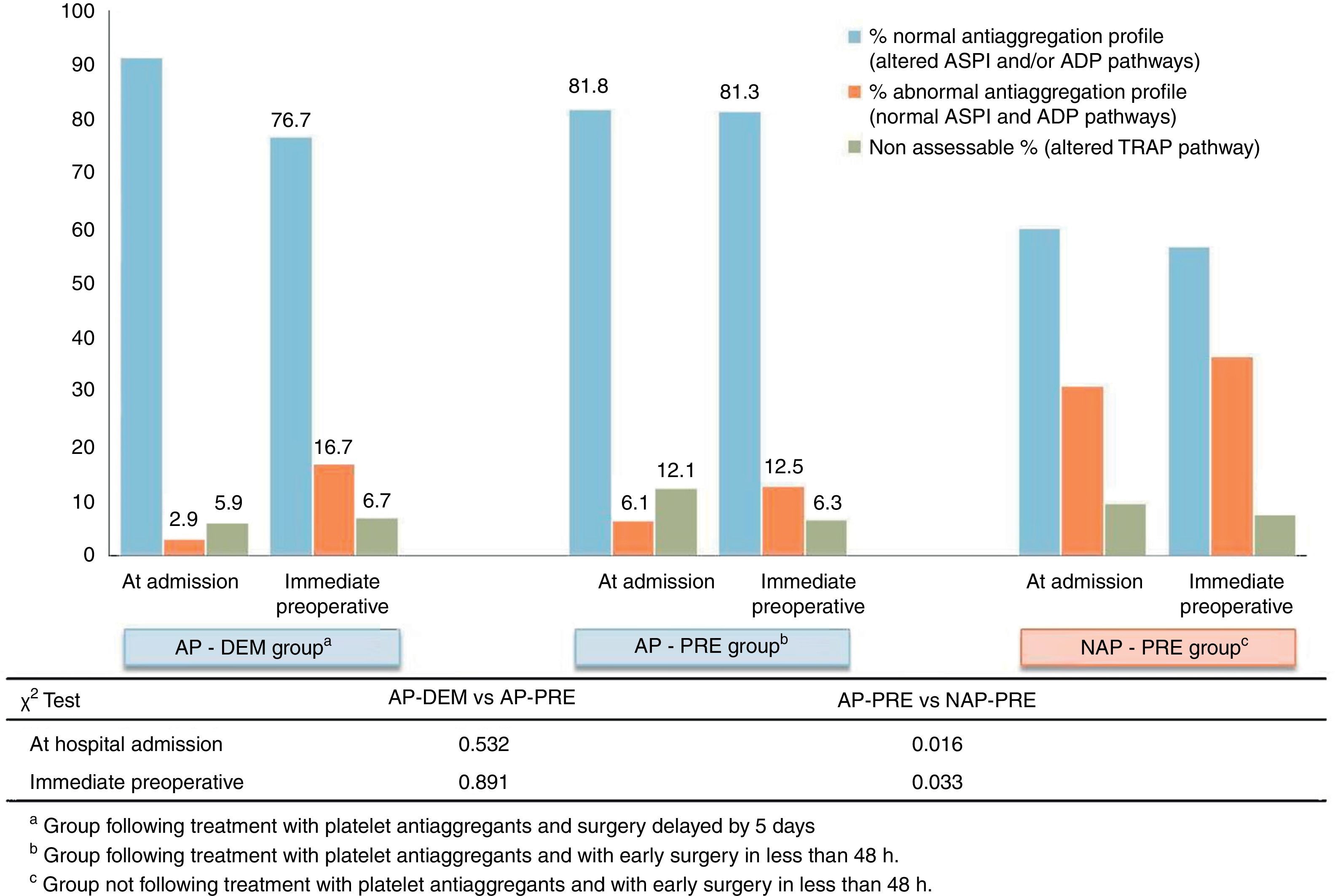
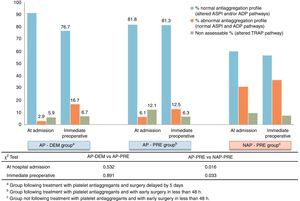
The results of impedance aggregometry showed a low correlation between the measured antiaggregation and the reports by patients upon admission. These results are summarised in Fig. 3. Patients who referred taking antiplatelet agents at admission presented the expected aggregation in 86.5% of cases. However, 59.8% of patients assigned to the group which reported not taking antiplatelet agents at admission (NAP-PRE), presented a state of platelet antiaggregation through some of the pathways analysed (ASPI or ADP) by Multiplate®.
Through analysis we detected a subgroup, corresponding to 9% of all tested patients, which presented an alteration of the common pathway of platelet aggregation (TRAP pathway). This analytical result invalidated the measurement of common platelet activation pathways performed by Multiplate®, since the results of the other pathways (ADP and ASPI) were altered and lost reliability. This subgroup was present in all groups (NAP-PRE, AP-PRE and AP-DEM) in a consistent and similar manner. These patients were counted in a separate section for the statistical analysis, as shown in Fig. 3.
In the early surgery group, measurements between admission and the immediate preoperative were concordant. The percentage of patients who were taking antiplatelet agents at the time of admission and maintained their antiaggregation profile in the immediate preoperative period in the AP-PRE group only varied by 0.5%, and this figure was 3.4% for the NAP-PRE group, whereas in the group in which the intervention was delayed 5 days after admission there was a decrease of 14.5% in the percentage of patients presenting antiaggregation, with 76.7% patients retaining antiaggregation observed analytically at the time of the intervention.
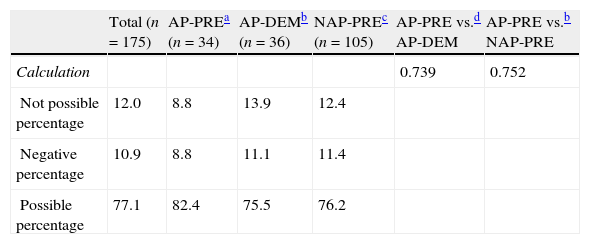
The calculation of perioperative blood loss (judged from haematocrit in the immediate preoperative period and 24h postoperatively) was obtained in 88% of patients in the study. The comparison could not take place for patients who lacked collection of a sample (either at admission or in the immediate preoperative period). Up to 10.9% presented negative absolute bleeding values and were also excluded from the evaluation. The estimated values for bleeding showed a tendency to be higher in the AP-PRE group (308±182ml) compared to the AP-DEM group (278±210ml), and the NAP-PRE group (265±128ml), without these differences presenting statistical significance (Table 2).
Calculation of blood loss (n=175).
| Total (n=175) | AP-PREa (n=34) | AP-DEMb (n=36) | NAP-PREc (n=105) | AP-PRE vs.d AP-DEM | AP-PRE vs.b NAP-PRE | |
| Calculation | 0.739 | 0.752 | ||||
| Not possible percentage | 12.0 | 8.8 | 13.9 | 12.4 | ||
| Negative percentage | 10.9 | 8.8 | 11.1 | 11.4 | ||
| Possible percentage | 77.1 | 82.4 | 75.5 | 76.2 |
| Total (n=135) | AP-PRE (n=28) | AP-DEM (n=27) | NAP-PRE (n=80) | AP-PRE vs. AP-DEMb | AP-PRE vs. NAP-PREb | |
| Value of loss (ml)e | 276±159 | 308±182 | 278±210 | 265±128 | 0.587 | 0.193 |
Transfusion requirements are detailed in Table 3: the groups with the early pattern, NAP-PRE and AP-PRE, had results of 1.3±2 and 1.4±2 packed red blood cells per patient, respectively, while the AP-DEM group with a delayed pattern had a result of 2.3±4. There was ample variability and it was not possible to demonstrate statistical significance (Table 3).
Results of analytical determinations (n=175).
| Total (n=175) | AP-PREc (n=34) | AP-DEMd (n=36) | NAP-PREe (n=105) | AP-PRE vs.b AP-DEM | AP-PRE vs.b NAP-PRE | |
| At admission | ||||||
| Haemoglobin (g/dl)a | 12.5±1.6 | 12.2±1.7 | 12.5±2 | 12.6±1.6 | 0.415 | 0.113 |
| Haematocrit (%)a | 39.4±5.1 | 38.7±5.3 | 39.3±6.2 | 39.6±4.5 | 0.674 | 0.339 |
| APTT ratioa | 0.9±0.12 | 0.88±0.08 | 0.89±0.12 | 0.91±0.14 | 0.459 | 0.196 |
| INR a | 1.05±0.12 | 1.02±0.07 | 1.06±0.12 | 1.05±0.13 | 0.159 | 0.343 |
| Platelets (×109/l)a | 222±71 | 210±51 | 237±96 | 220±65 | 0.155 | 0.406 |
| Total protein (g/dl) a | 6.2±0.7 | 6.0±0.6 | 6.2±0.6 | 6.3±0.7 | 0.338 | 0.077 |
| Albumin (g/dl)a | 3.7±0.4 | 3.6±0.4 | 3.7±0.4 | 3.7±0.3 | 0.322 | 0.555 |
| Urea (mg/dl)a | 56.3±28.3 | 57.8±21.1 | 57.8±24.5 | 55.3±31.7 | 0.989 | 0.682 |
| Creatinine (mg/dl)a | 1.1±0.75 | 1.4±1.4 | 1.1±0.4 | 1.0±0.4 | 0.275 | 0.005 |
| Sodium (mmol/l)a | 138.1±4.1 | 137.5±5.3 | 138.3±4.9 | 138.2±3.2 | 0.522 | 0.351 |
| Potassium (mmol/l)a | 4.0±0.7 | 4.1±0.7 | 3.9±0.9 | 4.1±0.6 | 0.313 | 0.788 |
| Leukocytes (×109/l)a | 11.1±3.9 | 11.5±5.2 | 11.3±4.3 | 11.0±3.1 | 0.858 | 0.477 |
| Iron (μg/dl)a | 41.6±26.6 | 42.2±22.3 | 42.0±26.6 | 41.3±28.1 | 0.971 | 0.879 |
| Preoperative | ||||||
| Haemoglobin (g/dl)a | 11.45±1.7 | 11.12±1.7 | 11.65±1.8 | 11.5±1.6 | 0.238 | 0.261 |
| Haematocrit (%)a | 35.8±5.2 | 34.7±5.2 | 36.5±6.0 | 35.9±4.9 | 0.589 | 0.980 |
| APTT ratio a | 0.92±0.15 | 0.90±0.1 | 0.95±0.2 | 0.91±0.1 | 0.176 | 0.739 |
| INRa | 1.07±0.1 | 1.07±0.1 | 1.07±0.2 | 1.07±0.1 | 0.996 | 0.900 |
| Platelets (×109/l)a | 214±88 | 187±50 | 272±126 | 203±72 | 0.001 | 0.261 |
| Urea (mg/dl)a | 48.7±31.2 | 47.7±20.9 | 50.2±32.6 | 48.4±34.0 | 0.724 | 0.912 |
| Sodium (mmol/l)a | 137.23±3.8 | 137.1±4.7 | 137.3±4.5 | 137.3±3.3 | 0.847 | 0.784 |
| Potassium (mmol/l)a | 4.0±0.7 | 3.9±0.7 | 4.1±0.6 | 3.9±0.7 | 0.207 | 0.956 |
| Leukocytes (×109/l)a | 8.9±3.0 | 8.6±4.0 | 8.6±2.7 | 9.1±2.7 | 0.984 | 0.430 |
| Postoperative | ||||||
| Haemoglobin (g/dl)a | 10.3±1.7 | 10.0±1.8 | 10.9±1.9 | 10.2±1.7 | 0.238 | 0.261 |
| Haematocrit (%)a | 32.5±5.5 | 31.7±5.5 | 34.3±5.9 | 32.1±5.4 | 0.198 | 0.224 |
APTT ratio: ratio of activated partial thromboplastin time; INR: international normalised ratio or prothrombin time.
The mean hospital stay was 9.8±7 days, but in the AP-DEM group this increased to 4.1 days on average compared to the AP-PRE group (9.7±7 vs. 13.8±10) in a statistically significant manner (P=.046). In the NAP-PRE group this value (8.6±5) remained below the mean, although with no significant differences compared to the AP-PRE group (Table 3).
The overall mortality of the 3 groups at 12 months was 30.3%. In the NAP-PRE control group it reached 23.8%, in the AP-PRE group it amounted to 32.4%, and in the AP-DEM group it was even higher, reaching 47.2%, although these values failed to reach statistical significance (Table 3).
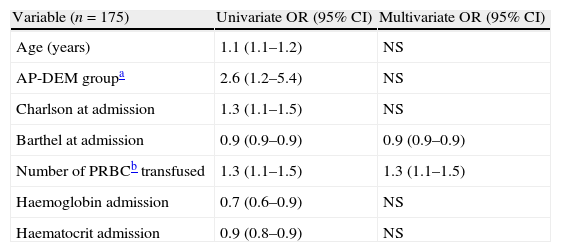
In the logistic remission analysis of variables associated to mortality at 12 months, the following factors showed significant odds ratios in the univariate assessment: age (1.1–1.2), belonging to the AP-DEM group (1.2–5.4), a higher Charlson index at admission (1.1–1.5), a lower Barthel index at admission (0.9–0.9), the number of transfused packed red blood cells (1.1–1.5) and low haemoglobin (0.6–0.9) and haematocrit (0.8–0.9) values at admission. However, of all these factors, the only variables which remained statistically significant in the full multivariate analysis were a low Barthel index at admission and the number of transfused packed red blood cells (Table 4).
Multiple logistic remission analysis. Only the variables which are independently associated with mortality at 12 months are shown.
| Variable (n=175) | Univariate OR (95% CI) | Multivariate OR (95% CI) |
| Age (years) | 1.1 (1.1–1.2) | NS |
| AP-DEM groupa | 2.6 (1.2–5.4) | NS |
| Charlson at admission | 1.3 (1.1–1.5) | NS |
| Barthel at admission | 0.9 (0.9–0.9) | 0.9 (0.9–0.9) |
| Number of PRBCb transfused | 1.3 (1.1–1.5) | 1.3 (1.1–1.5) |
| Haemoglobin admission | 0.7 (0.6–0.9) | NS |
| Haematocrit admission | 0.9 (0.8–0.9) | NS |
PRBC: packed red blood cells; NS: Non statistically significant difference; OR: odds ratio.
Model of multiple logistic remission with ABC, mean AUC of 0.81 (range: 0.73–0.89).
The differences observed in the descriptive analysis of the sample may represent some selection bias, but are justifiable in terms of:
- •
The larger number of comorbidities in the Charlson index in the AP-PRE group with antiplatelet therapy compared to the NAP-PRE group without antiplatelet therapy is explained because patients who were following antiplatelet therapy at admission had a higher frequency of health problems than those who were not. This is confirmed by the fact that this difference was not observed between AP-PRE and AP-DEM.
- •
The longer mean duration of the intervention in the AP-DEM group compared to the AP-PRE group could be explained because, being able to predict the date of surgery a few days in advance, a greater number of surgeries were performed according to a surgical schedule. In contrast, the groups with an early surgery pattern were intervened at the moment when there was availability of a surgical area, often in the emergency surgery area.
The type of anaesthesia employed was differently distributed between the study groups, and this could represent a bias in our results. General anaesthesia was the rule in the AP-PRE group, in order to avoid the risk of bleeding as a result of a spinal puncture. However, regarding the distribution of the types of anaesthesia in those groups in which the technique was left at the discretion of the anaesthesiologist, there was a clear predilection for subarachnoid anaesthesia: in the AP-DEM group there were 30 regional anaesthesias, 2 general anaesthesias and 4 without data, while in the NAP-PRE group there were 92 regional anaesthesias, 6 general anaesthesias and 9 without data.
Although the guidelines reflected in the multidisciplinary document for the treatment of hip fractures elaborated by the Osteoporosis Study and Research Group (GEIOS) of the Spanish Society of Orthopaedic Surgery and Traumatology, and also endorsed by the Spanish Society of Anaesthesiology, Resuscitation and Pain Therapeutics, which was published in 200947 indicate that there was insufficient evidence regarding which anaesthetic technique, whether general or spinal, offered better results, most anaesthesiologists preferred a regional technique, as long as it was not contraindicated.
As reported in the article published by Luger et al. in 2010,48 the choice of anaesthetic technique should be individualised according to patient preferences, associated comorbidities, postoperative complications expected and the experience of the anaesthesiologist. Therefore, in cases in which we opted for general anaesthesia we used multiple variants. It is clinically impossible to standardise a specific protocol for these patients, since they suffer from various severe pathologies and often require undergoing interventions in suboptimal conditions.
Perioperative bleeding at 24h did not show a statistically significant trend towards being higher in the AP-PRE group compared to the other groups, but the differences in absolute figures (30ml compared to the AP-DEM group and 43ml compared to the NAP-PRE group) did not imply clinical relevance. The measurement, based on the difference in haematocrit preoperatively and at 24h postoperatively, presented assessment problems, returning negative perioperative bleeding values contrary to logic, not in accordance with reality, and which could cause confusion. The explanation for these results lied in the variability in haematocrit levels under certain clinical conditions. Dehydration, haemoconcentration and haemodilution, as well as interference caused by blood transfusions during surgery and during the first postoperative hours, could all cause a biased result in either direction (underestimated or overestimated). This problem could not be avoided by changing the time interval to 48h or restricting it exclusively to the intraoperative period. For this reason, we believe that haematocrit is not a useful parameter to estimate perioperative bleeding.
The mean transfusion requirements were higher in the AP-DEM group, although the comparison did not show statistical significance. The transfusion requirements were not only a way of measuring perioperative bleeding indirectly, but could also be influenced by the previous health status of patients49 (nutrition, anaemia, etc.), and especially by their postoperative evolution. Those patients with a more torpid evolution often required more transfusions of packed red blood cells and also presented a higher risk of complications and exitus. This compendium of preoperative, perioperative and postoperative factors had an influence on this variable and justified its statistically significant association with a higher mortality, in both the univariate and multivariate analyses.
The mean stay in the AP-DEM group increased by 4.1 days on average compared to the AP-PRE group in a statistically significant manner. This is in accordance with the delayed intervention in that group, which took place between 4 and 5 days later than in the early protocol. This result is a direct consequence of the study design, but by postponing the intervention systematically the mean hospital stay was also increased proportionally, and this implied a higher direct health expenditure for each process in the group with a delayed pattern compared to the groups with an early pattern.
Mortality at 12 months was comparable with the rates published in the literature, although it was in the highest rank. The groups of patients following antiplatelet therapy presented a tendency towards higher overall mortality, since they also had higher rates of comorbidities. It was statistically significantly higher in the delayed intervention AP-DEM group. Therefore, belonging to this group was a factor associated with mortality in the univariate analysis, although it lost statistical significance in the multivariate analysis. Regardless of whether it was a major mortality factor or not, the results showed that a delay in intervention for patients treated with AP not only failed to improve clinical outcomes in terms of bleeding complications and transfusion requirements in our study, but even showed a tendency towards increased mortality. These data support the change in attitude which began in the latest revisions of anaesthesia societies,42 which called for maintaining perioperative antiplatelet therapy and not delaying interventions for this reason.
Although there are many factors that can affect platelet aggregation by different pathways, it is surprising that 59.8% of patients who reported not taking antiplatelet agents (NAP-PRE) presented pathological analytical results for some of the studied platelet activation pathways (ADP and ASPI). Therefore, we sometimes treated patients as if they were not following AP therapy when they actually were, and yet, we did not observe an increase in complications. This situation did not seem to have haemorrhagic consequences, although patients were exposed to the severe risk, albeit rare, derived from the use an anaesthetic technique such as spinal puncture when platelet aggregation was impaired. A preoperative analysis of platelet function could be of interest in these patients.
On the other hand, 86.5% of patients who reported taking antiplatelet agents (AP-PRE and AP-DEM) indeed presented antiaggregation as determined by the analyses and, conversely, 13.5% did not have adequate antiaggregation profiles. This situation could occur due to a lack of response to the drug, or else not taking it at a dose which maintained it within the therapeutic range. Regardless of the origin, this datum would be of little clinical relevance if the indication for antiplatelet therapy were as primary prophylaxis with low risk of thrombosis, but it would be more worrying if the origin of the therapy was the treatment of an acute thrombotic episode or secondary prophylaxis with a high thrombotic risk. Although there were no problems during the procedure, patients were at risk of suffering arterial thrombosis, which is relevant beyond the perioperative period. Therefore, it seems advisable to agree on a method of control and monitoring of therapeutic response to the AP drug, especially during the clinical monitoring of patients with an increased risk of thrombosis, as proposed in cardiology consensus guidelines.50
It is also interesting to note that the AP-DEM group showed 76.7% of antiaggregation through the aggregometry test after 5 days, in spite of the study protocol including maintenance treatment with 100mg of ASA during the delay. The true percentage of patients with correct antiaggregation profiles should increase, since there was no lack of compliance with the therapy, but it decreased by about 14.5 percentage points compared to admission. Once again, this may be important in patients at high risk of thrombosis. The explanation could be related to the type of antiplatelet drug and dose employed, but further studies and serial hospital determinations should be performed in order to confirm this point.
Several factors associated with mortality appeared following the univariate analysis, but in the full multivariate analysis, closer to reality, the variables which remained statistically significant were a low Barthel index at admission and the number of packed red blood cells transfused.
The fact that aggregometry values did not show a statistical relationship with mortality, with bleeding or with transfusion requirements, could cast doubt over the routine use of these agents in the perioperative period, although they seem highly recommended for the monitoring of patients at high risk of thrombosis. It is somewhat surprising that such a widely used treatment, which has traditionally conditioned the perioperative management of these patients and increased their economic cost, has so few resources to support its actual effect. We believe that studies which assess the cost/benefit of this determination would be warranted, perhaps being reserved for selected patients who were candidates for spinal anaesthesia.
There are inevitable weaknesses in the study design, in the sense that the medical team caring for patients cannot be kept blind to their surgical delays and medication histories, and this could perhaps have some relevance in the selection of the type of anaesthesia, as discussed. We believe that the study could be improved with a larger number of patients, by including data from other centres with similar treatment protocols, and also including data enabling the measurement of variables beyond mortality, such as the evolution of quality of life after fracture, as well as assessments of recovery of autonomy and the capacity for independent movement prior to the fracture.
Conclusions- 1.
The analytical determination of platelet function was able to detect a significant number of patients in whom objective aggregability did not match the expectations, based on medication history.
- 2.
The pattern of early surgery on patients following antiplatelet therapy showed an increase in intraoperative bleeding which was not significant or clinically relevant.
- 3.
The delayed surgery pattern for patients following antiplatelet therapy did not improve clinical and analytical outcomes, and also showed a tendency to increase mortality, although this was not statistically significant.
- 4.
The mean hospital stay in the group of patients with delayed surgical pattern was longer, and this led to a proportional increase in direct healthcare costs for each process.
Therefore, in the light of the conclusions reached, we do not recommend delaying intervention because of AP treatment. In any case the final decision regarding the optimal timing of the intervention should be adopted by each multidisciplinary care team, that is, surgeon, anaesthesiologist and geriatrician or internist, considering the best interest of each patient and, of course, depending on the availability of technical means. Studies like the present one can help to make decisions with more information.
Level of evidenceLevel of evidence i.
Ethical responsibilitiesProtection of people and animalsThe authors declare that this investigation did not require experiments on humans or animals.
Confidentiality of dataThe authors declare that this work does not reflect any patient data.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
FinancingThis study was funded through a research grant from Fundación SECOT.
Conflict of interestsThe authors have no conflict of interests to declare.
The authors wish to thank Fundación SECOT for the opportunity to carry out this study through the award of one of their research grants.
Please cite this article as: Mas-Atance J, Marzo-Alonso C, Matute-Crespo M, Trujillano-Cabello J, Català-Tello N, de Miguel-Artal M, et al. Estudio comparativo aleatorizado de la intervención quirúrgica temprana frente a la demorada en los pacientes con fractura de cadera tratados con antiagregantes plaquetarios. Determinación de la agregabilidad plaquetaria y el sangrado perioperatorio, y la revisión de la mortalidad al año. Rev Esp Cir Ortop Traumatol. 2013;57:240–53.