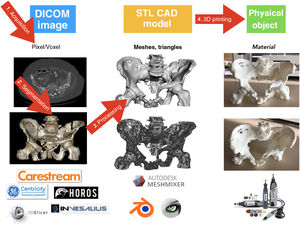
3D printing (I3D) is an additive manufacturing technology with a growing interest in medicine and especially in the specialty of Orthopaedic Surgery and Traumatology. There are numerous applications that add value to the personalised treatment of patients: advanced preoperative planning, surgeries with specific tools for each patient, customised orthotic treatments, personalised implants or prostheses and innovative development in the field of bone and cartilage tissue engineering.
This paper provides an update on the role that the orthopaedic surgeon and traumatologist plays as a user and prescriber of this technology and a review of the stages required for the correct integration of I3D into the hospital care flow, from the necessary resources to the current legal recommendations.
La impresión 3D (I3D) es una tecnología de fabricación aditiva con un creciente interés en medicina y sobre todo en la especialidad de Cirugía Ortopédica y Traumatología. Hay numerosas aplicaciones que aportan un valor añadido al tratamiento personalizado de los pacientes: planificación preoperatoria avanzada, cirugías con herramientas específicas para cada paciente, tratamientos ortésicos a medida, implantes o prótesis personalizadas y un desarrollo innovador en el campo de la ingeniería de tejidos óseos y cartilaginosos.
En el presente trabajo se realiza una actualización sobre el papel que el cirujano ortopédico y traumatólogo desempeña como usuario y como médico prescriptor de esta tecnología y un repaso a las etapas necesarias para una correcta integración de la I3D en el flujo asistencial hospitalario, desde los recursos necesarios hasta las recomendaciones legales actuales.
3D printing (3DP) groups together a series of manufacturing technologies that, applied to the medical sector, bring many advantages, and represent a paradigm shift in health. Although 3DP is not a new technology (it dates from 1983), it has become popular in the last 10 years. This is due, on the one hand, to the release of patents on the main manufacturing technologies stereolithography (SLA) and fused deposition modelling (FDM)) and, on the other, to the advent of new materials and 3DP techniques. 3DP is a growing technology also used in many applications in the industrial, aeronautical, automotive, and architectural sectors.
3DP is a type of additive manufacturing that allows a digital model to be transformed into a real and tangible three-dimensional object. Three-dimensional models are obtained by processing digital radiological imaging studies of patients, three-dimensional, external scanning techniques, computer-aided design (CAD) and reverse engineering techniques. Once the virtual model has been obtained, it can be printed. Objects are built layer-by-layer, using different technologies and materials depending on the final application. This layer-by-layer addition of material is what differentiates 3DP from other classical manufacturing technologies such as machining, casting, moulding, or forming.
Because 3DP enables manufacture by laying down successive layers of the object’s material, complex structures are created that could not be obtained with other technologies. This characteristic, together with the concept of personalised medicine, has resulted in the successful use of 3DP in medicine.1 The possibility of obtaining short series in a shorter time and at a lower cost than other industrial manufacturing techniques and avoiding waste are other advantages.
On the other hand, there are several disadvantages of the technology such as: 1) the need to use extra material as a support to prevent layers collapsing, 2) the low mechanical traction resistance of the part in the direction of the superimposed layers (Z axis) and 3) the time needed to print with certain technologies.2
There are many 3DP technologies, therefore, in 2015, the American Society for Testing and Materials (ASTM) developed the international standard ISO/ASTM 52900-2015 which classifies them into 7 processes which has resulted in 11 different technologies.3 Each technology has its advantages, disadvantages and potential applications, therefore various techniques will be used in medicine according to the intended utility.4
In any case, as with any recently introduced technology in the medical sector, caution is essential, since neither the regulations nor the legal regulation of the medical use of 3DP are fully in place and there are still numerous legal challenges that require further research and the development of specific medical regulations.
This article reviews the current status of 3DP in medicine, its different practical uses in the speciality of orthopaedic surgery and traumatology (OST), the working process from image acquisition to manufacture using 3DP and, finally, the most relevant technical and legal details for successful implementation in a hospital’s OST department.
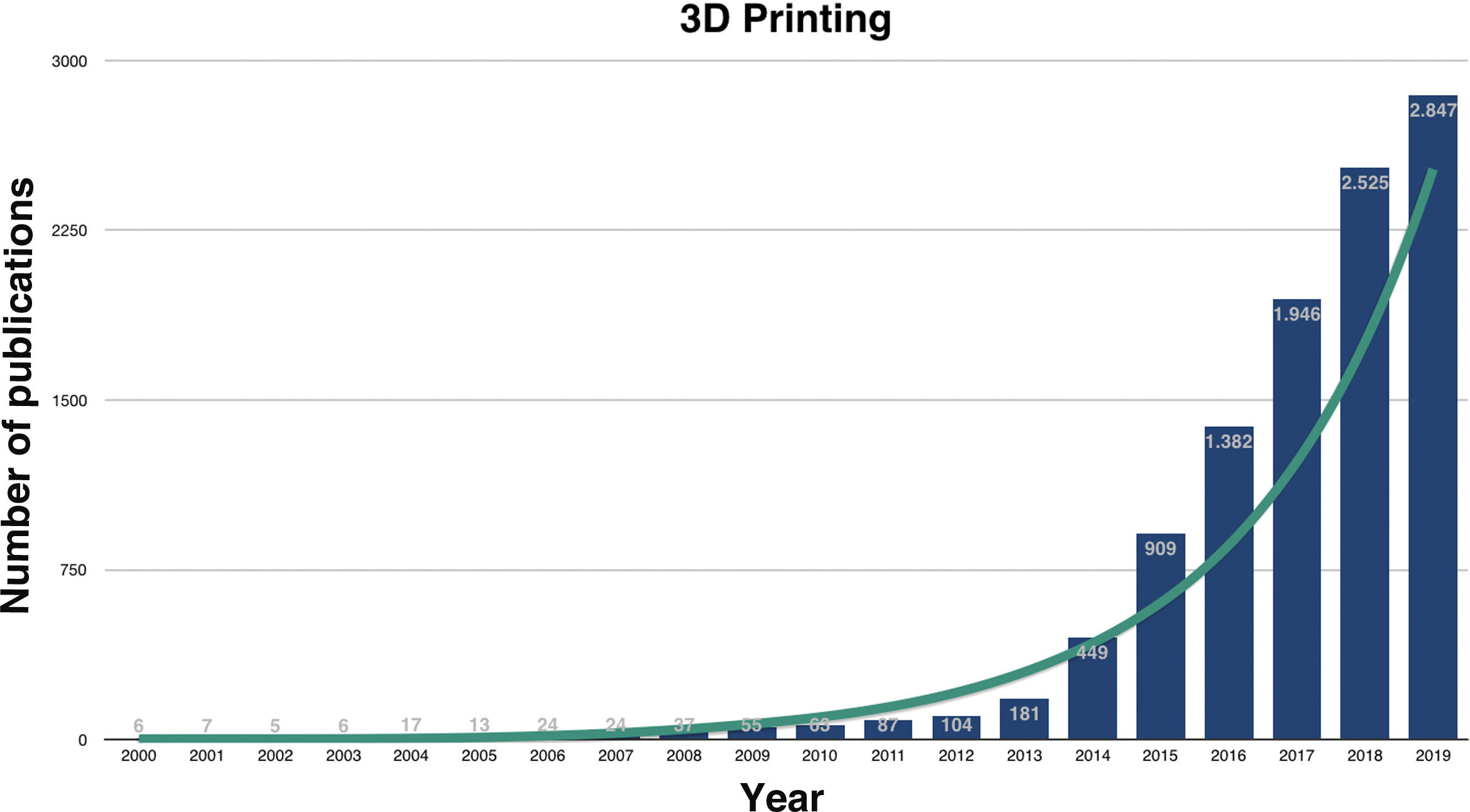
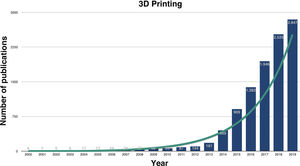
3D printing in medicineResearch on the utilities of medical 3DP has grown exponentially in recent years (Fig. 1) and, although numerous, they can be grouped into the manufacture: 1) of biomodels or bioreplicas, 2) of custom-made tools, 3) of custom-made implants, 4) of drugs and 5) of biocompatible tissues, also known as bioprinting.
The many utilities of 3DP are currently in different stages of maturity. The hype cycle of emerging technologies of the global consulting firm Gartner is a graphic representation of the maturity, adoption, and commercial application of specific technologies. It is published annually and establishes the key phases of a technology's life cycle: 1) the trigger, 2) the peak of inflated expectations, 3) the trough of disillusionment, 4) the slope of enlightenment, finally, 5) the plateau of productivity.5
The state of maturity of each application needs to be examined to make efficient use of medical 3DP. As an example, Gartner's 2018 emerging technologies hype cycle highlights that the 3D tools (where patient-specific surgical guides are included) are on the slope of enlightenment while the 3D anatomical models are about to start on it. By contrast, customised implants and human tissue obtained by bio-printing are in full disillusionment, and therefore they advise investing in research into them to establish their real usefulness and start their consolidation on the market.
The use of this technology in the medical sector still has some disadvantages that need to be known. Depending on the use, the additional time required for manufacture, the extra cost involved, the need for technical staff, the mechanical properties or the precision of some technologies must be considered.2
3D biomodels3D biomodels or bioreplicas are physical reproductions of a patient’s specific organ or anatomical region. Using 3DP, any anatomical region visualised on medical imaging can be manufactured, in real scale, in various materials, and with millimetric accuracy depending on the quality of the image and the technology chosen. While it is possible to obtain data from any conventional imaging test, the most used are computed tomography (CT), magnetic resonance imaging (MRI) and even ultrasound.6
Thanks to 3D biomodels, advance planning of certain medical procedures, such as surgical interventions, is possible.7 This enables the surgeon to make decisions or simulate surgery prior to the surgical act and facilitates communication with patients and teaching among professionals.8
The use of 3D biomodels enables innovation and improvement in medical education. In addition to manufacturing highly detailed and realistic anatomical models, it is possible to manufacture, at very low cost, simulators of medical techniques such as orotracheal intubation,9 sutures,10 endoscopies,11 endovascular12 and surgical interventions, among others.13–15 Furthermore, it is an alternative to the use of cadavers for university teaching of medicine in subjects such as anatomy.16–18
Customised toolsOne of the main advantages of 3DP is the creation of customised products in small quantities, which facilitates personalised medicine, i.e., treatment tailored to each patient’s individual characteristics.
Patient-specific guides are some of the most widely used tools manufactured by 3DP currently available. As they are exactly adapted to the patient’s specific location, they can be applied to a particular medical or surgical treatment such as an osteotomy, to insert an implant, to obtain grafts, to administer drugs or to use radiotherapy devices with exact dose control.19 These guides are manufactured with biocompatible material in the hospital itself, and therefore the entire process is under the permanent supervision of the surgeon or prescribing physician.
Surgical instruments manufactured by 3DP are adapted not only to the size of the patient, but also to the surgeon and to a specific surgical technique.20 Most of the surgical instruments used today in interventions have not changed over the years and have structural characteristics that are not optimally adapted to new materials and manufacturing techniques. 3DP enables advanced rapid prototyping, reduces verification and validation times, improves the shape, weight, and strength of medical instruments, and gives them unique properties such as radio-translucence.21
Customised implants3DP as a medical device manufacturing technique has many advantages over other traditional manufacturing techniques. It enables the creation of customised implants using the patient's own medical imaging data. These implants are manufactured with external and internal geometries that would probably not be possible with traditional methods.22
Furthermore, with 3DP it is possible to manufacture customised implants at a significantly lower cost than other traditional techniques.23 Manufacturing technologies by means of selective laser sintering enable the creation of metal implants safely and very cost-effectively.24 This means that these customised implants are being used in more hospitals and in more surgical techniques.25
Non-implantable medical devices, such as splints or corsets, are also manufactured using 3DP, which has many therapeutic advantages since, depending on the anatomical region, it is possible to manufacture them with improved characteristics and properties compared to traditional materials and techniques.
DrugsWith 3DP it is possible to manufacture highly complex, customised drugs with enhanced pharmaceutical properties. There are currently several drugs on the market that are approved by the Food and Drug Administration (FDA) and produced using additive pharmaceutical manufacturing techniques.26 The FDA itself highlights the great potential of 3DP to create new therapies and improve the adherence, safety, and efficacy of existing ones. Therefore, 3DP for drugs is a utility that the pharmaceutical industry will continue to develop in coming years.27,28
BioprintingBioprinting is the manufacture of biological tissues by layer-by-layer printing of structures or scaffolds made of a biocompatible material and coated with the patient's own live cells obtained by means of tissue engineering techniques.29,30 Cell coating is performed either a posteriori, using a bioreactor, or simultaneously with the manufacture of the scaffold, using bioinks.31
The possibilities of this technology have opened a wide range of research with countless potential applications. The manufacture of human tissues, using the patient's own living cells that can replace damaged ones, is a utility that is still in the early stages of research, but advancing rapidly.
3D printing in orthopaedic surgery and traumatologyOST is possibly the area of medicine that can benefit most from the advantages of 3DP.32,33 Almost all 3DP utilities are applicable to the various aspects of the specialty, from preoperative planning of orthopaedic interventions using 3D biomodels to the development of instruments, patient-specific surgical guides, or customised orthopaedic implants, among others.34
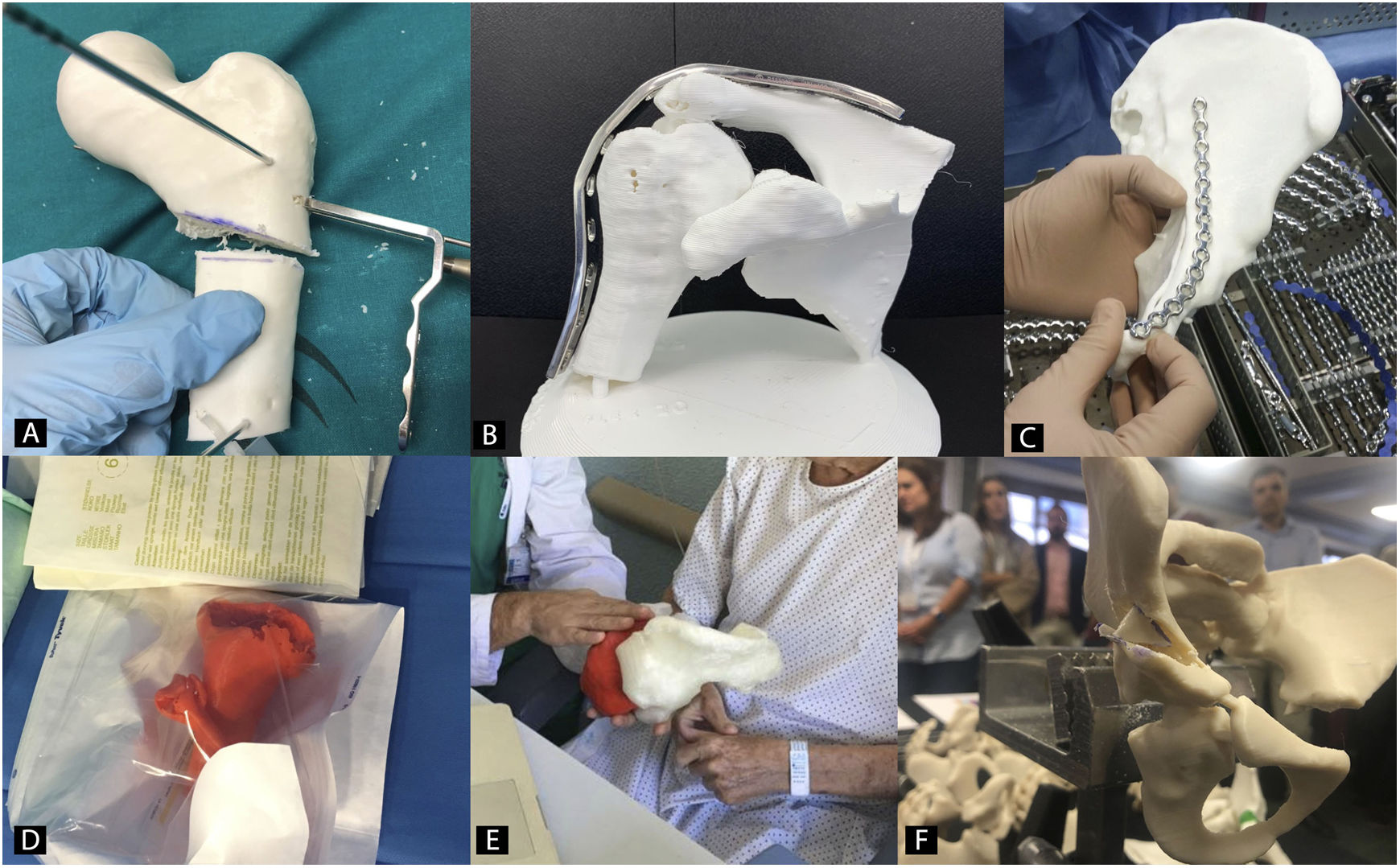
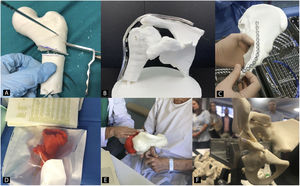
Preoperative planning in orthopaedic surgery and traumatology with 3D biomodelsThe use of 3D biomodels for preoperative planning in OST is one of the utilities where there has been most interest in recent years, since all subspecialties will be able to use it (Fig. 2).35 The 3D Special Interest Group RSNA has reviewed and classified the clinical cases in which it is more efficient to use 3D biomodels in OST and concludes that in simple fractures, the role of 3D is not as useful (1/10) as in complex fractures, hip dysplasia or bone tumours with joint involvement (8/10).36
Recently it has been demonstrated how the use of 3D biomodels reduces surgical time in certain interventions, with a saving in surgery costs of more than 3.700 $.37 It has also been shown that both the intraoperative radiation dose using 3D biomodels as support during surgery,38 and complications following the surgical intervention could be lower.39
Communication with patients is another advantage that has been studied and whose improvement using 3D biomodels has been quantified. Different studies have established that patients have a better understanding of their injury and planned surgery when the surgeon gives them information using 3D biomodels.40,41
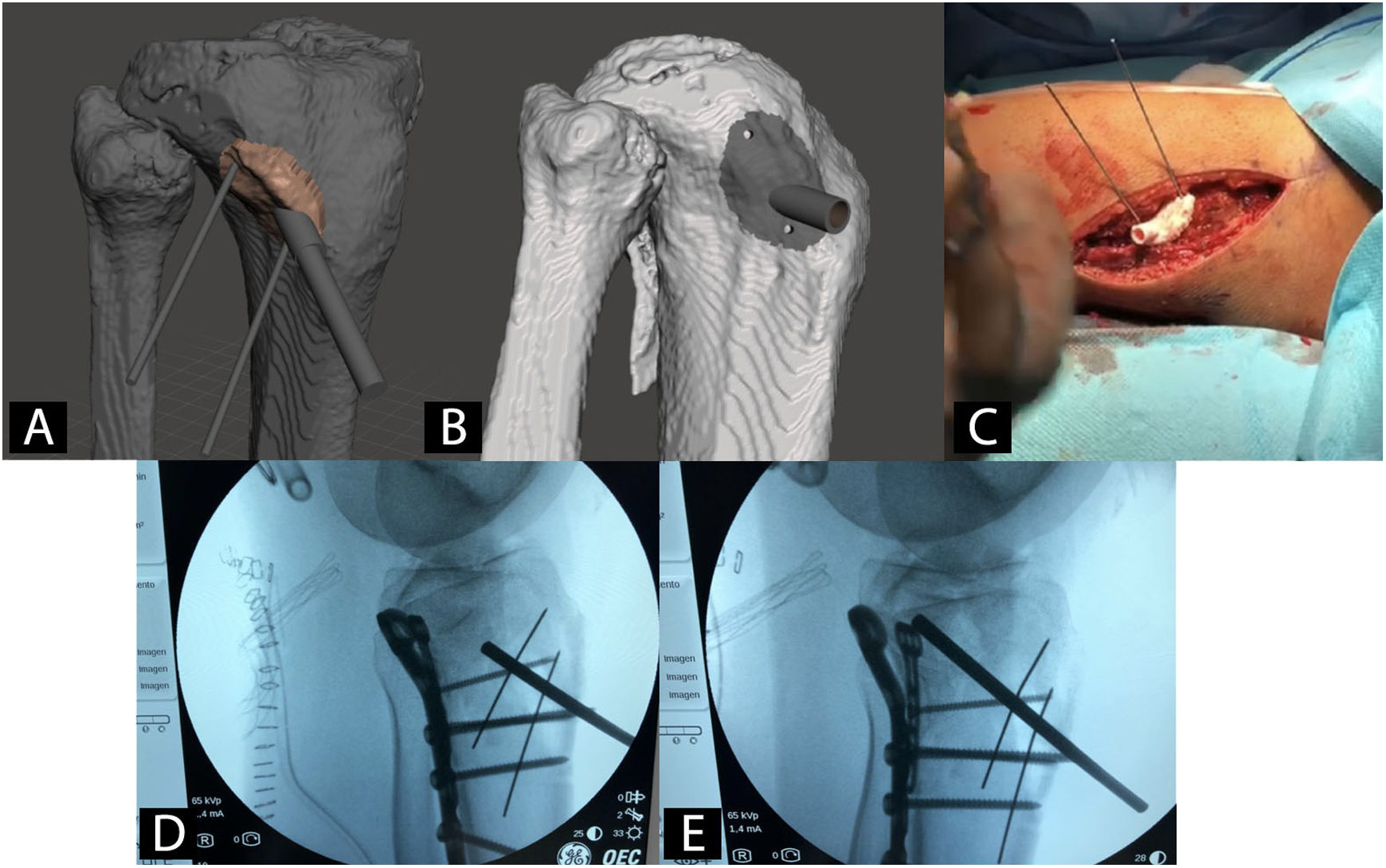
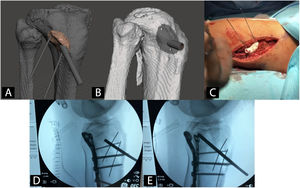
Patient-specific surgical guides in orthopaedics and traumatologyUsing patient-specific surgical guides manufactured by 3DP it is possible to perform osteotomies of the pelvis,42 hip43,44 and knee,45 tumour resections,46 correction of deformities,47 pedicle screw insertion,48 percutaneous trauma surgery49 and arthroplasties50 with greater precision than with generic instruments (Fig. 3).
Example of a patient-specific surgical guide for the elevation of a sunken joint fragment in a tibial plateau fracture. A) CAD model and virtual planning of guide. B) CAD model of the surgical guide. C) Intervention with patient-specific surgical guide. D) Radioscopy prior to joint fragment elevation. E) Radioscopy during joint fragment elevation.
Many studies highlight that the use of patient-specific surgical guides reduces radiation and surgical time, and some studies even confirm a saving of $3500 in overall operating costs.37
The manufacture of customised surgical instruments for OST operations enables hybridisation with other technologies such as surgical navigation or augmented reality.51,52
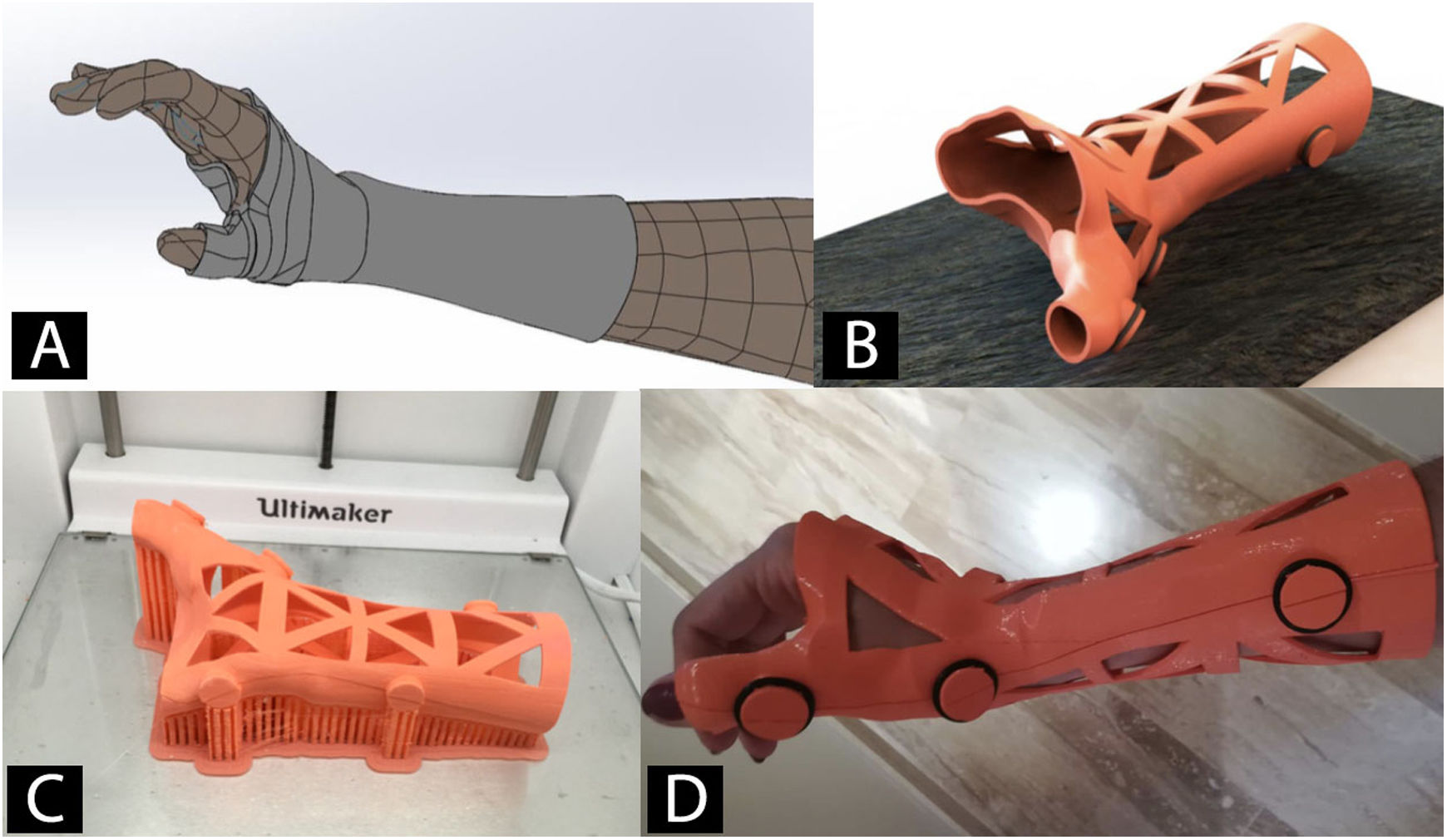
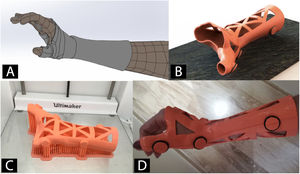
Orthoses and splints manufactured using 3D printingWith 3D scanning technology, orthoses and splints are manufactured using 3DP (Fig. 4).53 The main advantage is improved adaptation to the patient's anatomy.54 This together with the use of different 3DP technologies and new materials has facilitated the design and approval of orthoses and splints for the treatment of diseases of the lower limb,55,56 upper limb57,58 and even spinal deformities, such as scoliosis.59
Orthoses manufactured by means of 3D technology are a valid alternative to the traditional that are manufactured by shaping thermoplastic materials. Although their use is not yet very widespread, this brings numerous advantages, such as a reduction in manufacturing times, lower costs, and greater patient satisfaction.56 Recent papers have validated their biomechanical behaviour and confirmed them to be comparable to traditional orthoses.60
Customised implants in orthopaedic surgery and traumatologyCustomized implants manufactured by 3DP allow the reconstruction of bone defects after tumour resection operations,61–66 or complex prosthetic revision surgeries (especially when it is not possible to use modular implants).67,68 The fixation and stability of customised implants is excellent, and therefore clinical outcomes, still in the short term, are very favourable.69
Despite the numerous advantages of customised implants, most published papers refer to isolated clinical cases or case series, without the possibility of a comparative analysis with traditional implants.70
Most companies in the medical sector use direct metal laser sintering (DMLS) technology for the manufacture of customised orthopaedic implants. This, and because their design involves a more complex process than that of 3D biomodels and surgical guides, means that they are not yet widely used in the hospital setting and the participation of the medical industry is essential.
3D printing and tissue engineering in cartilage and boneBioprinting in OST has revolutionised the field of cartilage and bone tissue engineering.71 With respect to cartilage, bioprinting of mesenchymal stem cells in several layers has been performed in vivo together with an extracellular matrix formed by atelocollagen hydrogel and hyaluronic acid. The subsequent cell differentiation into mature chondrocytes allows the creation of personalised cartilage autografts for the reconstruction of chondral defects.72
Regarding the additive manufacture of bone tissue, thanks to bio-printing functional prototypes of clinically relevant bone tissue, mechanically resistant and with a functional bone marrow have been developed.73 In the experimental field, synthetic polymer hydrogels have been successfully used to create an extracellular matrix to which mesenchymal stem cells are added. These differentiate into mature bone tissue when stimulated by ceramics, such as hydroxyapatite or bioactive crystals.71 The main challenge at present is the vascularisation of bio-printed bone tissue.74
Combining and integrating imaging tests with bioprinting of cartilage and bone tissues will make it possible in the future to bioprint patient-specific autografts for the treatment of chondral and bone defects. Thanks to these bio-printed autografts, it will be possible to avoid both the problems of autografts (availability and morbidity) and those of allografts (compatibility and osteogenic capacity).75
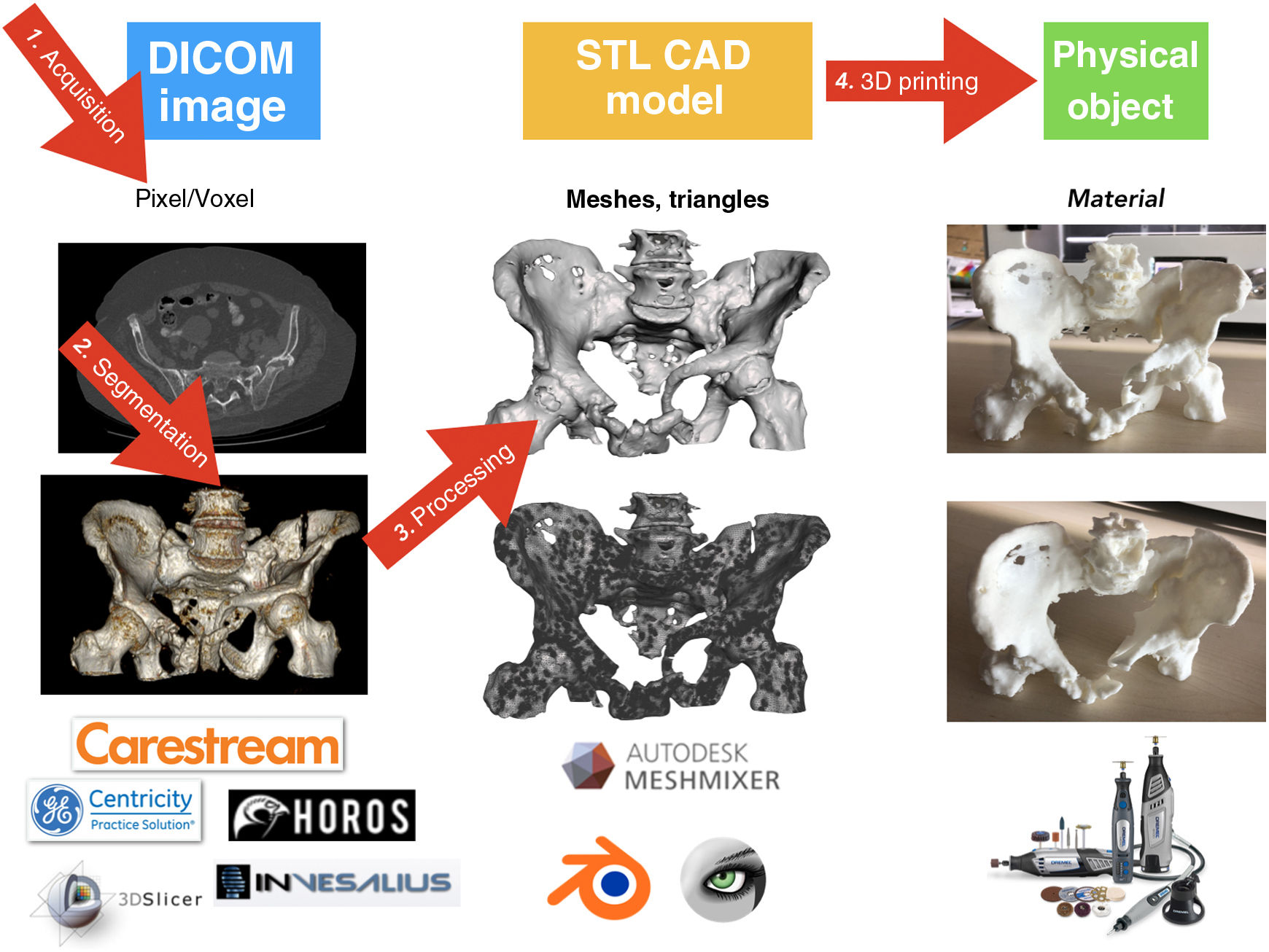
Medical 3D printing processThere are 4 well-differentiated phases in the medical 3DP process: 1) acquisition, 2) segmentation, 3) processing and 4) manufacturing by 3DP (Fig. 5).76
AcquisitionThe medical 3DP process begins before the medical imaging treatment. Correct acquisition and optimisation of imaging studies is essential to create the CAD object that will later be printed. It is possible to use CT or MRI studies from routine clinical practice, but it must be borne in mind that these may not have been performed with optimised protocols for bone segmentation (Fig. 6).
CT is the best study for 3DP of bone structures.77 There are certain technical characteristics that can be modified at the time of the study that will facilitate the segmentation of fractures or orthopaedic deformities: 1) sections: slice thickness of 1mm (or even less) with increments of .625–.75mm (less than 1mm); 2) Kernel filter: soft or moderate parts and 3) collimation: 1.25–1.50mm.78
If the anatomical region presents metal elements, protocols for the reduction of metal artifacts are necessary. The new dual-energy tomographs, at the same dose, reduce brightness produced around the bone and that prevent the correct segmentation of the anatomical structures.79
Medical imaging is stored in DICOM (Digital Imaging and Communication on Medicine) format for subsequent processing.
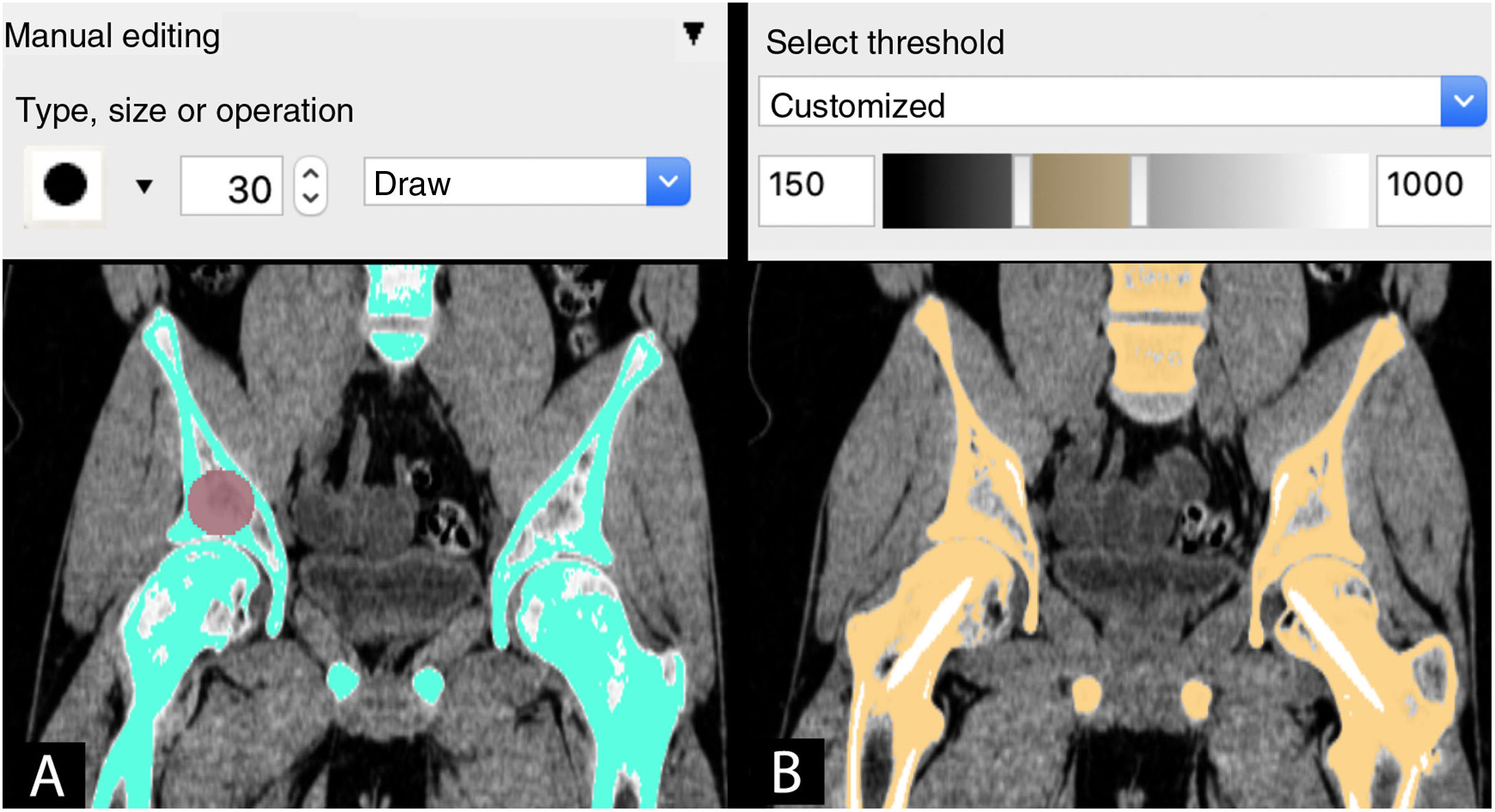
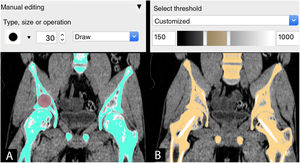
SegmentingThe next stage is to process the medical image by selecting the regions or areas of the anatomy to be reconstructed in the CAD model, a process known as segmentation.
Thanks to the development of commercial software tools such as Mimics (Materialise NV, Leuven, Belgium) and freely available tools such as Horos (Horos Project, Annapolis, MD, USA). Invesalius (Centro de Tecnologia da Informação Renato Archer, Campinas, SP, Brazil) and 3D-Slicer (BWH, Cambridge, MA, USA), the segmentation process has been simplified and, in certain situations, even automated.
Segmentation can be carried out by 3 methods:
- 1
Manual: by selecting or painting pixel by pixel the regions of interest (ROI) in each layer of the imaging study.
- 2
Automatic: using tools with algorithms to select regions of interest with similar characteristics for automatic segmentation in all the layers of the study.
- 3
Semi-automatic: combination of manual and automatic tools. The combination of the automatic tool for segmentation by grey intensity threshold of the Hounsfield scale together with the manual editing tool is one of the most widely used. This scale covers the different greys (from black to white) found in each pixel of a CT image, ranging from −1000 Hounsfield units (HU), which is equivalent to air density, to >1000 HU, which represent metal density.
The next step, once segmentation has been made and regardless of the method selected, is rendering to obtain a virtual 3D model. The visualisation and analysis of the virtual model are necessary to adjust those details of segmentation that may have gone unnoticed, such as areas of low contrast, artifacts or internal surfaces that do not require segmentation and that may compromise, as will be seen later, the result of 3DP.
After segmentation and rendering of the virtual model, the model is exported to a digital CAD file. This file is made up of a three-dimensional mesh of triangles whose most widespread format is STL (stereolithography), a format which, despite being the most widely used and supported by most computer applications, does not provide information on colour, textures, or physical properties, although it does provide information on size or spatial location. It is possible that in years to come the format will evolve to include more information, which will give the models more useful clinical characteristics.
ProcessingDepending on each case and the intended use, the CAD model usually requires further processing. Processing can range from slight smoothing of surface irregularities to the creation of additional elements such as surgical guides. In any case, it is essential not to vary the clinical information provided by the model.
There are numerous computer applications for processing CAD models; some of the most used for medical models are free distribution applications such as Meshmixer (Autodesk Inc, San Rafael, CA, USA) and MeshLab (ISTI-CNR, Pisa, TO, Italy).
The 5 key phases of processing are:
- 1
Mesh correction. The conversion of the virtual model to the STL file usually causes the appearance of small defects (such as holes or lack of triangles in certain areas of the mesh) that require correction. Automatic correction tools are available in most applications for this purpose.
- 2
Close the ends of the model. It is advisable to close open ends, such as the medullary cavity, to facilitate printing.
- 3
Optimise internal structures. If the interior of the model is not going to provide clinical information, it is advisable to eliminate those layers or internal regions to improve the printing phase. A clean model without internal structures will be produced faster and with less chance of error.
- 4
Smooth out artifacts. CT often produces a variety of artifacts that need to be smoothed, such as the steps between layers, brightness from metal artifacts or surface irregularities.
- 5
Modifications of the model. Sometimes it is necessary to join elements that have become separated (for example, joints). To do this, it is possible to create connections to give the model stability. It is also advisable to add labels to classify them, highlight certain anatomical details, divide the model to improve visualisation and even add colours. In any case, it is essential not to make significant changes to the original anatomy.
Digital 3D planning is a further step in the preoperative planning process. It provides the surgeon three-dimensional vision of the fundamental phases of the operation, since it allows the virtual simulation of the gestures that will be performed later during the surgery. It is particularly useful for preoperative planning of surgery on deformities, tumours or in complex locations, such as the pelvis or certain joints.
The surgical intervention is planned once the CAD model has been reconstructed and repaired and prior to its manufacturing phase. The various tools of the computer applications allow rotating, cutting, measuring, and selecting and moving fragments, among other utilities. In addition, during this planning phase it is possible to design guides, instruments, or implants to achieve patient-specific CAD models.
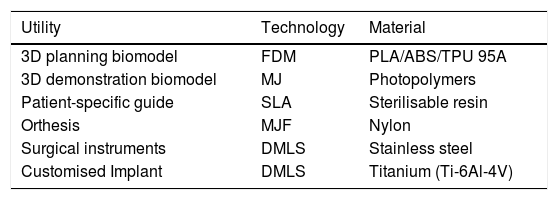
3D printing manufactureThe final step in the 3DP process is manufacturing. For this, depending on the intended use, it is essential to select the right 3DP technology and the most appropriate material (Table 1).
Most frequently used technologies and materials for 3D printing in OST.
| Utility | Technology | Material |
|---|---|---|
| 3D planning biomodel | FDM | PLA/ABS/TPU 95A |
| 3D demonstration biomodel | MJ | Photopolymers |
| Patient-specific guide | SLA | Sterilisable resin |
| Orthesis | MJF | Nylon |
| Surgical instruments | DMLS | Stainless steel |
| Customised Implant | DMLS | Titanium (Ti-6Al-4V) |
ABS: Acrylonitrile butadiene styrene; DMLS: Direct metal laser sintering; FDM: Fused deposition modelling; MJ: Material jetting or polyjet; MJF: Multijet fusion; PLA: Polylactic acid; SLA: Stereolithography; TPU 95A: Thermoplastic polyurethane.
Regardless of the technology used, the manufacturing process will be similar. The CAD model in STL format will be imported into a laminating software that will convert it into orders to be interpreted by the printer, which will manufacture the product layer by layer (Table 2).
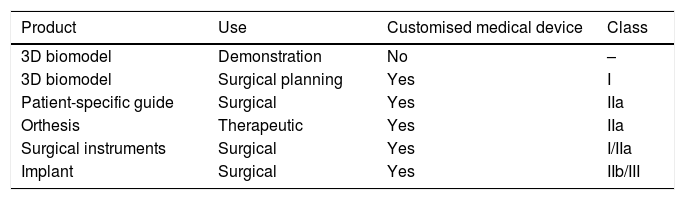
Classification of customised medical devices according to use and class.
| Product | Use | Customised medical device | Class |
|---|---|---|---|
| 3D biomodel | Demonstration | No | – |
| 3D biomodel | Surgical planning | Yes | I |
| Patient-specific guide | Surgical | Yes | IIa |
| Orthesis | Therapeutic | Yes | IIa |
| Surgical instruments | Surgical | Yes | I/IIa |
| Implant | Surgical | Yes | IIb/III |
Surgical instruments: reusable (class I), non-reusable (class IIa).
Implants: osteosynthesis material: IIb.
Total or partial joint prostheses III.
Source: compiled by the authors.
The technical printing settings will depend on the technology used: speed, resolution, fillers, or substrates are some of the basic features that will need to be adjusted before manufacturing begins. In addition, it is advisable to simulate the process using the different laminating applications to detect possible errors and determine those areas that require supports or those that can be optimised (such as fillers) to reduce the time and quantity of material required.
Once manufactured, a more or less complex post-processing will be necessary depending on the technology, material, and product. A simple 3D biomodel manufactured by FDM will only require the removal of the supports, but an implant or surgical instrument manufactured by DMLS may require several complex treatments to improve its surface finish and mechanical characteristics.
Implementation of 3D printing: 3D printing hospital unitsCreation of a digital hospital manufacturing workshopThere is currently growing interest in the creation of digital manufacturing workshops or laboratories in the hospital itself. 3D printing means the emergence of new forms of production, such as point of care (POC) manufacturing, allowing hospitals to produce biomodels, patient-specific surgical guides, custom implants, and other 3D printed applications at the point of care. Thus, larger hospitals with more complex case histories can have their own 3D printing hospital units (3DP-HU), while smaller centres may network with those and the industry contracted, according to needs.80,81
Many hospitals around the world already have 3DP-HUs, the OST speciality benefiting the most. Even some centres like the Hospital for Special Surgery in New York have a customised implant manufacturing workshop in the hospital itself thanks to collaboration with traditional orthopaedic implant manufacturers (https://trends.directindustry.es/project-190325.html).
Traditional manufacturers, who often complement POC projects in hospitals, are valuable entities when integrated into the care flow working closely with hospital professionals. The creation of 3DP-HUs, the strengthening of collaborative work between the different actors involved, additional clinical studies and regulatory guidance will help to encourage innovation and ensure that in-hospital manufacturing becomes the standard of care.
Elements needed to start a hospital 3D printing unitThe creation of a 3DP-HU begins with determining predicted utilities and coordinating the services involved. There are several human, technological, and material resources required to begin this activity:
Human capital- •
Clinical coordinator of the unit: a doctor with a global vision of the process, which involves manufacturing at the point of care, from the acquisition of the virtual model from digital studies or computer design to the manufacture of biomodels, guides, instruments, and patient-specific implants
- •
Medical specialists in radiodiagnosis: experts in obtaining and processing medical images. In the 3D printing process, their role in validating both the digital model prior to manufacture and the biomodel is particularly relevant.
- •
Specialist radio-diagnostic technicians: facilitate the acquisition and segmentation of imaging tests. As they are already present in most hospitals, this is a specialist profile to be considered to facilitate the creation of 3DP-HU.
- •
Bioengineers: the applications of 3DP in areas of specific knowledge, such as medicine, require the creation of new competence models. Engineers with anatomical knowledge, training in medical imaging, experience in CAD design and in additive manufacturing technologies, are specialist profiles to consider when equipping a 3DP-HU.
- •
Medical specialists: essential in cases where the utility is surgical planning and the need to manufacture patient-specific guides, instruments, or implants, which the legislation identifies as prescribing physicians responsible for the design.
- •
3D printers. In the initial stages, it may be sufficient to start with a semi-professional 3D printer using FDM technology that prints at an appropriate size (recommended print volume of 300×300×200mm), with a double head, is reliable and minimal maintenance. The next entry technology can be 3D printing using SLA, which will allow the creation of biocompatible surgical guides for sterilisation later in the hospital itself, which will facilitate accessibility and reduce production time and costs. Other technologies such as MJF or DMLS may require more complex facilities and specialized manufacturing technicians, they will therefore not be widely used in most hospitals.
- •
Computer equipment. Computer equipment of adequate power is essential to process the medical image smoothly. This equipment must be connected to the computer network and to the hospital PACS to import the patient's DICOM studies.
- •
Software. Depending on the budget free distribution (Invesalius, Horos, Meshmixer, MeshLab) and commercial (Mimics) will be used.
- •
Facility. The location of the 3DP-HU is important so that professionals have access for supervision and communication tasks with the staff in charge. It also needs to be ventilated and have workstations for the staff.
- •
Computer equipment. Computer equipment of adequate power is essential to process the medical image smoothly. This equipment must be connected to the computer network and to the hospital PACS to import the patient's DICOM studies.
- •
Post-processing tools. Basic working tools are necessary such as pliers, tweezers, files, and saws to work and process the manufactured pieces. It is also interesting to provide the workshop with basic reused surgical instruments with which to simulate the techniques, such as screwdrivers, screws or osteosynthesis plates. Some technologies require advanced tools and even special post-processing machinery.
3DP is a tool within any doctor’s reach. The rapid growth of this technology has allowed new utilities to reach the health services. Although its use is gradually being regulated, there are currently certain aspects that are not completely legislated, and which are a barrier to definitive implementation in routine clinical practice.82,83
The specific regulations according to the specific utility of the product manufactured by 3DP in a hospital should be checked. It can be considered a healthcare product and will therefore require specific regulation.
The regulation of medical devices at European level requires a high level of control over the phases of the product cycle to guarantee patient and user safety. All aspects related to design and development, production, marketing, distribution, and installation, among others, are regulated by Regulation (EU) 2017/745 of the European Parliament and of the Council, of 5 April 2017.
Medical deviceA medical device is any instrument or device intended to be used for human beings, which does not achieve its primary intended action by pharmacological, immunological, or metabolic means, used for:
- •
Diagnosis, prevention, monitoring, prediction, prognosis, treatment, or alleviation of disease.
- •
Diagnosis, monitoring, treatment, alleviation of or compensation for an injury or disability.
- •
Investigation, replacement, or modification of the anatomy or of a physiological or pathological process or state.
- •
Providing information by means of in vitro examination of specimens derived from the human body, including organ, blood, and tissue donations.
Medical devices are classified into 4 classes according to their purpose and inherent risks: class I, class IIa, class IIb and class III; the lowest risk is class I and the maximum is class III.
Customised medical deviceFor products manufactured for a specific patient, such as a 3D biomodel to support planning, a patient-specific surgical guide or a customised implant, the concept of a customised medical device must be considered.
A customised medical device is defined as any product specially made in accordance with a written prescription from any person authorised by national law by virtue of that person’s professional qualifications, which gives under that person’s responsibility, specific design characteristics, and is intended for the sole use of a particular patient exclusively to meet their individual conditions and needs.
Manufacturing hospitalWith reference to intra-hospital additive manufacture, the rules are that healthcare establishments must have the possibility of manufacturing, modifying, and using products internally and thus meet, on a non-industrial scale, the specific needs of the target patient groups which cannot be satisfied at the same level of performance by another equivalent product available on the market.
Legal recommendations in a 3D printing hospital unitFor a healthcare facility to manufacture, it must be stated in the documentation that the specific needs of the group of patients for which the products are intended cannot be met or cannot be met at the appropriate level of performance, by another equivalent product on the market.
Hospitals manufacturing any medical device must comply with the following requirements:
- -
Draw up a statement containing the following information:
- o
Name and address of the manufacturer and all manufacturing sites.
- o
If applicable, the name and address of the authorised representative.
- o
Data identifying the product in question.
- o
Declaration that the product is intended for use only by a specific patient or user, identified by a name, an acronym, or a numerical code.
- o
Name of the person issuing the prescription and who is authorised to do so by national law by virtue of their professional qualification and, if applicable, the name of the healthcare facility concerned.
- o
Specific characteristics of the product indicated by the prescription.
- o
Declaration that the product conforms to the general safety and performance requirements of Annex 1 and, where applicable, an indication of any general safety and performance requirements that it does not fully meet, with reasons.
- o
If applicable, indication that the device contains or incorporates a medicinal substance, including a human blood or human plasma derivative, or cells or tissues of human or animal origin as covered in Regulation (EU) No 722/2012.
- o
- -
Make the documentation available to the competent national authorities showing the place(s) of manufacture and allowing an understanding of the design, manufacture, and operation of the product, including the expected performance.
- -
Take the necessary measures to ensure that the manufacturing process guarantees the conformity of the products manufactured.
Therefore, in view of current legislation, the following documentation is advisable for intrahospital additive manufacturing:
- o
Technical documentation for each medical device.
- o
Medical device manufacturer's licence.
- o
Quality system ISO 13485:2016.
- o
In cases where required, POC manufacturing will allow networking with other health centres or manufacturing companies that are in possession of the above documentation.
ConclusionsMedical 3DP in OST is an innovative and growing technology with many practical applications. The use of 3D biomodels for preoperative planning, surgeries assisted by patient-specific surgical guides, custom-made splints or implants and the application of bioengineering techniques are some of the utilities that will reduce costs and surgical times and will increase the safety of interventions and patient and doctor satisfaction.
The current technological development enables the whole process of design, validation, and manufacturing of medical products to be integrated in the hospital itself. This requires the establishment of workflows and the implementation of existing legislation.
It is essential that surgeons, as the medical specialists responsible for prescribing and delivering treatment, know this technology and its uses for the correct implementation of personalised medicine.
Level of evidenceLevel of evidence IV.
Conflicts of interestThe authors have no conflict of interests to declare.
Please cite this article as: Andrés-Cano P, Calvo-Haro JA, Fillat-Gomà F, Andrés-Cano I, Perez-Mañanes R. Papel del cirujano ortopédico y traumatólogo en la impresión 3D: aplicaciones actuales y aspectos legales para una medicina personalizada. Rev Esp Cir Ortop Traumatol. 2021;65:138–151.